Key Points
This clinical study assessed idelalisib, a selective PI3Kδ inhibitor, in 64 patients with relapsed, indolent non-Hodgkin lymphoma.
Idelalisib treatment rapidly induced durable disease responses in heavily pretreated patients with a favorable safety profile.
Abstract
Idelalisib (GS-1101, CAL-101), an oral inhibitor of phosphatidylinositol 3-kinase-δ, was evaluated in a phase I study in 64 patients with relapsed indolent non-Hodgkin lymphoma (iNHL). Patients had a median (range) age of 64 (32-91) years, 34 (53%) had bulky disease (≥1 lymph nodes ≥5 cm), and 37 (58%) had refractory disease. Patients had received a median (range) of 4 (1-10) prior therapies. Eight dose regimens of idelalisib were evaluated; idelalisib was taken once or twice daily continuously at doses ranging from 50 to 350 mg. After 48 weeks, patients still benefitting (n = 19; 30%) enrolled into an extension study. Adverse events (AEs) occurring in 20% or more patients (total%/grade ≥3%) included diarrhea (36/8), fatigue (36/3), nausea (25/3), rash (25/3), pyrexia (20/3), and chills (20/0). Laboratory abnormalities included neutropenia (44/23), anemia (31/5), thrombocytopenia (25/11), and serum transaminase elevations (48/25). Twelve (19%) patients discontinued therapy due to AEs. Idelalisib induced disease regression in 46/54 (85%) of evaluable patients achieving an overall response rate of 30/64 (47%), with 1 patient having a complete response (1.6%). Median duration of response was 18.4 months, median progression-free survival was 7.6 months. Idelalisib is well tolerated and active in heavily pretreated, relapsed/refractory patients with iNHL. These trials were registered at clinicaltrials.gov as NCT00710528 and NCT01090414.
Introduction
Indolent non-Hodgkin lymphomas (iNHL) are a group of slow-growing, but incurable, B-cell malignancies constituting approximately one-third of all cases of NHL and include follicular lymphoma (FL), small lymphocytic lymphoma/leukemia (SLL), marginal zone lymphoma (MZL), and lymphoplasmacytic lymphoma [Waldenström’s macroglobulinemia (LPL/WM)].1-3 In 2013, it is estimated that ∼20 000 people in the United States will be diagnosed with iNHL, and ∼7000 will die of this disease4,5
Although initially effective in most patients, current chemotherapy treatments for iNHL demonstrate decreasing efficacy with repeated administrations and are associated with short- and long-term toxicities, including myelosuppression, cardiac toxicity, and secondary malignancies.6-9 The most recent advances in therapy for iNHL that had a major influence on disease control include anti-CD20 antibodies such as rituximab (approved in the US in 1997),10 bendamustine (approved in the US in 2008),11 which demonstrated good activity and tolerability, and radioimmunotherapies,12 including 131I-Tositumomab (Bexxar, approved in the US in 2003),13 and 90Y-Ibritumomab (Zevalin, approved in the US in 2002),14 which, while active, have potential long-lasting toxicities and are rarely used. Indeed, Bexxar has now been withdrawn from the market.15 Thus, for iNHL patients who relapse, there is a need for drugs with new mechanisms of action that can control disease with an acceptable safety profile, either when used as single agents or in combination with existing therapies.
Phosphatidylinositol 3-kinase (PI3K) is a lipid kinase having a catalytic subunit that exists in 4 different isoforms: α, β, γ, and δ. Activation of PI3K generates lipid second messengers at the cell membrane that recruit and activate multiple intracellular enzymes that are regulators of cell proliferation, survival, and motility.16,17 The α and β isoforms are widely expressed in many tissues, whereas the γ and δ isoforms are highly restricted to hematopoietic cells. In B lymphocytes, the δ isoform (PI3Kδ) plays a central role in normal B-cell development and function, transducing signals from the B-cell receptor as well as from receptors for various cytokines, chemokines, and integrins.18,19 PI3Kδ signaling pathways are commonly hyperactive in B-cell malignancies,20-22 making inhibition of PI3Kδ a promising target in the therapy of iNHL.
Idelalisib (5-fluoro-3-phenyl-2-[(s)-1-(9H-purin-6-ylamino)-propyl]-3H-quinazolin-4-one) is a potent, small-molecule inhibitor of PI3Kδ that is highly selective for the δ isoform compared with the α, β, and γ isoforms.20 In lymphoid cell lines and primary patient samples, idelalisib blocks PI3Kδ/AKT signaling and promotes apoptosis.20-22 Phase 1a testing in healthy volunteers established idelalisib oral bioavailability and safety at dose levels that achieved plasma concentrations shown to inhibit PI3Kδ in preclinical models.23
Based on these data, we conducted phase 1b dose-escalation and extension studies of idelalisib in patients with relapsed B-cell malignancies. Our objectives were to characterize the drug’s safety, pharmacokinetics, and clinical activity; here, we report on the outcomes in participants with previously treated iNHL.
Patients and methods
A primary dose-ranging study was performed that evaluated patients through 48 weeks of idelalisib treatment, with an extension study for patients who were benefiting from continued idelalisib therapy. The study protocols were approved by institutional review boards at each of the 8 enrolling study centers. All patients provided written informed consent before enrollment. These studies were conducted under a US Investigational New Drug Application in accordance with International Conference on Harmonization guidelines for Good Clinical Practice and the original principles embodied in the Declaration of Helsinki. All authors had full access to study data and were involved in data interpretation and manuscript preparation.
Patients and eligibility criteria
Inclusion criteria.
Patients were required to be ≥18 years of age and have a histologically confirmed diagnosis of iNHL, including histologic subtypes of FL (grades 1, 2, and 3a), SLL, MZL, and LPL/WM. Measurable disease was also required consisting of ≥1 lesion measuring >2 cm in a single dimension by computed tomography. Patients with LPL/WM who did not have measurable disease could enroll if they had measurable serum monoclonal immunoglobulin (Ig)M with symptomatic hyperviscosity or clinically relevant cytopenias. Patients must have received ≥1 prior chemotherapy regimen and prior rituximab and have a WHO performance status ≤2. Sexually active men and women of child-bearing potential were required to use adequate contraception for the duration of the study.
Exclusion criteria.
Key exclusion criteria were known active central nervous system lymphoma, active serious infection requiring systemic therapy, or prior stem-cell transplantation with active graft-versus-host-disease. Other reasons for exclusion were serum creatinine ≥2.0 mg/dL; serum bilirubin ≥2.0 mg/dL (unless due to Gilbert’s syndrome); serum transaminases ≥2 times the upper limit of normal; thrombocytopenia requiring platelet transfusion support; serological evidence of HIV, active hepatitis B, or hepatitis C; or poorly controlled diabetes mellitus. Female patients were excluded if they were pregnant or nursing.
