Key Points
This clinical study assessed idelalisib, a selective PI3Kδ inhibitor, in 40 patients with relapsed/refractory MCL.
In a dose-escalation trial in heavily pretreated patients, an overall response rate of 40% was observed with an acceptable safety profile.
Abstract
Idelalisib, an oral inhibitor of phosphatidylinositol-3-kinase δ (PI3Kδ), was evaluated in a 48-week phase 1 study (50-350 mg daily or twice daily) enrolling 40 patients with relapsed or refractory mantle cell lymphoma (MCL). Primary outcome was safety and dose-limiting toxicity (DLT). Secondary outcomes were pharmacokinetic parameters, pharmacodynamic effects, overall response rate (ORR), progression-free survival (PFS), and duration of response (DOR). Patients without DLT and no evidence of disease progression after 48 weeks enrolled in the extension study. Patients had median age of 69 years (range, 52-83) and received median of 4 prior therapies (1-14); 17 of 40 patients (43%) were refractory to their most recent treatment. Median duration of idelalisib treatment was 3.5 months (range, 0.7-30.7), with 6 (15%) continuing extension treatment. Common grade ≥3 adverse events (AEs) included (total%/grade ≥3%) diarrhea (40/18), nausea (33/5), pyrexia (28/0), fatigue (25/3), rash (23/3), decreased appetite (20/15), upper respiratory infection (20/0), pneumonia (13/10), and alanine transaminase or aspartate transaminase elevations (60/20). ORR was 16 of 40 patients (40%), with CR in 2 of 40 patients (5%). Median DOR was 2.7 months, median PFS was 3.7 months, and 1-year PFS was 22%. These data provide proof of concept that targeting PI3Kδ is a viable strategy and worthy of additional study in MCL. This trial was registered at www.clinicaltrials.gov as #NCT00710528.
Introduction
Mantle cell lymphoma (MCL) is a moderately aggressive and incurable B-cell malignancy that accounts for approximately 6% of all new lymphoma cases each year.1,2 The disease is characterized by the t(11;14) translocation, which results in juxtaposition of the immunoglobulin heavy chain enhancer on chromosome 14 to the cyclin D1 locus on chromosome 11, generating cyclin D1 overexpression, the biological hallmark of MCL. MCL is initially chemosensitive; however, it typically exhibits short remission durations and carries a poor prognosis, with a median survival of 3 to 5 years.3-5 Patients with MCL are often treated with rituximab-chemotherapy combinations, either with or without stem cell transplant consolidation.6 Relapse is typical, and MCL becomes increasingly resistant to therapy over time. Although a number of chemotherapy regimens and newer agents, including bortezomib, lenalidomide, and ibrutinib, are active in MCL, these approaches are often characterized by brief remissions. However, as the molecular pathogenesis of MCL is elucidated, new targets and agents acting against those targets are being discovered.7
A promising target in several B-cell malignancies is the B-cell receptor (BCR) signaling pathway. This pathway includes signaling through phosphatidylinositol-3-kinase (PI3K). PI3K-mediated phosphorylation activates the serine/threonine kinase AKT and, subsequently, mammalian target of rapamycin (mTOR). Overexpression of PI3K/AKT appears to contribute to the pathogenesis of MCL.8-10 AKT may play a role in stabilizing cyclin D1 mRNA, preventing nuclear export of cyclin D by phosphorylation of GSK-3b.11 Additionally, mTOR activation may increase cyclin D1 translation.12 Taken together, these observations suggest the PI3K-AKT-mTOR axis is a therapeutic target in MCL.
PI3Ks are lipid kinases that exist in 4 different isoforms: p110α, p110β, p110γ, and p110δ. The p110δ isoform is a key messenger in BCR signaling and is highly expressed in B lymphocytes.13 PI3Kδ plays a central role in normal B-cell development and function.14 PI3Kδ signaling is overactive in many B-cell malignancies and has been shown to drive proliferation, survival, and trafficking to lymphoid tissue.15 Idelalisib (GS-1101, CAL-101, 5-fluoro-3-phenyl-2-[(s)-1-(9H-purin-6-ylamino)-propyl]-3H-quinazolin-4-one) is a potent, highly selective, small-molecule inhibitor of PI3Kδ, with higher specificity for the δ isoform compared with the α, β, and γ isoforms.16 In lymphoid cell lines and primary patient samples, idelalisib blocks PI3K/AKT signaling and promotes apoptosis.16,17 Phase 1a testing in healthy volunteers established idelalisib oral bioavailability and safety at dose levels that achieve plasma concentrations shown to inhibit PI3Kδ in preclinical models.18
We conducted a dose-escalation and extension study of idelalisib in patients with relapsed B-cell malignancies, acute myelogenous leukemia, and multiple myeloma to characterize safety, pharmacodynamics, and pharmacokinetics and to examine clinical activity; here, we report on the outcomes of the cohort of enrolled patients with MCL.
Patients and methods
The study protocol was approved by the responsible institutional review board or ethics committee at each study center. All patients provided written informed consent before enrollment. This study was conducted under a US Investigational New Drug Application, in accordance with recognized international scientific and ethical standards, including but not limited to the International Conference on Harmonization guideline for Good Clinical Practice and the original principles embodied in the Declaration of Helsinki.
Patients and eligibility criteria
Inclusion criteria
Patients were ≥18 years in age with relapsed or refractory MCL, with measurable disease, defined as at least 1 lesion measuring >2 cm in a single dimension on computed tomography scan. Patients were refractory to or relapsed after at least 1 prior chemotherapy regimen and had received at least 1 prior rituximab-containing therapy as a single agent or in combination with other therapies. Men and women of childbearing potential agreed to use contraception for the duration of the study.
