Key Points
Idelalisib was evaluated in 54 patients with heavily pretreated chronic lymphocytic leukemia, and target inhibition was documented in vivo.
Oral idelalisib therapy demonstrated a favorable safety profile and rapidly induced durable disease control in the majority of patients.
Abstract
In a phase 1 trial, idelalisib (GS-1101, CAL-101), a selective inhibitor of the lipid kinase PI3Kδ, was evaluated in 54 patients with relapsed/refractory chronic lymphocytic leukemia (CLL) with adverse characteristics including bulky lymphadenopathy (80%), extensive prior therapy (median 5 [range 2-14] prior regimens), treatment-refractory disease (70%), unmutated IGHV (91%), and del17p and/or TP53 mutations (24%). Patients were treated at 6 dose levels of oral idelalisib (range 50-350 mg once or twice daily) and remained on continuous therapy while deriving clinical benefit. Idelalisib-mediated inhibition of PI3Kδ led to abrogation of Akt phosphorylation in patient CLL cells and significantly reduced serum levels of CLL-related chemokines. The most commonly observed grade ≥3 adverse events were pneumonia (20%), neutropenic fever (11%), and diarrhea (6%). Idelalisib treatment resulted in nodal responses in 81% of patients. The overall response rate was 72%, with 39% of patients meeting the criteria for partial response per IWCLL 2008 and 33% meeting the recently updated criteria of PR with treatment-induced lymphocytosis.1,2 The median progression-free survival for all patients was 15.8 months. This study demonstrates the clinical utility of inhibiting the PI3Kδ pathway with idelalisib. Our findings support the further development of idelalisib in patients with CLL. These trials were registered at clinicaltrials.gov as #NCT00710528 and #NCT01090414.
Introduction
Chronic lymphocytic leukemia (CLL) is a neoplasm resulting from the progressive accumulation of functionally incompetent monoclonal B lymphocytes in blood, bone marrow, lymph nodes, spleen, and liver.3 The initial treatment of CLL generally uses chemoimmunotherapy adding a first generation anti-CD20 antibody (rituximab) to chemotherapy. Across prospective phase 3 trials and retrospective studies, the addition of rituximab appears to provide a survival advantage with initial therapy.4,5 However, disease progression is inevitable and resistant disease emerges, resulting in patients succumbing to their CLL or to treatment complications including myelodysplastic syndrome and acute myeloid leukemia.6 Well-tolerated drugs with novel mechanisms of action are needed to maintain long-term control of CLL.
One promising approach is to target the B-cell receptor signaling pathway in CLL. This pathway plays a predominant role in CLL biology and can be disrupted through inhibition of phosphatidylinositol-3-kinase (PI3-K), an essential lipid kinase and signaling protein in many cell types.7 PI3K-mediated phosphorylation activates the serine/threonine kinases Akt and mammalian target of rapamycin (mTOR), exerting pleiotropic effects on cell metabolism, migration, proliferation, survival, and differentiation. In mammalian cells, PI3K signaling is mediated by 4 different catalytic isoforms (p110 α, β, γ, δ). The δ isoform is restricted to hematopoietic cells, is highly expressed in lymphoid cells, and is the most critical isoform for signaling in normal B and CLL cells.8-10 In CLL, hyperactive B-cell receptor signaling with sustained signaling of the PI3Kδ/Akt/mTOR pathway contributes to the malignant phenotype.9-13
Idelalisib (GS-1101, CAL-101, 5-fluoro-3-phenyl-2-[(S)-1-(9H-purin-6-ylamino)-propyl]-3H-quinazolin-4-one) is a potent small-molecule inhibitor that is highly specific for PI3Kδ.10 In lymphoid cell lines and primary patient samples, idelalisib abrogates PI3K/Akt signaling and promotes apoptosis.9,10,13 Phase 1a testing in healthy volunteers established idelalisib oral bioavailability and safety at dose levels that achieve plasma concentrations shown to inhibit PI3Kδ in preclinical models.14
Given the molecular role of PI3Kδ in B-cell malignancies and the pharmacologic profile of idelalisib, a phase 1 dose-finding and extension study of idelalisib in patients with hematologic malignancies was performed to evaluate the safety, pharmacodynamics, pharmacokinetics, and clinical activity of idelalisib. This report summarizes the findings in patients with previously treated CLL.
Patients and methods
Eligibility criteria
This paper reports on the evaluation of idelalisib in the CLL cohorts of a large dose−ranging study of idelalisib that included primarily patients with CLL, indolent non-Hodgkin lymphoma (iNHL) and mantle cell lymphoma, but also included small patient cohorts of diffuse large B-cell lymphoma (DLBCL), acute myeloid leukemia, and multiple myeloma. Eligible patients for the CLL cohorts were adults ≥18 years of age with a World Health Organization (WHO) performance status ≤2, who had CLL and required therapy, both according to international workshop on CLL (iwCLL) criteria. All patients had disease that had recurred after ≥2 prior therapies, at least one of which included fludarabine. Institutional review boards at each of the 10 study sites approved the protocols. All subjects provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki. Please see the supplemental Methods on the Blood Web site for additional eligibility criteria.
Study treatment
The primary dose-ranging trial evaluated subjects through 48 weeks of idelalisib treatment and was followed by an extension study of continued therapy for subjects who were deriving clinical benefit. The primary 48-week study was completed in January 2012, and the extension study is continuing. This final analysis is based on data up to May 2013.
The study drug was provided as capsules or tablets containing 50, 75, or 100 mg of active idelalisib. Depending on the cohort, patients took idelalisib once per day (qd) or twice per day (bid), fasting, at approximately the same time each day. For the bid schedule, doses were taken at ∼12-hour intervals.