Study design and treatment
Idelalisib was provided as capsules or tablets containing 50, 75, or 100 mg of active drug. Depending upon the cohort, patients took idelalisib orally once per day (QD) or twice per day (BID). For the BID schedule, doses were to be taken at ∼12-hour intervals.
Dose escalation was performed in a 3+3 design in sequential cohorts of patients with various hematological malignancies, including iNHL. Initially, patients were treated at 50, 100, 200, and 350 mg BID based on idelalisib safety and pharmacokinetic data from the prior phase 1a healthy volunteer experience.23 Dosing regimens of 150 mg BID, 150 or 300 mg QD, and 150 mg BID taken 3 weeks on and 1 week off in every 4-week cycle were subsequently added. Idelalisib was continued through 48 weeks on the primary study and indefinitely on the extension study provided there was continued clinical benefit.
Supportive care such as antiemetics, therapeutic or prophylactic antibiotics, transfusions, or growth factors was allowed based on investigator and institutional standards of care.
Safety assessments
All adverse events (AEs) were evaluated, and complete blood counts and standard serum chemistry tests were collected pretreatment, weekly for 4 weeks, every 2 weeks for 4 weeks, every 4 weeks for 12 weeks, and thereafter every 8 to 12 weeks. Fasting serum insulin and proinsulin C-peptide levels were measured at 8-week intervals through 24 weeks. Serum Igs and immunophenotyped peripheral blood leukocytes were assessed at 8- to 12-week intervals through 48 weeks. Electrocardiograms were performed at screening and pre- and 1.5 hours postdose on days 1 and 28.
AEs were coded using the Medical Dictionary for Regulatory Activities, and the severity of AEs and laboratory abnormalities were graded using the Common Terminology Criteria for Adverse Events, version 3.0.24 Dose limiting toxicities (DLTs) were defined as any AEs related to idelalisib that occurred within 28 days of starting therapy and were either grade 4 hematologic AEs persisting for ≥7 days or grade ≥3 nonhematologic AEs. Only serious AEs and grade ≥3 AEs were collected during the extension phase of the study.
Pharmacokinetic assessments
Plasma samples were obtained for pharmacokinetic analyses at 0, 0.5, 1, 1.5, 2, 3, 4, and 6 hours relative to the morning idelalisib dose on days 1 and 28. Additional trough values were collected on days 8, 15, and 22. Idelalisib concentrations were determined using a validated bioanalytical method with a lower limit of quantitation of 0.5 ng/mL.23
Efficacy assessments
Patients were assessed for disease response or progression at week 4, week 8, every 8 weeks until week 48, and then every 12 weeks using standard response criteria for lymphoma25 and additional criteria for LPL/WM where appropriate.26 Efficacy endpoints included overall response rate (ORR), time to response (TTR), duration of response (DOR), and progression-free survival (PFS). Lymph node changes relative to baseline were determined using the sum of the products of the perpendicular diameters for all measured nodal masses.
Statistical analysis
The maximum tolerated dose (MTD) was defined as the dose regimen in which the lower 95% confidence interval (CI) for DLT rate was >25%.
Safety, ORR, and PFS were assessed in all patients who received any amount of idelalisib. For TTR and DOR, we included only responding patients. Changes in lymph node area, and pharmacokinetic parameters were analyzed in all patients who had the necessary baseline and on-study measurements, as appropriate, to provide interpretable results for the specific parameters of interest.
Data were analyzed descriptively by dose regimen and histological iNHL subtype, as appropriate. Unless otherwise indicated, data from the dose-ranging study and the extension study were considered together. When required for the statistical analysis of a particular variable, the baseline value was the last recorded value prior to the administration of the first dose of study treatment. Pharmacokinetic variables of maximum concentration, area under the concentration-time curve over 12 or 24 hours, and trough concentration were derived using noncompartmental or population pharmacokinetic methods. For response rates, exact binomial 95% CIs were computed. In calculating time-to-event variables, Kaplan-Meier methods were employed.
Results
Patient characteristics
Between July 2008 and January 2011, 64 patients with iNHL were enrolled (Table 1). Data including the extension study are presented through July 2013. The median age was 64 years with a range of 32 to 91 years. Most patients were male (69%). Lymphoma types included: FL, 38 subjects (59%); SLL, 11 subjects (17%); MZL, 6 subjects (9%); and LPL, 9 subjects (14%). Of 64 patients, 28 (44%) presented with bulky lymphadenopathy, defined as having ≥1 lymph node ≥5 cm in diameter.27 Patients frequently presented with cytopenias (≥grade 1), including 41 (64%) with anemia, 36 (56%) with thrombocytopenia, and 7 (11%) with neutropenia. Patients were extensively pretreated with chemoimmunotherapy; the median number of prior therapies was 4 (range 1-10). Most patients had received rituximab (97%) or an alkylating agent (91%), more than one-half of the patients (52%) had previously received an anthracycline, 27 patients (42%) had been treated with a purine analog, and 17 patients (27%) had prior bendamustine. Thirty-seven patients (58%) were refractory to their last prior regimen, defined as a lack of response to that treatment or progressive disease (PD) ≤6 months from completion of the last prior therapy.
Patient baseline characteristics
| Patient characteristic . | N = 64 . |
|---|---|
| Age, median (range), years | 64 (32-91) |
| Sex, male/female, n (%) | 44 (69)/20 (31) |
| Disease type, n (%) | |
| FL | 38 (59) |
| SLL | 11 (17) |
| LPL | 9 (14) |
| MZL | 6 (9) |
| Bulky disease (≥1 lymph node ≥5 cm), n (%) | 28 (44) |
| Elevated LDH | 24 (38) |
| Cytopenias, n (%) any grade/n (%) grade ≥3 | |
| Anemia | 41 (64)/3 (5) |
| Thrombocytopenia | 36 (56)/2 (3) |
| Neutropenia | 7 (11)/2 (3) |
| Prior therapies, median (range), n | 4 (1-10) |
| Prior therapy type, n (%) | |
| Rituximab | 62 (97) |
| Alkylating agent | 58 (91) |
| Anthracycline/anthracenedione | 33 (52) |
| Purine analog | 27 (42) |
| Bendamustine | 17 (27) |
| Refractory to last prior therapy, n (%)* | 37 (58) |
| Patient characteristic . | N = 64 . |
|---|---|
| Age, median (range), years | 64 (32-91) |
| Sex, male/female, n (%) | 44 (69)/20 (31) |
| Disease type, n (%) | |
| FL | 38 (59) |
| SLL | 11 (17) |
| LPL | 9 (14) |
| MZL | 6 (9) |
| Bulky disease (≥1 lymph node ≥5 cm), n (%) | 28 (44) |
| Elevated LDH | 24 (38) |
| Cytopenias, n (%) any grade/n (%) grade ≥3 | |
| Anemia | 41 (64)/3 (5) |
| Thrombocytopenia | 36 (56)/2 (3) |
| Neutropenia | 7 (11)/2 (3) |
| Prior therapies, median (range), n | 4 (1-10) |
| Prior therapy type, n (%) | |
| Rituximab | 62 (97) |
| Alkylating agent | 58 (91) |
| Anthracycline/anthracenedione | 33 (52) |
| Purine analog | 27 (42) |
| Bendamustine | 17 (27) |
| Refractory to last prior therapy, n (%)* | 37 (58) |
Lack or response or iNHL progression ≤6 mo from completion of last prior therapy.