Exclusion criteria
Patients were excluded from the study if they had received radiotherapy, radioimmunotherapy, biological therapy, chemotherapy, or treatment with an investigational product within 4 weeks prior to visit 1. Patients were also excluded if they had active graft-versus-host-disease following transplantation; known active central nervous system involvement of the malignancy; active, serious infection requiring systemic therapy; or significant, ongoing comorbid conditions that would preclude safe delivery of the study drug. Patients were excluded if they had serum creatinine ≥2.0 mg/dL, serum bilirubin ≥2.0 mg/dL (unless due to Gilbert syndrome), or serum transaminases ≥2 times the upper limit of normal and positive HIV antibody. Patients with severe thrombocytopenia requiring platelet transfusion support, active hepatitis B or C infection, poorly controlled diabetes mellitus with hemoglobin A1c >10%, or those who had taken a medication that is a potent inhibitor or inducer of cytochrome P450 3A4 within 1 week prior to study entry were also ineligible.
Study design/treatment
The primary end point of this dose-escalation and extension study was to evaluate the safety and dose-limiting toxicity (DLT) of oral idelalisib administered for 48 weeks in patients with MCL. Secondary outcome measures included pharmacokinetic parameters, pharmacodynamic effects, and clinical response rate. An extension study was offered to patients who were deriving clinical benefit (http://www.clinicaltrials.gov; #NCT00710528).
The study was conducted in 2 phases: dose escalation and cohort expansion. In phase 1, idelalisib was provided as capsules or tablets containing 50, 75, or 100 mg active drug. Depending on the cohort, patients were administered oral idelalisib once per day or twice per day at approximately the same daily times. For the twice-daily schedule, doses were given at approximate 12-hour intervals. Dose escalation was performed in sequential cohorts of 3 to 6 patients followed by expansion in up to 12 patients per cohort. The dose range was based on idelalisib safety and pharmacokinetic data from the phase 1 studies. Patients received 50, 100, 150, 200, or 350 mg twice daily for 28 days, 150 or 300 mg daily for 28 days, or 150 mg twice daily 3 weeks on and 1 week off for 28 days. Idelalisib was continued through 48 weeks on the primary study and indefinitely on the extension study until progressive disease (PD) or intolerability.
Safety assessments
The safety analysis included patients in both the dose-escalation and extension studies. The primary outcome measure was to determine the safety and the DLT of idelalisib in patients with relapsed B-cell malignancies. DLTs were defined according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0 as any event with possible or probable relationship to study drug occurring up to day 28 that was either a grade 4 hematologic adverse event (AE) persisting for ≥7 days or any grade ≥3 nonhematologic toxicity. Complete blood counts and standard serum chemistry tests were collected pretreatment and weekly for 4 weeks, every 2 weeks for 4 weeks, every 4 weeks for 12 weeks, and then every 8 to 12 weeks. AEs and their severity were assessed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0.19
Serious AEs were defined as any AE that was fatal, life-threatening, or required prolonged hospitalization; was persistent or significantly disabling or incapacitating; resulted in a congenital anomaly or birth defect; or necessitated medical intervention to prevent any of these outcomes.
Pharmacokinetic assessments
Serial plasma samples were collected and analyzed for idelalisib concentration at 0, 0.5, 1, 1.5, 2, 3, 4, and 6 hours on treatment days 1 and 28. Additional trough values were collected on days 8, 15, and 22. Idelalisib concentrations were determined using a validated method with a lower limit of quantitation of 0.5 ng/mL.18
Efficacy assessments
The primary efficacy end point was overall response rate (ORR), and secondary end points were progression-free survival (PFS) and duration of response (DOR). Patients were assessed for clinical response using standard response criteria for lymphoma.20
First evaluation for clinical response was completed no later than day 32, and patients continued to receive study drug in the interim. Patients were assessed for disease response or progression every 8 weeks until week 48, and then, if on the extension study, every 12 weeks. Patients were withdrawn from the study if they developed PD, unacceptable toxicity, or, in the opinion of the investigator, were no longer deriving clinical benefit.
Statistical analysis
The safety and evaluable populations were used for the primary efficacy and safety analyses. The safety population consisted of all enrolled patients who received any amount of idelalisib; the evaluable population included patients who had the necessary baseline and on-study measurements to provide interpretable results for specific parameters of interest. All data summaries were descriptive. For data summaries involving continuous variables, data tables typically contained sample size, mean, median, standard deviation, standard error, minimum, and maximum. For categorical variables, sample size and proportion were typically presented. When required for the statistical analysis of a particular variable, the baseline value was the last recorded value prior to the administration of the first dose of study treatment.
Results
Patient characteristics
Between July 2008 and January 2011, 40 patients with MCL were enrolled in the 48-week dose-escalation study, with the extension study currently ongoing (Table 1). Most patients were male (87.5%), with a median age of 69 years (range, 52-83 years). Of 40 patients, 14 (35%) had high-risk Mantle Cell Lymphoma International Prognostic Index (MIPI)21 scores. By individual MIPI risk factors, 35 patients (88%) were age >60 years, 4 patients (10%) had World Health Organization performance status ≥2, 18 patients (45%) had elevated lactate dehydrogenase levels, and 6 patients (15%) had white blood cell counts above the upper limit of normal. In addition, 24 patients (60%) presented with bulky lymphadenopathy (>5 cm).