An initial 3+3 dose escalation included cohorts of patients with various hematologic malignancies treated at dose levels of 50 mg bid, 100 mg bid, 200 mg bid, and 350 mg bid. The dose range was based on idelalisib safety and pharmacokinetic data from the phase 1 healthy-volunteer experience.14 No dose-limiting toxicity (DLT) was observed, and the 350-mg bid cohort was expanded. Subsequent observation of grade ≥3 transaminase elevations (predominantly seen in patients with iNHL reported in a separate publication) led to further investigation of multiple lower-dose levels in cohorts of as large as 12 subjects per cohort. In total, idelalisib was administered at starting dose levels of 50 mg bid, 100 mg bid, 300 mg qd, 150 mg bid, 200 mg bid, and 350 mg bid to patients with CLL. Unless disease progression or intolerable toxicity occurred, patients could continue idelalisib at the initially assigned dose level through 48 weeks on the primary study and indefinitely on the extension study.
Statistics
Within each expanded dosing cohort, we evaluated for the determination of the maximum tolerated dose (MTD) under the premise that a dose level was not tolerated when the lower 95% exact binomial confidence interval (CI) for the DLT rate was >25%. DLTs were defined as treatment-emergent, drug-related toxicities occurring during the first 4 weeks of therapy that were grade ≥3 for nonhematologic adverse events (AEs) and grade ≥4 lasting for ≥7 days for hematologic AEs. Patients with CLL (reported here) or NHL (reported in the companion papers) were initially evaluated together in the initial 3+3 dose escalation, but after amendment and expansion, comprised different dose escalation levels. Unless otherwise noted, data from the dose-ranging study and the extension study were combined. Please see the supplemental Methods for additional statistical details and study assessments.
Results
Patient characteristics
Between July 2008 and January 2011, 54 patients with CLL were enrolled (Table 1). Eighty-three percent of the patients were male, and the median age was 63 years (range 37-82). Subjects were heavily pretreated with a median of 5 (range 2-14) prior therapies; all patients had received fludarabine, 96% had been exposed to rituximab, and 87% had previously been treated with alkylating agents. Seventy percent of patients were refractory to their most recent prior therapy. Sixty-seven percent (36/54) of patients presented with high-risk Rai-stage disease and 80% had bulky lymphadenopathy defined by the presence of at least 1 lymph node ≥5 cm. The median lymphocyte count (ALC) at baseline was 10.7 (range, 0.1-185.2) × 109/L, and grades 3-4 hematologic abnormalities were common at baseline. Unmutated IgHV was documented in 91% (49/54) of patients, and 24% (13/54) tested positive for del17p and/or TP53 mutation. Del11q was present in 28% (15/54), and NOTCH1 mutations were documented in 17% (9/54) of patients (see the supplemental material for analysis details).
Patient characteristics and disposition
| Description . | N = 54 . |
|---|---|
| Sex, n (%) | |
| Male | 45 (83.3) |
| Female | 9 (16.7) |
| Age, y, median (range) | 62.5 (37-82) |
| WHO performance status score, n (%) | |
| 0 | 17 (31.5) |
| 1 | 32 (59.3) |
| 2 | 5 (9.3) |
| Stage of disease at baseline (Rai classification) | |
| Low risk | 5 (9.3) |
| Intermediate risk | 12 (22.2) |
| High risk | 36 (66.7) |
| Missing | 1 (1.9) |
| Extent of CLL, n (%) | |
| Bulky lymphadenopathy (≥1 node ≥5 cm in diameter) | 43 (79.6) |
| Splenomegaly | 20 (37.0) |
| Hepatomegaly | 3 (5.6) |
| Thrombocytopenia (platelets <100 × 109/L) | 34 (63.0) |
| Anemia (Hb <110 g/L) | 25 (46.2) |
| Neutropenia (ANC <1.5 × 109/L) | 15 (27.7) |
| ALC cells × 109/L, median (range) | 10.8 (0.1-185.2) |
| CLL genetics, n (%) | |
| Unmutated IgHV | 49 (90.7) |
| del17p and/or TP53 mutation | 13 (20.4) |
| Prior therapies, n, median (range) | 5 (2-14) |
| Prior therapy type, n (%) | |
| Purine analog | 54 (100.0) |
| Rituximab | 52 (96.0) |
| Alkylating agent | 47 (87.0) |
| Alemtuzumab | 19 (35.2) |
| Anthracycline/anthracenedione | 18 (33.3) |
| Disease status (investigator judgment), n (%) | |
| Relapsed | 16 (29.6) |
| Refractory (to the most recent prior therapy) | 38 (70.4) |
| Idelalisib starting dose level, n (%) | |
| 50 mg/dose bid | 5 (9.3) |
| 100 mg/dose bid | 11 (20.4) |
| 300 mg/dose qd | 10 (18.5) |
| 150 mg/dose bid | 11 (20.4) |
| 200 mg/dose bid | 10 (18.5) |
| 350 mg/dose bid | 7 (13.0) |
| Duration of idelalisib therapy, mo, mean (range) | 15 (0.2-48.7) |
| Treatment disposition, n (%) | |
| Completed primary study (≥48 wk of idelalisib therapy) | 25 (46.3) |
| Discontinued primary study therapy | 29 (53.7) |