Patient disposition
Patients initially were treated at 50, 100, 200, and 350 mg BID. Following the occurrence of grade 3 serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations in a patient taking 350 mg BID, enrollment to this cohort was halted, and other cohorts were expanded (up to 12 patients/cohort). Primarily due to observations of serum AST/ALT elevations at other dosing levels, dosing regimens of 150 mg BID, 150 or 300 mg QD, and 150 mg BID taken 3 weeks on and 1 week off in every 4-week cycle were also explored.
Of 64 patients, 45 patients (70%) discontinued treatment during the primary 48-week study, while 19 (30%) completed the primary study and enrolled in the extension study (Table 2). Of the 19 patients who entered the extension trial, 1 patient was still receiving treatment as of the data cutoff for the analysis. The major reasons for study discontinuation were PD followed by AEs.
Patient disposition
| Disposition . | Primary study (N = 64) . | Extension study (N = 19) . |
|---|---|---|
| Idelalisib dose regimen, n (%) | ||
| 50 mg BID | 7 (11) | 0 |
| 150 mg QD | 9 (14) | 3 |
| 150 mg BID (3 wk on, 1 wk off) | 12 (19) | 1 |
| 100 mg BID | 7 (11) | 3 |
| 300 mg QD | 5 (8) | 3 |
| 150 mg BID | 10 (15) | 3 |
| 200 mg BID | 10 (15) | 4 |
| 350 mg BID | 4 (6) | 2 |
| Completed study (≥48 wk), n (%) | 19 (30) | 1 ongoing |
| Discontinued, n (%) | 45 (70) | 18 (95) |
| Disease progression | 23 (36) | 9 (47) |
| AE | 8 (13) | 4 (21) |
| Patient/investigator request | 2 (3) | 2 (11) |
| Withdrew consent | 2 (3) | 0 |
| Prohibited drug | 1 (2) | 0 |
| Patient noncompliance | 1 (2) | 0 |
| Death | 0 | 1 (5) |
| Other* | 8 (13) | 2 (11) |
| Disposition . | Primary study (N = 64) . | Extension study (N = 19) . |
|---|---|---|
| Idelalisib dose regimen, n (%) | ||
| 50 mg BID | 7 (11) | 0 |
| 150 mg QD | 9 (14) | 3 |
| 150 mg BID (3 wk on, 1 wk off) | 12 (19) | 1 |
| 100 mg BID | 7 (11) | 3 |
| 300 mg QD | 5 (8) | 3 |
| 150 mg BID | 10 (15) | 3 |
| 200 mg BID | 10 (15) | 4 |
| 350 mg BID | 4 (6) | 2 |
| Completed study (≥48 wk), n (%) | 19 (30) | 1 ongoing |
| Discontinued, n (%) | 45 (70) | 18 (95) |
| Disease progression | 23 (36) | 9 (47) |
| AE | 8 (13) | 4 (21) |
| Patient/investigator request | 2 (3) | 2 (11) |
| Withdrew consent | 2 (3) | 0 |
| Prohibited drug | 1 (2) | 0 |
| Patient noncompliance | 1 (2) | 0 |
| Death | 0 | 1 (5) |
| Other* | 8 (13) | 2 (11) |
Other in primary study includes: investigator decision/mild progression, need of additional therapy, deterioration of functional status, limited benefit; poor tolerance, patient decision/head injury; difficulty with transportation, and incarceration.
Treatment administration and safety
Idelalisib median (range) treatment duration for the 64 iNHL patients was 3.8 (0.3-41) months, with a mean (SD) of 9.3 (±10.52) months. A clear MTD of idelalisib was not identified. In the initial dose escalation, 1 patient had grade 3 elevation in hepatic transaminases while receiving 350 mg BID, leading to the suspension of enrollment at that dose. A further 4 patients had grade ≥3 ALT/AST elevations occurring with 3 different dosing regimens: 150 mg BID (n = 1), 200 mg BID (n = 2), and 300 mg QD (n = 1). As specified by the protocol, all of these serum transaminase abnormalities were DLTs, because they occurred within the first 28 days of the start of idelalisib therapy. However, additional patients treated with these and other dosing regimens had grade ≥3 increases in serum ALT/AST that first became apparent >28 days after initiation of idelalisib. Based on these further events in 7 of 8 dose regimens, this AE was not dose related.
Table 3 summarizes the treatment-emergent AEs of any grade together with rates of corresponding grade ≥3 AEs. Most treatment-emergent AEs were grade 1 to 2 in severity and likely reflected the underlying disease or previous therapy. The most common AEs were fatigue, diarrhea, nausea, rash, and chills and pyrexia. The most common grade ≥3 AEs were pneumonia and diarrhea. There was no clear relationship between dose regimen and AEs.