Patient baseline characteristics
| Characteristic . | N = 40 . |
|---|---|
| Median age (range), y | 69 (52-83) |
| Sex, male/female | 35/5 |
| WHO PS score, n (%) | |
| 0 | 14 (35.0) |
| 1 | 21 (52.5) |
| 2 | 4 (10.0) |
| Missing | 1 (2.5) |
| MIPI score, n (%) | |
| Low (<5.7) | 10 (25) |
| Intermediate (5.7-6.2) | 16 (40) |
| High (>6.2) | 14 (35) |
| Risk factors, n (%) | |
| Age >60 y | 35 (88) |
| WHO PS ≥2 | 4 (10) |
| LDH >ULN | 18 (45) |
| WBC >ULN | 6 (15) |
Bulky disease (>5 cm) | 24 (60) |
Treatment history | |
| Median number of prior therapies (range) | 4 (1-14) |
| ≥6 regimens, n (%) | 13 (32) |
| Prior therapy type, n (%) | |
| Rituximab | 40 (100) |
| Alkylating agent | 39 (98) |
| Anthracycline | 35 (88) |
| Bortezomib | 24 (60) |
| Bendamustine | 12 (40) |
| Autologous stem cell transplantation | 9 (22) |
| Purine analog | 9 (23) |
| Lenalidomide | 8 (20) |
| Refractory to last regimen, n (%) | 17 (43) |
| Characteristic . | N = 40 . |
|---|---|
| Median age (range), y | 69 (52-83) |
| Sex, male/female | 35/5 |
| WHO PS score, n (%) | |
| 0 | 14 (35.0) |
| 1 | 21 (52.5) |
| 2 | 4 (10.0) |
| Missing | 1 (2.5) |
| MIPI score, n (%) | |
| Low (<5.7) | 10 (25) |
| Intermediate (5.7-6.2) | 16 (40) |
| High (>6.2) | 14 (35) |
| Risk factors, n (%) | |
| Age >60 y | 35 (88) |
| WHO PS ≥2 | 4 (10) |
| LDH >ULN | 18 (45) |
| WBC >ULN | 6 (15) |
Bulky disease (>5 cm) | 24 (60) |
Treatment history | |
| Median number of prior therapies (range) | 4 (1-14) |
| ≥6 regimens, n (%) | 13 (32) |
| Prior therapy type, n (%) | |
| Rituximab | 40 (100) |
| Alkylating agent | 39 (98) |
| Anthracycline | 35 (88) |
| Bortezomib | 24 (60) |
| Bendamustine | 12 (40) |
| Autologous stem cell transplantation | 9 (22) |
| Purine analog | 9 (23) |
| Lenalidomide | 8 (20) |
| Refractory to last regimen, n (%) | 17 (43) |
LDH, lactic dehydrogenase; PS, performance status; ULN, upper limit of normal; WBC, white blood cell count; WHO, World Health Organization.
The median number of prior therapies was 4 (range, 1-14), with 13 patients (32%) receiving ≥6 prior therapies. All patients had received rituximab; 98% of patients had received a prior alkylating agent, and 35 patients (88%) had received an anthracycline. Twenty-four patients (60%) had received prior bortezomib, and 9 patients (22%) had received prior autologous stem cell transplant. A total of 17 patients (43%) were refractory to their last prior therapy, defined as lack of response or PD ≤6 months from completion of last prior therapy.
Patient disposition
The dose-escalation study tested idelalisib in doses ranging from 50 to 350 mg daily or twice daily for 48 weeks. Of the 40 patients entering the 48-week dose-escalation trial, 5 received 50 mg twice daily, 7 received 100 mg twice daily, 7 received 150 mg daily, 5 received 150 mg twice daily for 21 days, 4 received 300 mg daily, 6 received 150 mg twice daily, 3 received 200 mg twice daily, and 3 received 350 mg twice daily. Of 40 enrolled patients, 6 (15%) completed the primary 48-week dose-escalation study and enrolled in the extension study. The 34 patients who discontinued the primary study did so because of PD in 24 (60%), AEs in 8 (20%), withdrawn consent in 1 (3%), or investigator request in 1 (3%). Of the 6 patients who completed the 48-week study and entered the extension trial, 5 have discontinued, 4 due to PD and 1 for AE.
Pharmacokinetics
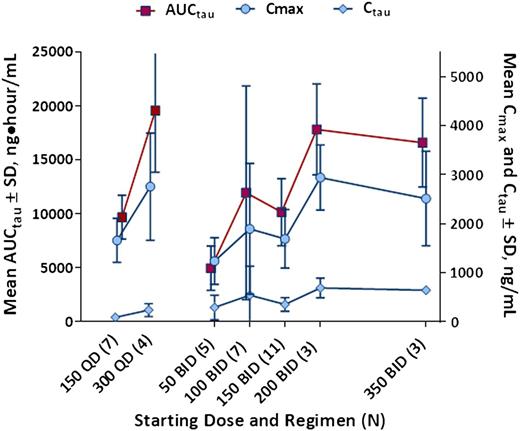
Pharmacokinetic parameters measured on days 1 and 28 demonstrated that there was no appreciable accumulation or loss of idelalisib with repeated dosing. Steady state was achieved by day 8. Cmax, C, and AUCτ demonstrated only modest increases in exposure above the dose level of 150 mg twice daily. In addition, as expected, twice-daily dosing better maintained Cτ than daily dosing (Figure 1).