| Enrolled in extension study | 23 (38.9) |
| Ongoing | 8 (14.8) |
| Discontinued extension study therapy | 15 (27.8) |
| Primary reasons for discontinuation from primary or extension study therapy* | |
| Disease progression | 25† (46.3) |
| AE | 7‡ (12.9) |
| Death | 4 (7.4) |
| Patient request | 3 (5.6) |
| Other (lost to follow-up, other therapy, referred for transplantation) | 3 (5.6) |
| Investigator request | 2§ (3.7) |
| Description . | N = 54 . |
|---|---|
| Sex, n (%) | |
| Male | 45 (83.3) |
| Female | 9 (16.7) |
| Age, y, median (range) | 62.5 (37-82) |
| WHO performance status score, n (%) | |
| 0 | 17 (31.5) |
| 1 | 32 (59.3) |
| 2 | 5 (9.3) |
| Stage of disease at baseline (Rai classification) | |
| Low risk | 5 (9.3) |
| Intermediate risk | 12 (22.2) |
| High risk | 36 (66.7) |
| Missing | 1 (1.9) |
| Extent of CLL, n (%) | |
| Bulky lymphadenopathy (≥1 node ≥5 cm in diameter) | 43 (79.6) |
| Splenomegaly | 20 (37.0) |
| Hepatomegaly | 3 (5.6) |
| Thrombocytopenia (platelets <100 × 109/L) | 34 (63.0) |
| Anemia (Hb <110 g/L) | 25 (46.2) |
| Neutropenia (ANC <1.5 × 109/L) | 15 (27.7) |
| ALC cells × 109/L, median (range) | 10.8 (0.1-185.2) |
| CLL genetics, n (%) | |
| Unmutated IgHV | 49 (90.7) |
| del17p and/or TP53 mutation | 13 (20.4) |
| Prior therapies, n, median (range) | 5 (2-14) |
| Prior therapy type, n (%) | |
| Purine analog | 54 (100.0) |
| Rituximab | 52 (96.0) |
| Alkylating agent | 47 (87.0) |
| Alemtuzumab | 19 (35.2) |
| Anthracycline/anthracenedione | 18 (33.3) |
| Disease status (investigator judgment), n (%) | |
| Relapsed | 16 (29.6) |
| Refractory (to the most recent prior therapy) | 38 (70.4) |
| Idelalisib starting dose level, n (%) | |
| 50 mg/dose bid | 5 (9.3) |
| 100 mg/dose bid | 11 (20.4) |
| 300 mg/dose qd | 10 (18.5) |
| 150 mg/dose bid | 11 (20.4) |
| 200 mg/dose bid | 10 (18.5) |
| 350 mg/dose bid | 7 (13.0) |
| Duration of idelalisib therapy, mo, mean (range) | 15 (0.2-48.7) |
| Treatment disposition, n (%) | |
| Completed primary study (≥48 wk of idelalisib therapy) | 25 (46.3) |
| Discontinued primary study therapy | 29 (53.7) |
| Enrolled in extension study | 23 (38.9) |
| Ongoing | 8 (14.8) |
| Discontinued extension study therapy | 15 (27.8) |
| Primary reasons for discontinuation from primary or extension study therapy* | |
| Disease progression | 25† (46.3) |
| AE | 7‡ (12.9) |
| Death | 4 (7.4) |
| Patient request | 3 (5.6) |
| Other (lost to follow-up, other therapy, referred for transplantation) | 3 (5.6) |
| Investigator request | 2§ (3.7) |
As reported by investigators.
Three fatal outcomes.
Three fatal outcomes.
One fatal outcome.
Patient disposition
The mean dosing duration for all 54 patients was 15 months (range 0.2-48.7). Twenty-nine patients (54%) discontinued therapy during the primary study, with the most common primary reasons for discontinuation being progressive disease (PD) (15, 28%), AEs (5, 9%), and 3 early deaths (6%) as a result of AEs (infection/sepsis, fungal sinusitis, Pneumocystis jirovecii pneumonia). Of the 25 (46%) patients who completed 48 weeks of treatment in the primary study, 23 (43%) elected to continue idelalisib therapy in the extension study. The mean dosing duration for these 23 patients was 29 months (range 15-49), with 8 subjects (15%) remaining in the study as of May 2013. Fifteen subjects (28%) discontinued treatment on the extension study, with the most common reasons for discontinuation being PD (10, 19%) and AEs (2, 4%). In total, 11 deaths occurred during the study: 8 during the primary study (3 within the first 3 weeks of the study) and 3 during the extension study. The causes in the primary study included 5 pulmonary events (pneumonia, fungal pneumonia, Pneumocystis spp. pneumonia, adult respiratory distress syndrome, respiratory failure), one case of PD, one suspected infection, and one case of fungal sinusitis. A total of 4 patients had no follow-up disease assessment, including the 3 patients who died within the first 3 weeks of the study and another patient who was removed from study because the early treatment-induced lymphocytosis was misinterpreted as PD.
Treatment administration and safety
We did not observe any DLTs during the first 4 weeks of therapy and thus did not define a MTD within the tested dose range. Seven patients (13%) had a dose reduction at any time during the primary study: 1 patient at 100 mg bid (grade 3 oral mucositis/stomatitis, day 94), 1 patient at 200 mg/dose bid (grade 3 diarrhea, day 169), 1 at 300 mg/dose qd (grade 3 febrile neutropenia, day 40), and 4 at 350 mg/dose bid (grade 2 cough/hydronephrosis/gout, grade 3 transaminase elevation, grade 3 colitis, grade 4 radiation gastroenteritis).