Incidence of treatment-emergent AEs and selected laboratory abnormalities reported in ≥10% of patients (N = 64)
| Description . | Dosing regimen . | Overall (N = 64) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg BID 28 d (N = 7) . | 150 mg QD 28 d (N = 9) . | 100 mg BID 28 d (N = 7) . | 150 mg BID 21 d (N = 12) . | 300 mg QD 28 d (N = 5) . | 150 mg BID 28 d (N = 10) . | 200 mg BID 28 d (N = 10) . | 350 mg BID 28 d (N = 4) . | |||||||||||
| Grade . | ||||||||||||||||||
| Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | |
| AE, n (%) | ||||||||||||||||||
| Diarrhea | 4 (57.1) | 1 (14.3) | 3 (33.3) | 0 | 0 | 0 | 6 (50.0) | 1 (8.3) | 2 (40.0) | 2 (40.0) | 4 (40.0) | 1 (10.0) | 2 (20.0) | 0 | 2 (50.0) | 1 (25.0) | 23 (35.9) | 6 (9.4) |
| Fatigue | 4 (57.1) | 0 | 2 (22.2) | 1 (11.1) | 2 (28.6) | 1 (14.3) | 6 (50.0) | 0 | 1 (20.0) | 0 | 2 (20.0) | 0 | 5 (50.0) | 0 | 1 (25.0) | 0 | 23 (35.9) | 2 (3.1) |
| Nausea | 3 (42.9) | 1 (14.3) | 1 (11.1) | 0 | 2 (28.6) | 0 | 4 (33.3) | 0 | 0 | 0 | 1 (10.0) | 0 | 4 (40.0) | 0 | 1 (25.0) | 0 | 16 (25.0) | 1 (1.6) |
| Rash | 1 (14.3) | 0 | 1 (11.1) | 0 | 0 | 0 | 3 (25.0) | 0 | 3 (60.0) | 2 (40.0) | 4 (40.0) | 0 | 3 (30.0) | 0 | 1 (25.0) | 0 | 16 (25.0) | 2 (3.1) |
| Chills | 3 (42.9) | 0 | 1 (11.1) | 0 | 2 (28.6) | 0 | 1 (8.3) | 0 | 1 (20.0) | 0 | 2 (20.0) | 0 | 2 (20.0) | 0 | 1 (25.0) | 0 | 13 (20.3) | 0 |
| Pyrexia | 1 (14.3) | 0 | 2 (22.2) | 1 (11.1) | 1 (14.3) | 0 | 2 (16.7) | 0 | 0 | 0 | 4 (40.0) | 0 | 2 (20.0) | 1 (10.0) | 1 (25.0) | 0 | 13 (20.3) | 2 (3.1) |
| Cough | 0 | 0 | 1 (11.1) | 0 | 1 (14.3) | 0 | 1 (8.3) | 0 | 0 | 0 | 4 (40.0) | 1 (10.0) | 5 (50.0) | 0 | 0 | 0 | 12 (18.8) | 1 (1.6) |
| Pneumonia | 1 (14.3) | 1 (14.3) | 0 | 0 | 2 (28.6) | 2 (28.6) | 3 (25.0) | 3 (25.0) | 2 (40.0) | 1 (20.0) | 2 (20.0) | 2 (20.0) | 1 (10.0) | 1 (10.0) | 1 (25.0) | 1 (25.0) | 12 (18.8) | 11 (17.2) |
| URI | 1 (14.3) | 0 | 1 (11.1) | 0 | 2 (28.6) | 0 | 3 (25.0) | 0 | 2 (40.0) | 0 | 0 | 0 | 2 (20.0) | 0 | 0 | 0 | 11 (17.2) | 0 |
| Edema peripheral | 2 (28.6) | 0 | 1 (11.1) | 1 (11.1) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (20.0) | 0 | 3 (30.0) | 0 | 1 (25.0) | 1 (25.0) | 9 (14.1) | 2 (3.1) |
| Constipation | 2 (28.6) | 0 | 1 (11.1) | 0 | 0 | 0 | 0 | 0 | 1 (20.0) | 0 | 2 (20.0) | 0 | 1 (10.0) | 0 | 1 (25.0) | 0 | 8 (12.5) | 0 |
| Insomnia | 1 (14.3) | 0 | 1 (11.1) | 0 | 1 (14.3) | 0 | 1 (8.3) | 0 | 1 (20.0) | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 | 1 (25.0) | 0 | 8 (12.5) | 0 |
| Night sweats | 3 (42.9) | 0 | 0 | 0 | 1 (14.3) | 0 | 0 | 0 | 0 | 0 | 1 (10.0) | 0 | 2 (20.0) | 0 | 1 (25.0) | 0 | 8 (12.5) | 0 |
| Vomiting | 4 (57.1) | 0 | 0 | 0 | 0 | 0 | 2 (16.7) | 0 | 0 | 0 | 0 | 0 | 1 (10.0) | 0 | 1 (25.0) | 0 | 8 (12.5) | 0 |
| Selected laboratory abnormality, n (%) | ||||||||||||||||||
| AST, increased | 3 (42.9) | 2 (28.6) | 4 (44.4) | 0 | 4 (57.1) | 1 (14.3) | 3 (25.0) | 0 | 3 (60.0) | 1 (20.0) | 7 (70.0) | 3 (30.0) | 7 (70.0) | 4 (40.0) | 3 (75.0) | 2 (50.0) | 34 (53.1) | 13 (20.3) |
| ALT, increased | 2 (28.6) | 2 (28.6) | 3 (33.3) | 1 (11.1) | 3 (42.9) | 0 | 3 (25.0) | 0 | 3 (60.0) | 1 (20.0) | 7 (70.0) | 4 (40.0) | 8 (80.0) | 5 (50.0) | 2 (50.0) | 2 (50.0) | 31 (48.4) | 15 (23.4) |
| AP, increased | 2 (28.6) | 0 | 3 (33.3) | 1 (11.1) | 2 (28.6) | 1 (14.3) | 3 (25.0) | 0 | 1 (20.0) | 0 | 5 (50.0) | 1 (10.0) | 8 (80.0) | 0 | 1 (25.0) | 0 | 25 (39.1) | 3 (4.7) |
| Bilirubin, increased | 1 (14.3) | 0 | 1 (11.1) | 0 | 0 | 0 | 3 (25.0) | 1 (8.3) | 2 (40.0) | 0 | 1 (10.0) | 1 (10.0) | 3 (30.0) | 0 | 1 (25.0) | 0 | 12 (18.8) | 2 (3.1) |
| Glucose, increased | 1 (14.3) | 0 | 1 (11.1) | 0 | 7 (100.0) | 0 | 6 (50.0) | 1 (8.3) | 1 (20.0) | 0 | 3 (30.0) | 0 | 5 (50.0) | 0 | 1 (25.0) | 0 | 25 (39.1) | 1 (1.6) |
| Glucose, decreased | 2 (28.6) | 0 | 2 (22.2) | 0 | 1 (14.3) | 0 | 2 (16.7) | 1 (8.3) | 1 (20.0) | 0 | 4 (40.0) | 0 | 0 | 0 | 1 (25.0) | 0 | 13 (20.3) | 1 (1.6) |
| ANC, decreased | 4 (57.1) | 3 (42.9) | 3 (33.3) | 2 (22.2) | 2 (28.6) | 0 | 5 (41.7) | 4 (33.3) | 3 (60.0) | 1 (20.0) | 2 (20.0) | 1 (10.0) | 6 (60.0) | 2 (20.0) | 3 (75.0) | 2 (50.0) | 28 (43.8) | 15 (23.4) |
| Hgb, decreased | 2 (28.6) | 1 (14.3) | 2 (22.2) | 1 (11.1) | 0 | 0 | 2 (16.7) | 0 | 2 (40.0) | 0 | 5 (50.0) | 0 | 5 (50.0) | 1 (10.0) | 2 (50.0) | 0 | 20 (31.3) | 3 (4.7) |
| Platelets, decreased | 0 | 0 | 4 (44.4) | 2 (22.2) | 1 (14.3) | 0 | 5 (41.7) | 2 (16.7) | 0 | 0 | 1 (10.0) | 0 | 2 (20.0) | 1 (10.0) | 3 (75.0) | 2 (50.0) | 16 (25.0) | 7 (10.9) |
| Description . | Dosing regimen . | Overall (N = 64) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg BID 28 d (N = 7) . | 150 mg QD 28 d (N = 9) . | 100 mg BID 28 d (N = 7) . | 150 mg BID 21 d (N = 12) . | 300 mg QD 28 d (N = 5) . | 150 mg BID 28 d (N = 10) . | 200 mg BID 28 d (N = 10) . | 350 mg BID 28 d (N = 4) . | |||||||||||