Dose-exposure relationship. Steady-state (day 28) idelalisib plasma exposures by idelalisib dosing regimen (N = 40). AUCτ indicates area under the concentration-time curve over 12 (daily) or 24 hours (twice daily); BID, twice daily; Cτ, trough concentration; Cmax, maximum concentration; QD, daily; SD, standard deviation.
Dose-exposure relationship. Steady-state (day 28) idelalisib plasma exposures by idelalisib dosing regimen (N = 40). AUCτ indicates area under the concentration-time curve over 12 (daily) or 24 hours (twice daily); BID, twice daily; Cτ, trough concentration; Cmax, maximum concentration; QD, daily; SD, standard deviation.
Safety
Median idelalisib treatment duration for the 40 MCL patients was 3.5 months (range, 0.7-30 months), with a mean of 6.3 ± 7.4 months. The maximum tolerated dose of idelalisib was not reached. Table 2 summarizes the most frequently reported AEs (>10% of patients) and rates of corresponding grade ≥ 3 AEs. Most AEs were grade 1 or 2 in severity. The most common AEs of any grade were diarrhea (40.0%), nausea (32.5%), pyrexia (27.5%), fatigue (25%), rash (22.5%), and decreased appetite and upper respiratory infection (each in 20%). The most common grade ≥3 events were diarrhea in 7 patients (17.5%), decreased appetite in 6 patients (15%), and pneumonia in 4 patients (10%). No tumor lysis syndrome or tumor flare was seen. An analysis of AEs by dose is shown in Table 3. No clear relationship between any AE or laboratory abnormality and dose was evident.
Treatment-emergent AEs reported in more than 10% of patients and grade 3 or higher AEs in patients treated with idelalisib (N = 40)
| AEs >10% . | All AEs . | Grade ≥3 AEs . |
|---|---|---|
| Diarrhea | 16 (40) | 7 (17.5) |
| Nausea | 13 (32.5) | 2 (5) |
| Pyrexia | 11 (27.5) | 0 |
| Fatigue | 10 (25) | 1 (2.5) |
| Rash | 9 (22.5) | 1 (2.5) |
| Decreased appetite | 8 (20) | 6 (15) |
| Upper respiratory infection | 8 (20) | 0 |
| Asthenia | 7 (17.5) | 0 |
| Constipation | 6 (15) | 0 |
| Headache | 6 (15) | 0 |
| Cough | 5 (12.5) | 0 |
| Pneumonia | 5 (12.5) | 4 (10) |
| Vomiting | 5 (12.5) | 0 |
| Weight loss | 5 (12.5) | 0 |
| Laboratory abnormality | ||
| ALT/AST elevation* | 24 (60) | 8 (20) |
| Neutropenia | 12 (30) | 4 (10) |
| Anemia | 9 (22.5) | 1 (2.5) |
| Thrombocytopenia | 8 (20) | 2 (5) |
| AEs >10% . | All AEs . | Grade ≥3 AEs . |
|---|---|---|
| Diarrhea | 16 (40) | 7 (17.5) |
| Nausea | 13 (32.5) | 2 (5) |
| Pyrexia | 11 (27.5) | 0 |
| Fatigue | 10 (25) | 1 (2.5) |
| Rash | 9 (22.5) | 1 (2.5) |
| Decreased appetite | 8 (20) | 6 (15) |
| Upper respiratory infection | 8 (20) | 0 |
| Asthenia | 7 (17.5) | 0 |
| Constipation | 6 (15) | 0 |
| Headache | 6 (15) | 0 |
| Cough | 5 (12.5) | 0 |
| Pneumonia | 5 (12.5) | 4 (10) |
| Vomiting | 5 (12.5) | 0 |
| Weight loss | 5 (12.5) | 0 |
| Laboratory abnormality | ||
| ALT/AST elevation* | 24 (60) | 8 (20) |
| Neutropenia | 12 (30) | 4 (10) |
| Anemia | 9 (22.5) | 1 (2.5) |
| Thrombocytopenia | 8 (20) | 2 (5) |
Data are presented as n (%) of patients.
ALT, alanine transaminase; AST, aspartate transaminase.
Occurred in >1 patient.