Table 2 describes the overall incidence of treatment-emergent AEs and the laboratory abnormalities of any grade, regardless of causal attribution, and occurring in ≥10% of patients. Most AEs and laboratory abnormalities were grade 1-2 and/or could be considered related to the underlying disease or previous therapy (eg, fatigue, pyrexia, cytopenias). Transaminase elevations of any grade were observed in 15 subjects (28%); only 1 subject experienced a grade ≥3 transaminase elevation with bilirubin grade ≤1. Diarrhea was reported in 16 subjects (29.6%), with 3 (5.6%) having grade ≥3. Among the subjects experiencing diarrhea, colitis was reported in 4 (7.4%), including 3 grade 3 events. Rash was reported in 12 subjects (22%), all of which were grade ≤2.
Overall incidence of selected treatment-emergent AEs and laboratory abnormalities in ≥10% of patients
| . | N = 54 . | |
|---|---|---|
| AE, n (%) . | Any grade* . | Grade ≥3 . |
| Fatigue | 17 (31.5) | 1 (1.9) |
| Diarrhea | 16 (29.6) | 3 (5.6) |
| Pyrexia | 15 (27.8) | 2 (3.7) |
| Cough | 13 (24.1) | 2 (3.7) |
| Back pain | 12 (22.2) | 0 |
| Rash | 12 (22.2) | 0 |
| URI | 12 (22.2) | 0 |
| Pneumonia | 12 (22.2) | 11 (20.4) |
| Night sweats | 10 (18.5) | 0 |
| Chills | 9 (16.7) | 0 |
| Neutropenic fever | 6 (11.1) | 6 (11.1) |
| Laboratory abnormality, n (%) | ||
| ANC, decreased | 31 (57.4) | 23 (42.6) |
| Hb, decreased | 20 (37.0) | 6 (11.1) |
| Platelets, decreased | 16 (29.6) | 9 (16.7) |
| AP, increased | 18 (33.3) | 0 |
| GGT, increased | 11 (20.4) | 1 (1.9) |
| AST, increased | 13 (24.1) | 1 (1.9) |
| ALT, increased | 10 (18.5) | 1 (1.9) |
| Glucose, increased | 25 (46.3) | 3 (5.6) |
| Glucose, decreased | 14 (25.9) | 2 (3.7) |
| . | N = 54 . | |
|---|---|---|
| AE, n (%) . | Any grade* . | Grade ≥3 . |
| Fatigue | 17 (31.5) | 1 (1.9) |
| Diarrhea | 16 (29.6) | 3 (5.6) |
| Pyrexia | 15 (27.8) | 2 (3.7) |
| Cough | 13 (24.1) | 2 (3.7) |
| Back pain | 12 (22.2) | 0 |
| Rash | 12 (22.2) | 0 |
| URI | 12 (22.2) | 0 |
| Pneumonia | 12 (22.2) | 11 (20.4) |
| Night sweats | 10 (18.5) | 0 |
| Chills | 9 (16.7) | 0 |
| Neutropenic fever | 6 (11.1) | 6 (11.1) |
| Laboratory abnormality, n (%) | ||
| ANC, decreased | 31 (57.4) | 23 (42.6) |
| Hb, decreased | 20 (37.0) | 6 (11.1) |
| Platelets, decreased | 16 (29.6) | 9 (16.7) |
| AP, increased | 18 (33.3) | 0 |
| GGT, increased | 11 (20.4) | 1 (1.9) |
| AST, increased | 13 (24.1) | 1 (1.9) |
| ALT, increased | 10 (18.5) | 1 (1.9) |
| Glucose, increased | 25 (46.3) | 3 (5.6) |
| Glucose, decreased | 14 (25.9) | 2 (3.7) |
ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase.
The severity of AEs was graded using CTCAE (version 3.0).
Serious AEs (SAEs) were reported for 36 patients (67%). The most common SAEs reported in ≥3 subjects were pneumonia (11, 20.4%), febrile neutropenia (5, 9.3%), colitis (3, 5.6%), cellulitis (3, 5.6%) and Pseudomonas bacteremia (3, 5.6%). Pneumonia was reported in 11 subjects (20%), and all were grade ≥3 events. Most pneumonias appeared to be bacterial based on culture results or responses to conventional antibiotics. Two subjects (4%) developed Pneumocystis jirovecii pneumonia; it was determined that, of those, one subject was determined to have signs of infection before starting study treatment. Two subjects were reported to have fungal pneumonia, and another 2 subjects to have organizing pneumonia considered to be potentially related to the study drug. Both of the latter were treated with steroids; one discontinued use per investigator request and the other completed the primary study after resolution of the event. A third subject was also reported to have interstitial pneumonitis considered to be possibly related to the study drug and also treated with steroids. After resolution, this subject completed the primary study and enrolled on the extension study at a reduced dose (150 mg bid, initially 200 mg bid). One subject had documented cytomegalovirus reactivation. No tumor lysis syndrome was reported in the study.
In general, treatment-emergent grade ≥3 cytopenias resulted largely from minor fluctuations in hemoglobin (Hb), neutrophil, and platelet counts in patients with preexisting hematologic abnormalities. All patients with grade ≥3 anemia (n = 3) and neutropenia (n = 9), and 11 of 16 patients with grade ≥3 thrombocytopenia at baseline saw improvement or normalization at ≥1 time point during study participation. Idelalisib treatment did not result in significant changes in serum immunoglobulin levels (IgA, IgE, IgG, or IgM) or in absolute numbers of CD4+/CD3+ T-helper cells, CD8+/CD3+ cytotoxic T cells, or CD56+/CD16+/CD3– natural killer cells (data not shown). Analysis of electrocardiograms obtained at baseline and during the study did not show treatment-emergent abnormalities. Idelalisib had no detectable effects on serum glucose or triglyceride concentrations. Similarly, no treatment-related changes in serum insulin or proinsulin C-peptide values were observed (data not shown).