| Grade . | ||||||||||||||||||
| Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | |
| AE, n (%) | ||||||||||||||||||
| Diarrhea | 4 (57.1) | 1 (14.3) | 3 (33.3) | 0 | 0 | 0 | 6 (50.0) | 1 (8.3) | 2 (40.0) | 2 (40.0) | 4 (40.0) | 1 (10.0) | 2 (20.0) | 0 | 2 (50.0) | 1 (25.0) | 23 (35.9) | 6 (9.4) |
| Fatigue | 4 (57.1) | 0 | 2 (22.2) | 1 (11.1) | 2 (28.6) | 1 (14.3) | 6 (50.0) | 0 | 1 (20.0) | 0 | 2 (20.0) | 0 | 5 (50.0) | 0 | 1 (25.0) | 0 | 23 (35.9) | 2 (3.1) |
| Nausea | 3 (42.9) | 1 (14.3) | 1 (11.1) | 0 | 2 (28.6) | 0 | 4 (33.3) | 0 | 0 | 0 | 1 (10.0) | 0 | 4 (40.0) | 0 | 1 (25.0) | 0 | 16 (25.0) | 1 (1.6) |
| Rash | 1 (14.3) | 0 | 1 (11.1) | 0 | 0 | 0 | 3 (25.0) | 0 | 3 (60.0) | 2 (40.0) | 4 (40.0) | 0 | 3 (30.0) | 0 | 1 (25.0) | 0 | 16 (25.0) | 2 (3.1) |
| Chills | 3 (42.9) | 0 | 1 (11.1) | 0 | 2 (28.6) | 0 | 1 (8.3) | 0 | 1 (20.0) | 0 | 2 (20.0) | 0 | 2 (20.0) | 0 | 1 (25.0) | 0 | 13 (20.3) | 0 |
| Pyrexia | 1 (14.3) | 0 | 2 (22.2) | 1 (11.1) | 1 (14.3) | 0 | 2 (16.7) | 0 | 0 | 0 | 4 (40.0) | 0 | 2 (20.0) | 1 (10.0) | 1 (25.0) | 0 | 13 (20.3) | 2 (3.1) |
| Cough | 0 | 0 | 1 (11.1) | 0 | 1 (14.3) | 0 | 1 (8.3) | 0 | 0 | 0 | 4 (40.0) | 1 (10.0) | 5 (50.0) | 0 | 0 | 0 | 12 (18.8) | 1 (1.6) |
| Pneumonia | 1 (14.3) | 1 (14.3) | 0 | 0 | 2 (28.6) | 2 (28.6) | 3 (25.0) | 3 (25.0) | 2 (40.0) | 1 (20.0) | 2 (20.0) | 2 (20.0) | 1 (10.0) | 1 (10.0) | 1 (25.0) | 1 (25.0) | 12 (18.8) | 11 (17.2) |
| URI | 1 (14.3) | 0 | 1 (11.1) | 0 | 2 (28.6) | 0 | 3 (25.0) | 0 | 2 (40.0) | 0 | 0 | 0 | 2 (20.0) | 0 | 0 | 0 | 11 (17.2) | 0 |
| Edema peripheral | 2 (28.6) | 0 | 1 (11.1) | 1 (11.1) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (20.0) | 0 | 3 (30.0) | 0 | 1 (25.0) | 1 (25.0) | 9 (14.1) | 2 (3.1) |
| Constipation | 2 (28.6) | 0 | 1 (11.1) | 0 | 0 | 0 | 0 | 0 | 1 (20.0) | 0 | 2 (20.0) | 0 | 1 (10.0) | 0 | 1 (25.0) | 0 | 8 (12.5) | 0 |
| Insomnia | 1 (14.3) | 0 | 1 (11.1) | 0 | 1 (14.3) | 0 | 1 (8.3) | 0 | 1 (20.0) | 0 | 1 (10.0) | 0 | 1 (10.0) | 0 | 1 (25.0) | 0 | 8 (12.5) | 0 |
| Night sweats | 3 (42.9) | 0 | 0 | 0 | 1 (14.3) | 0 | 0 | 0 | 0 | 0 | 1 (10.0) | 0 | 2 (20.0) | 0 | 1 (25.0) | 0 | 8 (12.5) | 0 |
| Vomiting | 4 (57.1) | 0 | 0 | 0 | 0 | 0 | 2 (16.7) | 0 | 0 | 0 | 0 | 0 | 1 (10.0) | 0 | 1 (25.0) | 0 | 8 (12.5) | 0 |
| Selected laboratory abnormality, n (%) | ||||||||||||||||||
| AST, increased | 3 (42.9) | 2 (28.6) | 4 (44.4) | 0 | 4 (57.1) | 1 (14.3) | 3 (25.0) | 0 | 3 (60.0) | 1 (20.0) | 7 (70.0) | 3 (30.0) | 7 (70.0) | 4 (40.0) | 3 (75.0) | 2 (50.0) | 34 (53.1) | 13 (20.3) |
| ALT, increased | 2 (28.6) | 2 (28.6) | 3 (33.3) | 1 (11.1) | 3 (42.9) | 0 | 3 (25.0) | 0 | 3 (60.0) | 1 (20.0) | 7 (70.0) | 4 (40.0) | 8 (80.0) | 5 (50.0) | 2 (50.0) | 2 (50.0) | 31 (48.4) | 15 (23.4) |
| AP, increased | 2 (28.6) | 0 | 3 (33.3) | 1 (11.1) | 2 (28.6) | 1 (14.3) | 3 (25.0) | 0 | 1 (20.0) | 0 | 5 (50.0) | 1 (10.0) | 8 (80.0) | 0 | 1 (25.0) | 0 | 25 (39.1) | 3 (4.7) |
| Bilirubin, increased | 1 (14.3) | 0 | 1 (11.1) | 0 | 0 | 0 | 3 (25.0) | 1 (8.3) | 2 (40.0) | 0 | 1 (10.0) | 1 (10.0) | 3 (30.0) | 0 | 1 (25.0) | 0 | 12 (18.8) | 2 (3.1) |
| Glucose, increased | 1 (14.3) | 0 | 1 (11.1) | 0 | 7 (100.0) | 0 | 6 (50.0) | 1 (8.3) | 1 (20.0) | 0 | 3 (30.0) | 0 | 5 (50.0) | 0 | 1 (25.0) | 0 | 25 (39.1) | 1 (1.6) |
| Glucose, decreased | 2 (28.6) | 0 | 2 (22.2) | 0 | 1 (14.3) | 0 | 2 (16.7) | 1 (8.3) | 1 (20.0) | 0 | 4 (40.0) | 0 | 0 | 0 | 1 (25.0) | 0 | 13 (20.3) | 1 (1.6) |
| ANC, decreased | 4 (57.1) | 3 (42.9) | 3 (33.3) | 2 (22.2) | 2 (28.6) | 0 | 5 (41.7) | 4 (33.3) | 3 (60.0) | 1 (20.0) | 2 (20.0) | 1 (10.0) | 6 (60.0) | 2 (20.0) | 3 (75.0) | 2 (50.0) | 28 (43.8) | 15 (23.4) |
| Hgb, decreased | 2 (28.6) | 1 (14.3) | 2 (22.2) | 1 (11.1) | 0 | 0 | 2 (16.7) | 0 | 2 (40.0) | 0 | 5 (50.0) | 0 | 5 (50.0) | 1 (10.0) | 2 (50.0) | 0 | 20 (31.3) | 3 (4.7) |
| Platelets, decreased | 0 | 0 | 4 (44.4) | 2 (22.2) | 1 (14.3) | 0 | 5 (41.7) | 2 (16.7) | 0 | 0 | 1 (10.0) | 0 | 2 (20.0) | 1 (10.0) | 3 (75.0) | 2 (50.0) | 16 (25.0) | 7 (10.9) |
The most common laboratory abnormalities included thrombocytopenia, anemia, neutropenia, and serum AST/ALT elevations. The most frequent grade ≥3 laboratory abnormalities included AST/ALT elevations in 16 (25%) patients and neutropenia in 16 (25%) patients. Other grade ≥3 cytopenias included thrombocytopenia in 8 patients (12.5%) and anemia in 2 patients (3%). There was no obvious relationship between the dose regimen and the pattern of laboratory abnormalities. No tumor flare reactions were noted, and no tumor lysis syndrome was seen.