Incidence of treatment-emergent AEs and selected laboratory abnormalities reported in 10% or more of patients
| Description . | Dosing regimen . | Overall N = 40 (%) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg bid 28 d (n = 5) . | 150 mg qd 28 d (n = 7) . | 100 mg bid 28 d (n = 7) . | 150 mg bid 21 d (n = 5) . | 300 mg qd 28 d (n = 4) . | 150 mg bid 28 d (n = 6) . | 200 mg bid 28 d (n = 3) . | 350 mg bid 28 d (n = 3) . | |||||||||||
| Grade . | Grade . | Grade . | Grade . | Grade . | Grade . | Grade . | Grade . | Grade . | ||||||||||
| Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | |
| Adverse event, n | ||||||||||||||||||
| Diarrhea | 1 | 0 | 4 | 2 | 3 | 3 | 2 | 0 | 1 | 0 | 3 | 1 | 2 | 1 | 0 | 0 | 16 (40.0%) | 7 (17.5%) |
| Nausea | 0 | 0 | 1 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 3 | 1 | 2 | 0 | 3 | 0 | 13 (32.5%) | 2 (5.0%) |
| Pyrexia | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 11 (27.5%) | 0 |
| Fatigue | 1 | 0 | 2 | 0 | 3 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 10 (25.0%) | 1 (2.5%) |
| Rash | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 9 (22.5%) | 1 (2.5%) |
| Anorexia | 0 | 0 | 1 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 8 (20.0%) | 6 (15.0%) |
| URI | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 8 (20.0%) | 0 |
| Asthenia | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 7 (17.5%) | 0 |
| Constipation | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 6 (15.0%) | 0 |
| Headache | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 6 (15.0%) | 0 |
| Cough | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 5 (12.5%) | 0 |
| Pneumonia | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 (12.5%) | 5 (12.5%) |
| Vomiting | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 5 (12.5%) | 0 |
| Weight loss | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 5 (12.5%) | 2 (5.0%) |
| Dizziness | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 (10.0%) | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 4 (10.0%) | 0 |
| Laboratory abnormality in ≥10% patients, n | ||||||||||||||||||
| AST, increased | 1 | 0 | 6 | 2 | 4 | 1 | 1 | 0 | 3 | 2 | 4 | 0 | 2 | 1 | 3 | 0 | 24 (60.0%) | 6 (15.0%) |
| ALT, increased | 0 | 0 | 5 | 2 | 3 | 2 | 2 | 1 | 3 | 2 | 4 | 0 | 1 | 1 | 2 | 0 | 20 (50.0%) | 8 (20.0%) |
| AP, increased | 2 | 0 | 5 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 14 (35.0%) | 0 |
| GGT, increased | 0 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 10 (25.0%) | 0 |
| Bilirubin, increased | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 7 (17.5%) | 0 |
| Glucose, increased | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 11 (27.5%) | 0 |
| Glucose, decreased | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 (12.5%) | 0 |
| BUN, increased | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 7 (17.5%) | 0 |
| Cr, increased | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 8 (20.0%) | 0 |
| ANC, decreased | 3 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 2 | 1 | 12 (30.0%) | 4 (10.0%) |
| Hgb, decreased | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 1 | 0 | 9 (22.5%) | 1 (2.5%) |
| Plts, decreased | 0 | 0 | 2 | 1 | 4 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 8 (20.0%) | 2 (5.0%) |
| Description . | Dosing regimen . | Overall N = 40 (%) . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg bid 28 d (n = 5) . | 150 mg qd 28 d (n = 7) . | 100 mg bid 28 d (n = 7) . | 150 mg bid 21 d (n = 5) . | 300 mg qd 28 d (n = 4) . | 150 mg bid 28 d (n = 6) . | 200 mg bid 28 d (n = 3) . | 350 mg bid 28 d (n = 3) . | |||||||||||
| Grade . | Grade . | Grade . | Grade . | Grade . | Grade . | Grade . | Grade . | Grade . | ||||||||||
| Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | Any . | ≥3 . | |
| Adverse event, n | ||||||||||||||||||
| Diarrhea | 1 | 0 | 4 | 2 | 3 | 3 | 2 | 0 | 1 | 0 | 3 | 1 | 2 | 1 | 0 | 0 | 16 (40.0%) | 7 (17.5%) |
| Nausea | 0 | 0 | 1 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 3 | 1 | 2 | 0 | 3 | 0 | 13 (32.5%) | 2 (5.0%) |
| Pyrexia | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 11 (27.5%) | 0 |
| Fatigue | 1 | 0 | 2 | 0 | 3 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 10 (25.0%) | 1 (2.5%) |
| Rash | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 9 (22.5%) | 1 (2.5%) |
| Anorexia | 0 | 0 | 1 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 8 (20.0%) | 6 (15.0%) |
| URI | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 8 (20.0%) | 0 |
| Asthenia | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 7 (17.5%) | 0 |
| Constipation | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 6 (15.0%) | 0 |
| Headache | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 6 (15.0%) | 0 |
| Cough | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 5 (12.5%) | 0 |
| Pneumonia | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 5 (12.5%) | 5 (12.5%) |
| Vomiting | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 5 (12.5%) | 0 |
| Weight loss | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 5 (12.5%) | 2 (5.0%) |
| Dizziness | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 (10.0%) | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 4 (10.0%) | 0 |
| Laboratory abnormality in ≥10% patients, n | ||||||||||||||||||
| AST, increased | 1 | 0 | 6 | 2 | 4 | 1 | 1 | 0 | 3 | 2 | 4 | 0 | 2 | 1 | 3 | 0 | 24 (60.0%) | 6 (15.0%) |
| ALT, increased | 0 | 0 | 5 | 2 | 3 | 2 | 2 | 1 | 3 | 2 | 4 | 0 | 1 | 1 | 2 | 0 | 20 (50.0%) | 8 (20.0%) |
| AP, increased | 2 | 0 | 5 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 14 (35.0%) | 0 |
| GGT, increased | 0 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 10 (25.0%) | 0 |
| Bilirubin, increased | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 7 (17.5%) | 0 |
| Glucose, increased | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 11 (27.5%) | 0 |
| Glucose, decreased | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 (12.5%) | 0 |
| BUN, increased | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 7 (17.5%) | 0 |
| Cr, increased | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 8 (20.0%) | 0 |
| ANC, decreased | 3 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 2 | 1 | 12 (30.0%) | 4 (10.0%) |
| Hgb, decreased | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 1 | 0 | 9 (22.5%) | 1 (2.5%) |
| Plts, decreased | 0 | 0 | 2 | 1 | 4 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 8 (20.0%) | 2 (5.0%) |
ALT, alanine transaminase; ANC, absolute neutrophil count; AP, alkaline phosphatase; AST, aspartate transaminase; bid, twice daily; BUN, blood urea nitrogen; Cr, creatine; d, days; GGT, gamma-glutamyl transpeptidase; Hgb, hemoglobin; Plts, platelets; qd, daily; URI, upper respiratory infection.