Pharmacokinetics and dose-response relationships
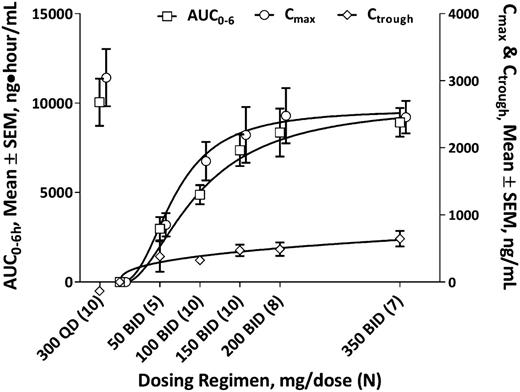
Pharmacokinetic data through day 28 were available on 50 patients (data not shown). Trough values for idelalisib were similar on days 8, 15, 22, and 28, indicating that steady state was achieved by day 8. No substantive changes in plasma exposure occurred with repeated dosing; mean (SD) accumulation ratios comparing day 28 with day 1 for maximum concentration (Cmax) and area under the concentration-time curve over 6 hours (AUC0-6h) were 1.1 (± 0.5) and 1.2 (± 0.7), respectively. There was no evidence of variation in idelalisib Cmax, AUC0-6, or trough concentration (Ctrough) by age (over a range of 40-84 years) or sex (42 males, 8 females) (data not shown).
An analysis of changes in Day 28 mean Cmax, AUC0-6h, or Ctrough relative to dose for bid dosing regimens showed modest increments in plasma exposure above the dose level of 150 mg/dose bid (Figure 1). Daily dosing did not maintain a continuous plasma exposure in the same manner as did BID administration; mean (SD) day 28 Ctrough values for 300 mg/dose qd (n = 10) vs 150 mg/dose bid (n = 10) were 153 ng/mL(± 83 ng/mL) vs 451 ng/mL (± 267 ng/mL), respectively.
Dose-exposure evaluation. Steady-state (day 28) idelalisib plasma exposures by idelalisib dosing regimen (N = 50). AUC0-6, area under the concentration-time curve over 6 hours; Cmax, maximum concentration; Ctrough, trough concentration; SEM, standard error of the mean.
Dose-exposure evaluation. Steady-state (day 28) idelalisib plasma exposures by idelalisib dosing regimen (N = 50). AUC0-6, area under the concentration-time curve over 6 hours; Cmax, maximum concentration; Ctrough, trough concentration; SEM, standard error of the mean.
Pharmacodynamic activity
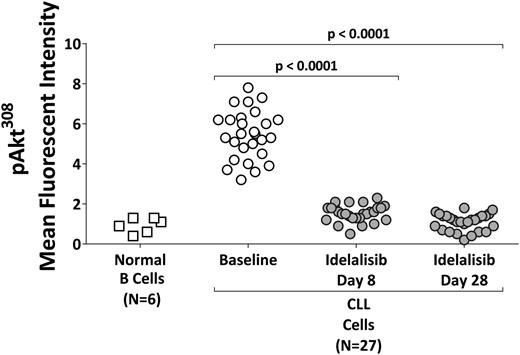
CLL cells from subjects receiving idelalisib doses of 100 mg bid (n = 7) and ≥150 mg BID (n = 20) were assessed for phospho-AktT308. Hyperactivation of the PI3K pathway in CLL cells as indicated by elevated levels of phospho-AktT308 was present at baseline in all evaluated subjects. Within 1 week of idelalisib treatment, Akt activation was uniformly, significantly, and persistently reduced to levels similar to that of normal B cells (Figure 2).
Pharmacodynamic effects. Phospho-Akt308 concentrations in circulating B cells (from normal subjects, N = 6) or CLL cells (from patients receiving idelalisib at 100 mg/dose bid (N = 7) or ≥150 mg/dose bid (N = 20)).
Pharmacodynamic effects. Phospho-Akt308 concentrations in circulating B cells (from normal subjects, N = 6) or CLL cells (from patients receiving idelalisib at 100 mg/dose bid (N = 7) or ≥150 mg/dose bid (N = 20)).
Plasma concentrations of CLL-derived chemokines (CCL3, CCL4, CCL17, CCL22) (supplemental Figure 1A-D) and of stroma-derived factors (soluble CD40 ligand [CD40L], CCL2, CXCL13, and tumor necrosis factor [TNF-α]) (supplemental Figure 1E-H) were commonly elevated at baseline and decreased significantly within 4 weeks of idelalisib therapy.
Efficacy
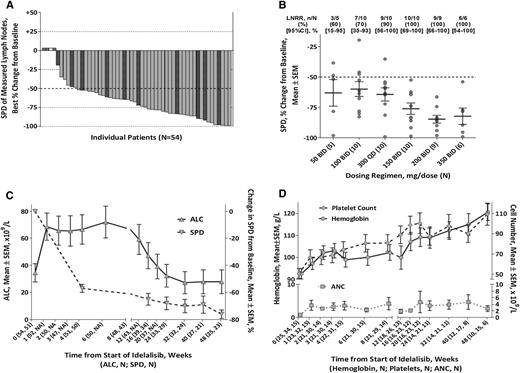
Overall, 44 of all 54 subjects (81.5%) achieved a lymph node response (≥50% reduction in the sum of the products of the perpendicular diameters (of measured lymph nodes) of the measured nodal lesions), and all subjects with a follow-up assessment (n = 50) saw a reduction in lymphadenopathy (Figure 3A). Four patients did not undergo a posttreatment tumor assessment owing to their early deaths as a result of AEs (in 1 patient with Pneumocystis jirovecii pneumonia [day 6] and 1 patient with fungal sinusitis [day 21], respectively) and/or clinical PD (in 2 patients who did not have follow-up on-study SPD assessments). Best-on-treatment changes in the SPD of ≥50% were most consistently seen at doses of 150 mg bid or higher (Figure 3B). Subjects had rapid reductions in lymph node area concurrent with asymptomatic increases in ALC (Figure 3C). Lymphocytosis was typically maximal within the first 8 weeks of therapy and generally declined from peak values thereafter but could persist above baseline for extended periods.