AEs leading to idelalisib discontinuation included serum ALT/AST elevations in 4 (6.3%) patients, pneumonia in 3 (4.7%) patients, and diarrhea, acute renal failure, and thrombocytopenia in 2 (3%) patients each. The most common serious AEs included pneumonia in 11 (17.2%) patients and acute renal failure, diarrhea, febrile neutropenia, and pulmonary embolism in 4 (6%) patients each. There were 2 deaths in the primary study (both had discontinued due to pneumonia) and 1 death in the extension study in a patient who developed bowel obstruction, sepsis, and acute renal insufficiency.
Grade 3 diarrhea occurred in 6 patients (2 were also reported as colitis). Two of these 6 patients presented early (at 1.2 and 1.3 months). One resolved with continued treatment, the other resolved after idelalisib was temporarily withheld and was successfully rechallenged at a lower dose. Four patients presented between 11 and 28 months. Two patients had other medical conditions, including renal failure and pneumonia, and both discontinued study. Two patients were managed with temporary interruption of idelalisib, one received budesonide in addition, and both resolved and were successfully rechallenged at a lower dose. One patient had a subsequent recurrence 6 months later and discontinued study.
Asymptomatic transaminase elevations occurred in 31 (48%) of patients, 16 (25%) of which were grade ≥3 in severity. Grade 1 and 2 elevations were transient and reverted to normal despite continued idelalisib dosing. Grade ≥3 serum transaminase elevations occurred with a median onset of 5.3 weeks (range 2-9 weeks) and were managed with temporary idelalisib interruption. All resolved to grade ≤1, with a median time of 3.7 weeks (range 2-6 weeks). Of the 16 patients with grade ≥3 serum transaminase elevations, 8 were rechallenged with idelalisib and 6 (75%) had no recurrence. Two patients had recurrent serum ALT/AST elevations on rechallenge and were discontinued from study. Of the 8 patients who were not rechallenged, 3 were discontinued because of the serum transaminase elevations, 3 had PD at the 8-week evaluation and were withdrawn from the study, and 2 withdrew from the study. There were no cases of associated grade ≥3 elevations of serum bilirubin, and there were no cases of liver failure.
Serial changes in hematological parameters were evaluated. An overt pattern of time-dependent myelosuppression due to idelalisib was not apparent. Frequently, the occurrence of treatment-emergent hematological laboratory abnormalities arose from fluctuations in blood counts, particularly for neutropenia. Trends in improvements in baseline cytopenias were noted, particularly for anemia (not shown).
Increases in absolute lymphocyte count were occasionally noted, particularly in patients with SLL. A threefold or greater increase in absolute lymphocyte count was found in 15/64 patients overall. Incidence by subtype was SLL, 7/11(64%); FL, 7/38 (18%); MZL, 1/6 (16%); and 0/9 in LPL/WM. Consistent with this finding, peripheral lymphocytosis has been more commonly seen in CLL patients on idelalisib.28
There was no evidence of idelalisib-mediated, dose-dependent effects on serum glucose concentrations (Table 3). Similarly, no treatment-related changes in serum insulin or pro-insulin C-peptide values were observed (data not shown). Idelalisib treatment did not result in significant changes in serum Ig (IgA, IgE, IgG, or IgM) levels or in absolute numbers of CD4+/CD3+ T helper cells, CD8+/CD3+ cytotoxic T cells or CD56+/CD16+/CD3− natural killer cells (data not shown). Analysis of electrocardiograms obtained at baseline and during the study did not reveal evidence of treatment-emergent abnormalities (data not shown).
Pharmacokinetics
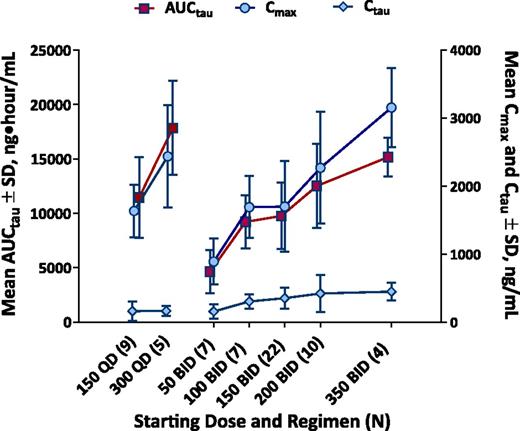
Idelalisib displayed linear pharmacokinetics (ie, not time dependent), a less-than dose-proportional increase in exposure, and achieved steady state by day 8. Idelalisib twice-daily administration resulted in higher trough concentrations, as expected, vs once-daily dosing at the corresponding dose level (Figure 1).
Dose-exposure relationship. Steady-state (day 28) idelalisib plasma exposures by idelalisib dosing regimen (total N = 64). AUCτ indicates area under the concentration-time curve over 12 (BID) or 24 (QD) hours; Cmax, maximum concentration; Cτ, trough concentration; SD, standard deviation.
Dose-exposure relationship. Steady-state (day 28) idelalisib plasma exposures by idelalisib dosing regimen (total N = 64). AUCτ indicates area under the concentration-time curve over 12 (BID) or 24 (QD) hours; Cmax, maximum concentration; Cτ, trough concentration; SD, standard deviation.