Overall, 9 (18%) patients discontinued therapy due to AEs. AEs leading to discontinuation in the primary study (n = 8) included diarrhea in 2 patients and alanine transaminase/aspartate transaminase elevations, acute renal failure, malignant pleural effusion, muscle spasms, rash/stomatitis, and pneumonia in 1 patient each. Diarrhea was the only AE leading to discontinuation in the extension study (n = 1). Temporary drug hold was noted for transaminase elevations (n = 5), diarrhea (n = 2), and for individual patients with fever/neutropenia, cytomegalovirus colitis, sepsis, pneumonia, pyrexia, and rash/cellulitis. Drug dose was reduced for patients with transaminase elevations (n = 5) and for individual patients with anorexia, diarrhea, fatigue/paresthesia, and reduced visual acuity.
The most common serious AEs (occurring in >1 patient) included pneumonia in 4 patients (10%), diarrhea in 3 patients (7.5%), and acute renal failure, pulmonary embolism, and pyrexia each in 2 patients (5%). Four deaths were reported on study, including 3 patients with PD and 1 with pneumonia.
In contrast to idelalisib-treated patients with chronic lymphocytic leukemia22 and small lymphocytic lymphoma,23 transient lymphocytosis in MCL was infrequent. Five patients (12.5%) had a >3-fold increase in absolute lymphocyte count during the study (range, 3.5-12×). Levels decreased over a 2- to 3-month period.
Alanine transaminase or aspartate transaminase elevations were seen in 24 (60%) of the 40 patients, 8 (20%) of which were grade ≥3 in severity. In patients with grade 1 or 2 elevations, the transaminase abnormalities were transient and reverted to normal despite continued dosing. Grade ≥3 transaminase elevations were asymptomatic, with onset occurring between 4 to 9 weeks after idelalisib initiation. For the 8 patients with grade ≥3 elevations, idelalisib was temporarily withheld, and the abnormalities resolved to grade ≤1 in all patients within 2 to 5 weeks. Two patients experienced PD either concurrently or during the drug hold, and 1 patient with stable disease (SD) opted to discontinue protocol therapy. Five patients were rechallenged with idelalisib at a 1-level dose reduction. Three patients had no further transaminase elevations. One patient required a second dose reduction, and another patient required a second and third dose reduction due to recurrent transaminase elevations.
Efficacy
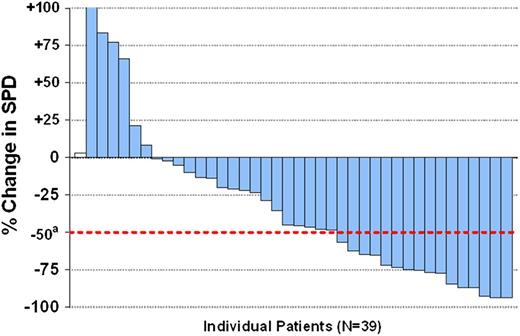
Among the 40 MCL patients, the ORR was 40% (95% confidence interval [CI], 24.9-56.7). Overall, 33 of 39 evaluable patients (84.6%) (1 patient did not have a tumor assessment) had some reduction in lymph node size. A waterfall plot of best overall response in tumor size is shown in Figure 2.
Lymph node response. The best response with respect to tumor size during idelalisib treatment. There were 40 patients in total, and 1 patient (white bar) did not have an evaluation for response. A total of 32 out of 39 patients (82%) had some decrease in measurable disease. Shown are on-treatment percent changes in the sum of greatest perpendicular dimension (SPD) of measured lymph nodes. The dotted line shows the percentage change that represents the criterion for lymphadenopathy response, according to Cheson et al,20 where a indicates criterion for lymphadenopathy response.
Lymph node response. The best response with respect to tumor size during idelalisib treatment. There were 40 patients in total, and 1 patient (white bar) did not have an evaluation for response. A total of 32 out of 39 patients (82%) had some decrease in measurable disease. Shown are on-treatment percent changes in the sum of greatest perpendicular dimension (SPD) of measured lymph nodes. The dotted line shows the percentage change that represents the criterion for lymphadenopathy response, according to Cheson et al,20 where a indicates criterion for lymphadenopathy response.
Table 4 shows tabulated response rates to idelalisib monotherapy. CR was reported in 2 patients (5%), and PR in 14 patients (35%); 19 patients (47.5%) had SD. Four patients were judged to have PD at first evaluation, and 1 patient was not evaluable. The ORR was higher in patients receiving ≥150 mg twice daily (11/16 [69%]) compared with patients receiving <150 mg twice daily (5/24 [21%]). The median time to response for the 16 evaluable patients was 1.1 months (range, 0.9-9.4 months). Six of the responders developed a response after an initial assessment of SD, 3 at the 2-month evaluation and 1 each at the 4-, 6-, and 9.4-month evaluation. Both patients with CR were in the 300-mg daily cohort, and attained PR by 2 months and CR by 4 months.