Changes in disease-related parameters. (A) Best on-treatment changes in the SPD of measured lymph nodes, by patient( ).
).  denotes nonevaluable patients (without a follow-up on-treatment tumor assessment) and
denotes nonevaluable patients (without a follow-up on-treatment tumor assessment) and  denotes the presence of a del17p or TP53 mutation. Forty-one patients were assessed by computed tomography scan and 9 by physical examination. Four patients did not have a follow-up assessment. (B) Rate of best on-treatment lymph node response (≥50% reduction in SPD of measured lymph nodes) and best on-treatment change from baseline in SPD of measured lymph nodes by dosing regimen (N = 50). (C) Mean changes in the SPD of measured lymph nodes and in the ALC by time during the primary study. (D) Hb concentration, platelet counts, and ANC by time during the primary study; includes only patients with baseline anemia (Hb <110 g/L), thrombocytopenia (platelets <100 × 109/L), or neutropenia (ANC <1.5 × 109/L). ALC, absolute lymphocyte count; LNRR, lymph node response rate.
denotes the presence of a del17p or TP53 mutation. Forty-one patients were assessed by computed tomography scan and 9 by physical examination. Four patients did not have a follow-up assessment. (B) Rate of best on-treatment lymph node response (≥50% reduction in SPD of measured lymph nodes) and best on-treatment change from baseline in SPD of measured lymph nodes by dosing regimen (N = 50). (C) Mean changes in the SPD of measured lymph nodes and in the ALC by time during the primary study. (D) Hb concentration, platelet counts, and ANC by time during the primary study; includes only patients with baseline anemia (Hb <110 g/L), thrombocytopenia (platelets <100 × 109/L), or neutropenia (ANC <1.5 × 109/L). ALC, absolute lymphocyte count; LNRR, lymph node response rate.
Changes in disease-related parameters. (A) Best on-treatment changes in the SPD of measured lymph nodes, by patient( ).
).  denotes nonevaluable patients (without a follow-up on-treatment tumor assessment) and
denotes nonevaluable patients (without a follow-up on-treatment tumor assessment) and  denotes the presence of a del17p or TP53 mutation. Forty-one patients were assessed by computed tomography scan and 9 by physical examination. Four patients did not have a follow-up assessment. (B) Rate of best on-treatment lymph node response (≥50% reduction in SPD of measured lymph nodes) and best on-treatment change from baseline in SPD of measured lymph nodes by dosing regimen (N = 50). (C) Mean changes in the SPD of measured lymph nodes and in the ALC by time during the primary study. (D) Hb concentration, platelet counts, and ANC by time during the primary study; includes only patients with baseline anemia (Hb <110 g/L), thrombocytopenia (platelets <100 × 109/L), or neutropenia (ANC <1.5 × 109/L). ALC, absolute lymphocyte count; LNRR, lymph node response rate.
denotes the presence of a del17p or TP53 mutation. Forty-one patients were assessed by computed tomography scan and 9 by physical examination. Four patients did not have a follow-up assessment. (B) Rate of best on-treatment lymph node response (≥50% reduction in SPD of measured lymph nodes) and best on-treatment change from baseline in SPD of measured lymph nodes by dosing regimen (N = 50). (C) Mean changes in the SPD of measured lymph nodes and in the ALC by time during the primary study. (D) Hb concentration, platelet counts, and ANC by time during the primary study; includes only patients with baseline anemia (Hb <110 g/L), thrombocytopenia (platelets <100 × 109/L), or neutropenia (ANC <1.5 × 109/L). ALC, absolute lymphocyte count; LNRR, lymph node response rate.
Idelalisib treatment resulted in significant improvements of cytopenias present at baseline (Figure 3D), inducing Hb, platelet, and absolute neutrophil count (ANC) response rates of 68% (17/25), 79% (27/34), and 100% (15/15), respectively. The ANC response in 4 patients was potentially confounded by the use of granulocyte colony-stimulating factor. In patients with palpable organomegaly present at baseline, idelalisib induced resolution of hepatomegaly or splenomegaly in 100% (3/3) and 78% (14/18) of patients, respectively (data not shown).