Efficacy
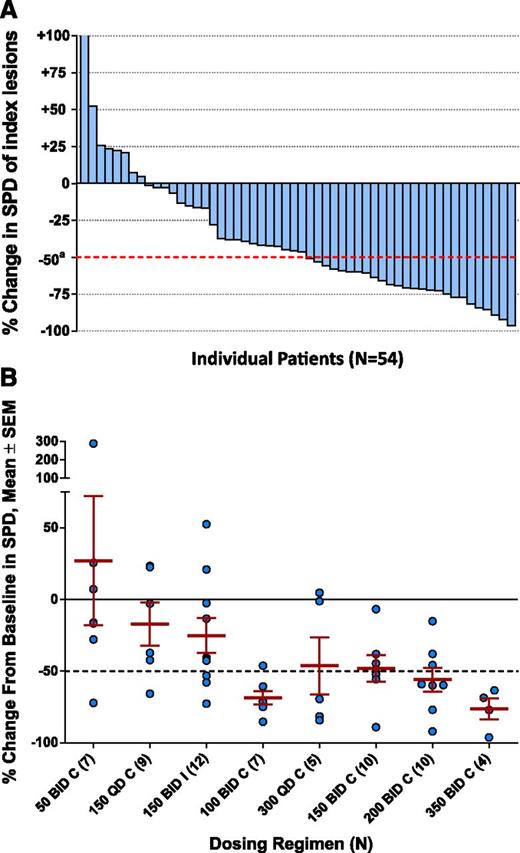
Of the 64 patients enrolled, 54 patients were evaluable for lymph node response, 5 had LPL/WM without sufficient lymphadenopathy, and 5 other patients did not have on-treatment follow-up scans. Of the evaluable patients, 46/54 (85%) had reductions in lymph node area from baseline during idelalisib therapy (Figure 2A). Nodal shrinkage mirrored the dose-exposure relationships, with reductions in nodal size being maximal at higher continuous BID dose levels (Figure 2B).
Lymph node response. (A) Best on-treatment percent changes in the SPD of measured lymph nodes in 54 evaluable patients (blue bars). Graph excludes 10 nonevaluable patients (5 patients with LPL/WM who had no measurable lymphadenopathy and 5 patients without a follow-up, on-treatment tumor assessment). (B) Best on-treatment change from baseline in SPD of measured lymph nodes by dosing regimen in 54 evaluable patients. SEM, standard error of the mean; SPD, sum of the products of the perpendicular diameters (of measured lymph nodes).
Lymph node response. (A) Best on-treatment percent changes in the SPD of measured lymph nodes in 54 evaluable patients (blue bars). Graph excludes 10 nonevaluable patients (5 patients with LPL/WM who had no measurable lymphadenopathy and 5 patients without a follow-up, on-treatment tumor assessment). (B) Best on-treatment change from baseline in SPD of measured lymph nodes by dosing regimen in 54 evaluable patients. SEM, standard error of the mean; SPD, sum of the products of the perpendicular diameters (of measured lymph nodes).
Table 4 shows response rates to idelalisib monotherapy. Considering all iNHL patients in the trial, 30/64 (47%) (95% CI: 34% to 60%) achieved an objective response (CR or PR: MR for WM/LPL). CR was reported in 1 patient (1.6%), PR in 25 patients (39%), and MR in 4 patients (6%), and 25 patients (39%) had SD, 4 patients (6%) had PD, and 5 patients (8%) were not evaluable. Among patients taking lower dose or intermittent dosing regimens (50 mg BID, 150 mg QD, or 100 mg BID continuously; and 150 mg BID for 21 days with 7 days off every 4 weeks), the ORR was 13/35 (37%) (95% CI: 21% to 55%), and among those receiving continuous higher doses (300 mg QD, 150 mg BID, 200 mg BID, and 350 mg BID), the ORR was 17/29 (59%) (95% CI: 39% to 76%) (Table 4). Patients with all subtypes of iNHL responded; response by disease subtype included FL, 17/38 (45%); SLL, 6/11 (55%); MZL, 2/6 (33%); and LPL/WM, 5/9 (55%).
Response rates, overall and by dosing regimen
| Response category . | All patients (N = 64) . | Receiving lower dosing regimens* (N = 35) . | Receiving higher dosing regimens† (N = 29) . |
|---|---|---|---|
| n (%) . | |||
| ORR | 30 (47) | 13 (37) | 17 (59) |
| CR | 1 (1.6) | 0 | 1 (3) |
| PR | 25 (41) | 11 (31) | 14 (48) |
| MR‡ | 4 (6) | 2 (6) | 2 (7) |
| SD | 25 (39) | 16 (46) | 9 (31) |
| PD | 4 (6) | 4 (11) | 0 |
| NE | 5 (8) | 2 (6) | 3 (10) |
| Response category . | All patients (N = 64) . | Receiving lower dosing regimens* (N = 35) . | Receiving higher dosing regimens† (N = 29) . |
|---|---|---|---|
| n (%) . | |||
| ORR | 30 (47) | 13 (37) | 17 (59) |
| CR | 1 (1.6) | 0 | 1 (3) |
| PR | 25 (41) | 11 (31) | 14 (48) |
| MR‡ | 4 (6) | 2 (6) | 2 (7) |
| SD | 25 (39) | 16 (46) | 9 (31) |
| PD | 4 (6) | 4 (11) | 0 |
| NE | 5 (8) | 2 (6) | 3 (10) |
NE, not evaluable; SD, stable disease.
Dosing regimens include 150 mg BID for 21 d with 7 d off every 4 wk, or 150 mg QD, 50 mg BID, and 100 mg BID continuously.
Dosing regimens include 300 mg QD, 150 mg BID, 200 mg BID, and 350 mg BID continuously.
Category for WM only.
The 30 responding patients experienced rapid reduction in lymphadenopathy; the median (range) TTR for these patients was 1.3 (0.7-14) months (Figure 3A). The median (range) DOR was 18.4 (0.03-34) months (Figure 3B), and the median (range) PFS in all patients was 7.6 (0.03-37) months (Figure 3C). At 48 weeks, 46.4% of patients remained progression free. PFS was also longer at higher dose levels (Figure 3D): the 35 patients taking lower dose or intermittent dosing regimens had a median (range) PFS of 3.7 (0.5-33) months. This contrasted with data from the 29 patients receiving continuous higher dose dosing regimens, who had a median (range) PFS of 16.8 (1-37) months.
Time-to-event endpoints. TTR (A), DOR (B), PFS overall (C), and PFS by higher or lower dosing regimen (D). TTR and PFS are measured from the start of therapy, whereas DOR is measured from the start of response. TTR and DOR comprise responding patients only; PFS is evaluated in all patients. A tick on the curve denotes a censored time point.
Time-to-event endpoints. TTR (A), DOR (B), PFS overall (C), and PFS by higher or lower dosing regimen (D). TTR and PFS are measured from the start of therapy, whereas DOR is measured from the start of response. TTR and DOR comprise responding patients only; PFS is evaluated in all patients. A tick on the curve denotes a censored time point.
Discussion
This evaluation of idelalisib constitutes the first clinical assessment of a selective PI3Kδ inhibitor to treat iNHL. The data from this study demonstrate that continuous inhibition of PI3Kδ can achieve substantial and prolonged activity in heavily pretreated patients with this disease.