Response rate in relapsed/refractory MCL patients receiving idelalisib
| Category . | All patients (N = 40) . | Receiving <150 mg bid* (n = 24) . | Receiving ≥150 mg qd† (n = 16) . |
|---|---|---|---|
| ORR | 16 (40) | 5 (21) | 11 (69) |
| CR | 2 (5) | 0 | 2 (12.5) |
| PR | 14 (35) | 5 (21) | 9 (56.3) |
| SD | 19 (47.5) | 15 (62.5) | 4 (25) |
| PD | 4 (10) | 4 (16.7) | 0 |
| NE | 1 (2.5) | 0 | 1/16 (6.3) |
| Category . | All patients (N = 40) . | Receiving <150 mg bid* (n = 24) . | Receiving ≥150 mg qd† (n = 16) . |
|---|---|---|---|
| ORR | 16 (40) | 5 (21) | 11 (69) |
| CR | 2 (5) | 0 | 2 (12.5) |
| PR | 14 (35) | 5 (21) | 9 (56.3) |
| SD | 19 (47.5) | 15 (62.5) | 4 (25) |
| PD | 4 (10) | 4 (16.7) | 0 |
| NE | 1 (2.5) | 0 | 1/16 (6.3) |
Data are presented as number (%) of patients.
bid, twice daily; CR, complete response; NE, not evaluable; PR, partial response; qd, daily.
Dose cohorts include 150 mg daily, 150 mg twice daily × 21 days, 50 mg twice daily, and 100 mg twice daily.
Dose cohorts include 300 mg daily, 150 mg twice daily, 200 mg twice daily, and 350 mg twice daily.
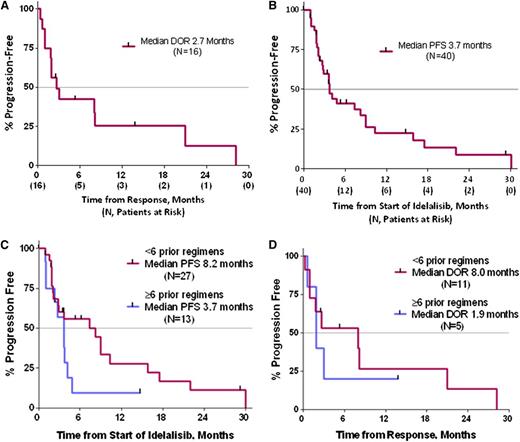
Kaplan-Meier (KM) estimates for DOR, inclusive of the 48-week dose-response trial and ongoing extension study, showed that idelalisib monotherapy was associated with a median DOR of 2.7 months (95% CI, 1-8.1 months; range, 0.3-28.2 months) in the 16 responding patients (Figure 3A). The median PFS (Figure 3B) for all 40 MCL patients was 3.7 months (95% CI, 2.7-8.2 months; range, 0.03-30.1 months). The KM estimate of PFS at 1 year is 22.5%. Three patients had long-term SD, with progressions at 16 and 18 months, and 1 patient remains free of PD at 29.5 months. The KM estimates for PFS did not differ when compared between the high- and low-dose treatment groups, where the median PFS values are identical at 3.7 months and the curves are essentially overlapping.
Duration of response and progression-free survival. (A) KM estimate for DOR in patients with MCL (n = 16). (B) KM estimate for PFS in patients with MCL (N = 40). PFS (C) and DOR (D) in subgroups based on high (≥6) or low (<6) numbers of prior treatment regimens.
Duration of response and progression-free survival. (A) KM estimate for DOR in patients with MCL (n = 16). (B) KM estimate for PFS in patients with MCL (N = 40). PFS (C) and DOR (D) in subgroups based on high (≥6) or low (<6) numbers of prior treatment regimens.
The amount of prior therapy tended to correlate with the PFS. As shown in Figure 3C-D, the subgroup of patients who had received <6 prior regimens (n = 27) had a median PFS of 8.2 months (range, 0.9-30.1 months). For responders in this group (n = 11), the median DOR was 8.0 months (range, 0.3-28.2 months). The patients receiving ≥6 prior regimens (n = 13) had a median PFS of 3.7 months (range, 0.03-14.8 months), with responders in this group (n = 5) having a median DOR of 1.9 months (range, 0.6-13.8 months).
Discussion
Relapsed MCL is characterized by relatively short remissions after conventional cytotoxic therapy.24-26 As a result, investigators have searched for novel agents with unique activity in MCL. Bortezomib, a first-in-class proteasome inhibitor, demonstrated an ORR of 33% and median DOR of 9.2 months in relapsed MCL,27 leading to US Food and Drug Administration approval in 2006. The mTOR inhibitor temsirolimus demonstrated an ORR of 22%, leading to regulatory approval in the European Union.28 More recently, based on an ORR of 26% in relapsed MCL patients with prior bortezomib exposure, lenalidomide received US Food and Drug Administration approval.29 Although each of these discoveries has provided valuable new treatment options for patients, the relatively low response rates and continuing pattern of relapse highlight the need to develop additional therapies in this patient population.
Our study demonstrates that targeting PI3K is a viable therapeutic strategy in MCL. PI3K is a family of lipid kinases that regulate multiple intracellular signaling networks. There are 4 distinct subfamilies (class I, II, III, and IV), with class I being the most clearly implicated in human cancers. Class I PI3K exists in 4 isoforms: p110α, p110β, p110γ, and p110δ. The δ isoform appears to be important for B-cell signaling, development, and survival.30,31 B-cell lymphoma cell lines and primary patient samples from different B-cell malignancies revealed that constitutive PI3K pathway activation was p110δ dependent. Idelalisib is a potent and selective inhibitor of p110δ (EC50 = 8 nM).16 In support of idelalisib’s selectivity for PI3Kδ, patients in this trial did not show elevations of serum glucose levels that are characteristically observed in patients receiving pan-PI3K inhibitors that have activity against PI3Kα.32,33
In this phase 1 trial, idelalisib produced an ORR of 40%, a median DOR of 2.7 months, and a median PFS of 3.7 months. A subset of patients did receive long-term clinical benefit. At 1 year, the KM estimate of PFS was 22%. Five patients had response durations >8 months (8, 8, 14, 21, and 28 months), and 3 patients had long-term SD (16, 18, and 29+ months). Interestingly, 7 out of 8 of these patients were less heavily pretreated and had <6 prior treatments.