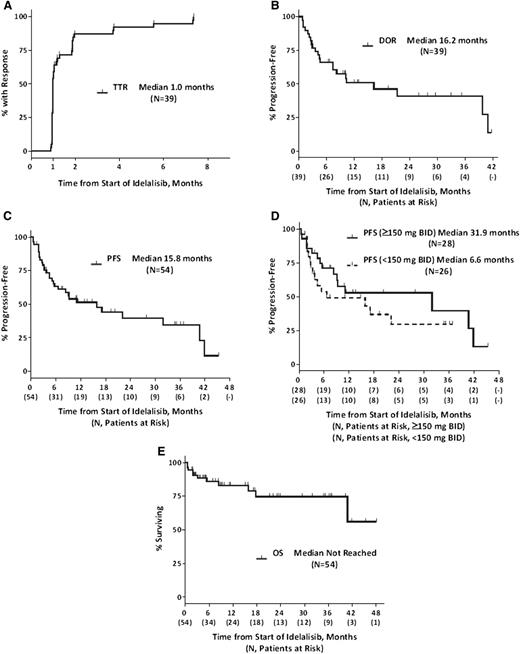
Using both iwCLL 2008 criteria15 and updated response criteria,1,2 the intention-to-treat overall response rate was 72% (95% CI, 58.4%-83.5%) (39/54), with 39% (21/54) of subjects meeting the criteria for partial response (PR) per iwCLL 2008 criteria and 33% (18/54) meeting the criteria of PR in the presence of treatment-induced lymphocytosis. The median time to response (TTR) (n = 39) was 1.0 month, and the median duration of response (DOR) was 16.2 months (Figure 4A-B). The median PFS for all 54 subjects was 15.8 months, and median OS was not reached with 75% of patients surviving at 36 months (Figure 4C,E). The median PFS for patients (n = 28) treated at ≥150 mg bid (the now recommended dose for phase 2/3 trials) was 32 months compared with 7 months for those (n = 26) treated at lower doses (Figure 4D). The ORR for the 13 subjects with del17p and/or TP53 mutation who had a median of 5 prior regimens, and 85% of whom were refractory to their most recent prior regimen, was 54% (7/13), and the median PFS was 3 months. Of this subgroup, 7 subjects were treated at doses of ≥150 mg bid, and their median PFS was 5 months. The median PFS for subjects without del17p and/or TP53 mutation and treated at doses of ≥150 mg bid or higher was 41 months (data not shown).
Time-to-event endpoints. (A) TTR, (B) DOR, (C-D) PFS, and (E) OS. TTR, PFS, and OS are measured from the start of therapy, whereas DOR is measured from the start of response. TTR and DOR are composed of responding subjects only; PFS and OS represent ITT analyses. A tick on the curve (┴) denotes a censored time point. OS, overall survival; PFS, progression-free survival.
Time-to-event endpoints. (A) TTR, (B) DOR, (C-D) PFS, and (E) OS. TTR, PFS, and OS are measured from the start of therapy, whereas DOR is measured from the start of response. TTR and DOR are composed of responding subjects only; PFS and OS represent ITT analyses. A tick on the curve (┴) denotes a censored time point. OS, overall survival; PFS, progression-free survival.
No significant difference in DOR was seen in the 11 responding patients with del11q (15 total) compared with those without. The DOR for the 6 responding patients with NOTCH1 mutation (9 total) was 7.8 months compared with 21.2 months for the patients without (P = .034; data not shown).
Discussion
We report here the results of the first clinical evaluation of selective inhibition of PI3Kδ to treat human disease. The data indicate that idelalisib, an orally administered, targeted, and selective inhibitor of the lipid kinase PI3Kδ, can be safely given as a continuous oral therapy over periods extending to >3 years and can offer substantial and sustained antileukemic effects in patients with CLL. As a result of the high selectivity of idelalisib for PI3Kδ, patients did not show the elevations of serum glucose, insulin, or proinsulin C-peptide levels that are characteristically observed in patients receiving pan–PI3K-inhibitors that also inhibit PI3Kα.16,17 As measured in vivo using patient CLL cells and serum samples, treatment with idelalisib led to dose-dependent reduction of Akt activity as well as chemokine and cytokine levels associated with CLL, confirming the pharmacologic activity of idelalisib in achieving target inhibition in vivo.
Idelalisib was well tolerated without observed DLTs as defined in the protocol. A recommended idelalisib dosing regimen of 150 mg bid was selected for future studies based on the overall safety profile observed in all diseases at higher doses, the observed plateau in plasma exposure at doses >150 mg bid, and the consistent clinical response in lymph nodes at doses of ≥150 mg bid. Given improving cytopenias during treatment, idelalisib did not induce myelosuppression, nor did it discernibly increase the risk of infection above the level already present in these heavily pretreated CLL patients. The pneumonias we observed were usually the bacterial pneumonias commonly seen in CLL patients. The rate of pneumonia in patients with CLL receiving idelalisib over ∼405 patient months of observation in the primary study was ∼0.037 pneumonias/patient months, a rate within the range of ∼0.060 pneumonias/patient months reported in the literature for a similar CLL population.18 Similarly, the 2 instances of Pneumocystis spp. pneumonia observed are not surprising in this population of patients with relapsed/refractory CLL. In this study, 3 cases of possibly drug-related interstitial pneumonitis were reported. However, these events responded rapidly to steroids, and 2 of the 3 patients continued on idelalisib. Other drug-related events of interest include asymptomatic serum transaminase elevations as well as skin rashes and diarrhea, in some cases in the setting of colitis. Grade ≤2 transaminase elevations generally resolved spontaneously despite continued administration of idelalisib. Although only one patient with CLL experienced grade ≥3 transaminase elevation, the overall incidence of grade ≥3 transaminase elevations (including in patients with NHL) during this study was higher.19 The broader study population showed that grade ≥3 transaminase elevation can be managed with early recognition by routine monitoring, drug interruption to allow for recovery, and subsequent reinitiation of therapy with dose titration if needed. Events of skin rash or colitis have been successfully managed with administration of local, nonabsorbable or systemic steroids.
Idelalisib induced marked reductions in lymphadenopathy and hepatosplenomegaly in patients with relapsed/refractory CLL, even with bulky refractory disease and extensive prior therapy. Additional clinical benefits observed included improved cytopenias, suggesting an overall improvement in bone marrow function during idelalisib therapy, although data from bone marrow biopsies in this study are limited.