Across a range of dosing regimens, most treatment-emergent AEs were grade 1-2 in severity. Many AEs (eg, cytopenias, constitutional symptoms, fatigue) were expected as a consequence of underlying iNHL, intercurrent illness in an older population of patients, and/or previous therapy. Rash was observed in some patients but did not require permanent drug discontinuation. Diarrhea possibly related to drug tended to occur late in therapy, was not life-threatening, and was manageable with idelalisib interruption, supportive care, and/or enteric antiinflammatory agents.
Asymptomatic grade ≥3 serum transaminase elevations occurred in ∼25% of iNHL patients during idelalisib treatment. Onset generally occurred within the first 9 weeks of therapy, thus emphasizing the need for monitoring of serum transaminase values during this period. Grade 1 or 2 serum ALT/AST elevations resolved spontaneously despite continued administration of idelalisib. In patients with grade ≥3 serum ALT/AST elevations, idelalisib was temporarily withheld, and the abnormalities resolved to grade ≤1 in all patients within 6 weeks. Among patients with grade ≥3 serum transaminase elevations, retreatment was initiated with a 1-level dose reduction in 8 patients and the majority (75%) had no recurrence of increased serum ALT/AST values. After successful rechallenge, reescalation of dose was possible in most cases.
The mechanism of transaminase elevations is not known. When significant transaminase elevations (grade ≥3) did occur, they developed within a defined time period of <12 weeks. Transaminase elevations were not related to cumulative drug exposure. In contrast, patients tend to accommodate to idelalisib over time. Multivariate analysis was undertaken to try and identify factors associated with grade ≥3 transaminase elevations. No strong associations with risk of grade ≥3 transaminase elevations were noted. Weak associations were noted with less prior therapy and longer interval from prior therapy. But given the small number of patients, firm conclusions could not be drawn.
We did not identify an MTD for idelalisib and could not discern an obvious dose relationship with any AE or laboratory abnormality. Dose escalation was stopped at the 350-mg BID dose. This occurred because of the initial finding of a potential DLT of transaminase elevations. The expansion of other dose regimens led to the subsequent finding that these events were not dose related and could occur before or (mostly) after 28 days. So the transaminase elevations are not strictly DLTs, and they are best described as idiosyncratic reactions.
We observed consistent reductions in lymphadenopathy, higher response rates, and long PFS at doses ≥150 mg BID. We also observed flattening dose-exposure curves (particularly for trough concentrations) at values ≥100 mg BID and better maintenance of exposure with BID dosing as opposed to QD dosing. Thus, we selected an idelalisib dose of ≥150 mg BID for future studies in iNHL.
In this dose-escalation phase I trial, idelalisib produced objective responses in patients with iNHL. The ORR was 48% among all patients and 59% among those receiving continuous dosing with idelalisib at starting dose levels equivalent to the recommended phase 2 dose of 150 mg BID or higher. Therapy was associated with prolonged disease control in responding patients with a median DOR of 18.4 months. Objective responses were seen within each of the iNHL subtypes (FL, SLL, LPL/WM, and MZL) despite bulky adenopathy, a median of 4 prior therapies, and disease that had commonly become refractory to existing treatments. These efficacy findings are impressive when compared with results from single-agent studies evaluating available cytotoxic and noncytotoxic drugs as therapies for previously treated iNHL. For example, 2 phase 2 trials of the chemotherapeutic agent bendamustine in patients who had received a median of 2 prior therapies for iNHL indicate response rates of 75% and 79% but substantial myelotoxicity and a median DOR in both trials of only 9 months,29,30 half of the median 18-month DOR seen in this trial. Results with noncytotoxic agents like bortezomib,31 fostamatinib,32 ibrutinib,33 lenalidomide,34 and rituximab35 have been associated with ORR values ranging from 12 to 52% in less heavily pretreated populations than those taking idelalisib in this study.
Our findings indicate that idelalisib has the potential to be a promising new therapy in patients with a variety of indolent lymphomas and have supported further study of idelalisib in patients with previously treated iNHL. A phase 2 trial evaluating patients with rituximab and alkylator-refractory iNHL (NCT01282424) has completed accrual and demonstrated similar findings to this study in a well-defined, highly refractory iNHL population, and phase 3 trials of idelalisib in combination with rituximab (NCT01838434) and bendamustine/rituximab (NCT01569295) are underway.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The investigators are grateful to the patients who participated in this trial for their willingness to commit themselves to the evaluation of a novel, investigational drug. The authors wish to acknowledge the very important contributions of the site personnel. Their gratitude is extended to the contract research organizations, Novella of Morrisville, NC, and LabConnect of Seattle, WA. The authors thank Tara Walvatne of Gilead Sciences for assistance with monitoring of the trial.
This clinical study was sponsored and funded by Gilead Sciences, Inc (Foster City, CA and Seattle, WA) and by Calistoga Pharmaceuticals, Inc (Seattle, WA) (acquired by Gilead Sciences, Inc.).
Authorship
Contribution: I.W.F., B.S.K., R.R.F., J.R.B., J.C.B., D.M.J., A.S.Y., and R.G.U. designed the study; I.W.F., B.S.K., J.P.L., R.R.F., J.R.B., J.C.B., N.D.W.-J., S.E.C., D.M.B., W.R.G., and S.E.S. enrolled the patients; I.W.F., D.M.J., A.S.Y., R.G.U., B.S.K., J.P.L., R.R.F., J.R.B., J.C.B., N.D.W.-J., S.E.C., D.M.B., W.R.G., S.P., Y.C., H.K.W., L.L.M., and S.E.S. performed research and analyzed data; and I.W.F. and W.R.G. wrote the paper with input from all other authors. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: This clinical study was sponsored and funded by Gilead Sciences, Inc. (Foster City, CA and Seattle, WA), and by Calistoga Pharmaceuticals, Inc (Seattle, WA) (acquired by Gilead Sciences, Inc.). In addition, the authors declare the following conflicts of interest: H.K.W., D.M.J., S.P., D.L., B.J.L., R.G.U., A.S.Y., Y.C., W.R.G., and L.L.M. are current or former employees of Calistoga or Gilead. D.M.J., R.G.U., A.S.Y., and L.L.M. have equity ownership in Calistoga or Gilead. J.C.B., S.E.C., B.S.K., R.G.U., L.L.M., J.P.L., and R.R.F. are consultants for Calistoga or Gilead. J.R.B. and J.C.B. received support from the Leukemia and Lymphoma Society. B.S.K. is a consultant for Infinity. D.M.J. is an employee and shareholder of Acerta Pharmaceuticals. B.J.L. is an employee of Effector Therapeutics and a consultant for Acerta Pharmaceuticals. L.L.M. is on the scientific advisory board of Acerta Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Ian W. Flinn, Sarah Cannon Research Institute, 250 25th Ave North, Nashville, TN 37203; e-mail: iflinn@tnonc.com.