Rapid and marked reductions in lymphadenopathy were seen in the MCL patients, including those who demonstrated unfavorable prognostic characteristics, such as bulky disease, high MIPI scores, and extensive prior therapy. A maximum tolerated dose for idelalisib was not identified, but based on the observed plateau in exposure above a dose of 150 mg and the consistent clinical response in lymph nodes at doses of 150 mg and higher, as well as the AE profile, idelalisib at 150 mg twice daily was selected as the dose for future studies in MCL. The ORR for patients treated with doses ≥150 mg twice daily was 69%. The responses seen with idelalisib are comparable to those seen with bortezomib, temsirolimus, and lenalidomide in relapsed/refractory MCL.27-29,34 It is unclear why remissions tend to be brief in relapsed/refractory MCL, but presumably, MCL is adept at developing resistance mechanisms. Determining mechanisms of resistance would enable the development of rational combinations of targeted agents. For idelalisib, a highly selective PI3Kδ inhibitor, resistance and progression may be a function of PI3K p110α overexpression, suggesting a possible role for dual p110α/p110δ inhibitors.35
Notably, the toxicity profile was favorable, with no clear evidence of myelosuppression and no peripheral neuropathy, suggesting idelalisib will be compatible in combination with traditional cytotoxic agents or other novel targeted agents. The most frequent toxicity was an asymptomatic transaminase elevation. The elevations typically occurred in the first 2 months and were identified by routine laboratory monitoring. The protocol management algorithm developed during the study was to continue drug for grade 1 or 2 elevations and to hold drug for grade 3 or 4 elevations. All 8 grade >3 transaminase elevations normalized during the drug holiday, and 5 patients were successfully rechallenged with lower doses of idelalisib. No predisposing factors for transaminase elevations could be identified in this patient cohort.
One source of PI3K signaling is the BCR complex.36 Other agents targeting the BCR pathway have been investigated in relapsed/refractory MCL. The spleen tyrosine kinase inhibitor fostamatinib disodium demonstrated an ORR of 11% in a phase 1 study involving 9 MCL patients.37 Ibrutinib, an agent targeting Bruton tyrosine kinase, has demonstrated an ORR of 68%, with a median PFS of 13.9 months in a phase 2 study involving 111 patients with relapsed/refractory MCL.38 Ibrutinib is now approved for treatment of recurrent MCL.39 A phase 2 study testing idelalisib at 150 mg PO twice daily in a similar population is warranted.
In conclusion, this phase 1 trial demonstrates that oral idelalisib produces rapid response in patients with relapsed/refractory MCL, with an acceptable safety profile. A total of 22% of the patients have shown clinical benefit lasting >1 year. Our data support further study of idelalisib in MCL. Combination studies with an mTOR inhibitor (everolimus), bortezomib, or bendamustine/rituximab are underway in MCL. In addition, idelalisib is being evaluated in combination with lenalidomide and rituximab (NCT01838434) as well as with GS-9973, an investigational spleen tyrosine kinase inhibitor (NCT01796470).
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the study participants for their dedication to this clinical trial and the clinical personnel at each of the study sites for diligence in caring for patients and collecting study data.
This clinical study was sponsored and funded by Gilead Sciences, Inc. (Foster City, CA, and Seattle, WA) and Calistoga Pharmaceuticals, Inc. of Seattle, WA (acquired by Gilead Sciences, Inc.).
Authorship
Contribution: B.S.K., R.R.F., I.W.F., J.R.B., S.E.C., J.C.B., N.W.J., and A.Y. designed the study; B.S.K., S.E.S., R.R.F., I.W.F., S.E.C., J.R.B., D.M.B., J.C.B., and N.W.J. enrolled the patients; B.S.K., S.E.S., R.R.F., I.W.F., S.E.C., J.R.B., D.M.B., J.C.B., S.P., Y.C., A.Y., W.R.G., and N.W.J. performed research and analyzed data; B.S.K. wrote the paper with input from S.E.S., R.R.F., I.W.F., S.E.C., J.R.B., D.M.B., J.C.B., W.R.G., and N.W.J.; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: This clinical study was sponsored and funded by Gilead Sciences, Inc. (Foster City, CA and Seattle, WA) and by Calistoga Pharmaceuticals, Inc. (Seattle, WA) (now a wholly owned subsidiary of Gilead Sciences, Inc.). S.P., Y.C., A.Y., and W.R.G. are current or former employees of Calistoga or Gilead and have equity ownership in Calistoga or Gilead. J.C.B., S.E.C., B.S.K., and R.R.F. are consultants for Calistoga or Gilead. J.R.B. and J.C.B. received support from the Leukemia and Lymphoma Society. B.S.K. is a consultant for Infinity. The remaining authors declare no competing financial interests.
Correspondence: Brad S. Kahl, 4059 Wisconsin Institutes Medical Research, 1111 Highland Ave, Madison, WI 53705; e-mail: bsk@medicine.wisc.edu.