Idelalisib treatment caused an increase in ALC in most patients. Observed as early as 4 hours after drug initiation, this rapid increase is thought to indicate a mobilization of CLL cells from tissues rather than a proliferative event. In contrast to the disease flare seen with lenalidomide,20 the idelalisib-induced lymphocytosis is associated with quiescence of CLL cells as indicated by reductions in phospho-Akt and inflammatory mediators. This clinical observation is consistent with preclinical data showing that PI3Kδ inhibition reduces chemokine-mediated signaling between lymphocytes and stromal cells, releasing B cells from lymph nodes and other tissue sites.12,21 This treatment-induced lymphocytosis is not unique to idelalisib but has also been observed in patients with CLL who are administered other drugs that affect the B-cell receptor and Akt/mTOR signaling pathways, including agents inhibiting Syk,22 mTOR,23 and BTK.24-26 According to iwCLL 2008 criteria, a ≥50% increase in ALC (with a total ALC of ≥5.0 × 109 cells/L) represents CLL progression.15 The data from this study strongly suggest that the ALC alone is not adequate as a determinant of disease status in the evaluation of novel therapeutic agents like idelalisib that cause a redistribution of CLL cells. Based in part on observations from this study, the iwCLL response criteria have been modified to indicate that an increase in ALC caused by treatment-induced redistribution of CLL cells should not be confused with PD, especially when other disease parameters are improving. Furthermore, patients meeting all of the criteria for PR except for persistent lymphocytosis may be considered “partial responders.”1 In this study, we have reported responding patients in both categories, as partial responders according to iwCLL 2008 criteria and as partial responders with persistent lymphocytosis, to facilitate the evolving understanding and comparison of these 2 response categories. Although it was difficult to assess in this study because of the small sample size and varying dose levels, no significant difference in DOR or PFS was observed between PRs by standard iwCLL 2008 criteria, and PRs with persistent lymphocytosis (data not shown).
Among all patients with CLL in this study, disease control was durable, with a median PFS of 15.8 months; some patients have remained on therapy without CLL progression for periods approaching 4 years. These efficacy findings with idelalisib in an extremely heavily pretreated high-risk population compare very favorably with results from single-agent studies evaluating available noncytotoxic drugs as therapies for previously treated CLL, including rituximab,27-30 alemtuzumab,31,32 ofatumumab,33 and lenalidomide.34,35 These results appear similar to those reported recently with the BTK inhibitor ibrutinib, particularly given that patients in this phase 1 study of idelalisib appeared to have more heavily pretreated and refractory disease than those treated in the phase 2 study of ibrutinib.25 Although the PFS of patients with del17p was short in this study, the small number of patients enrolled had very refractory disease and were treated using a wide range of dose levels. Additional studies will be needed to clarify the activity of idelalisib in 17p deleted CLL.
The development of idelalisib represents a major scientific effort that translates a decade of PI3K-related research into a targeted oral therapy that can be chronically administered, with an acceptable safety profile and excellent control of recurrent CLL. Our data support further evaluation of idelalisib in patients with CLL—either previously treated or treatment-naïve—both as a single agent and in combination, including with rationally partnered targeted agents. As we look toward the future of CLL therapy, targeted agents hold the potential promise of prolonged efficacy and reduced toxicity compared with chemoimmunotherapy regimens.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the study participants for their dedication, the clinical personnel at each of the study sites for diligence in caring for patients and collecting study data, and Cancer Genetics, Inc. (Rutherford, NJ) for their expertise in systematically evaluating CLL genetics for the study. The authors acknowledge study team members at Novella Clinical, Inc. (Columbus, OH) and C3 Research Associates, (Seattle, WA).
This study was sponsored and funded by Gilead Sciences, Inc (Foster City, CA and Seattle, WA), and by Calistoga Pharmaceuticals, Inc (Seattle, WA) (now a wholly owned subsidiary of Gilead Sciences, Inc).
Authorship
Contribution: J.R.B., J.C.B., D.M.J., B.J.L., R.G.U., A.S.Y., L.L.M., and R.R.F. designed the study; J.R.B., J.C.B., S.E.C., I.W.F., N.W.J., S.E.S., B.S.K., C.B., and R.R.F. enrolled the patients; J.R.B., J.C.B., S.E.C., D.M.B., I.W.F., N.D.W.-J., S.E.S., B.S.K., H.K.W., D.M.J., S.P., D.L., T.M.J., B.J.L., A.S.Y., L.L.M., and R.R.F. performed research and analyzed data; J.R.B. wrote the paper with input from J.C.B., S.E.C., I.W.F., N.W.J., S.E.S., B.S.K., T.M.J., L.L.M., and RRF; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: H.K.W., D.M.J., S.P., D.L., T.M.J., B.J.L., R.G.U., A.S.Y., and L.L.M. are current or former employees of Calistoga or Gilead. D.M.J., D.L., T.M.J., B.J.L., R.G.U., A.S.Y., and L.L.M. have equity ownership in Calistoga or Gilead. J.R.B., J.C.B., S.E.C., N.W.J., B.S.K., C.B., R.G.U., L.L.M., and R.R.F. are consultants for Calistoga or Gilead. J.R.B. and J.C.B. received support from the Leukemia and Lymphoma Society. B.S.K. is a consultant for Infinity. D.M.J. is an employee and shareholder of Acerta Pharmaceuticals. B.J.L. is an employee of Effector Therapeutics and a consultant for Acerta Pharmaceuticals. L.L.M. is on the scientific advisory board of Acerta Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Jennifer R. Brown, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: Jennifer_brown@dfci.harvard.edu.