Key Points
Alternatively polarized macrophages are abundant constituents of the tumor microenvironment in T-cell lymphoproliferative disorders.
GATA-3 expression identifies a subset of PTCL, NOS with a distinct cytokine profile and inferior survival.
Abstract
The cell of origin and the tumor microenvironment’s role remain elusive for the most common peripheral T-cell lymphomas (PTCLs). As macrophages promote the growth and survival of malignant T cells and are abundant constituents of the tumor microenvironment, their functional polarization was examined in T-cell lymphoproliferative disorders. Cytokines that are abundant within the tumor microenvironment, particularly interleukin (IL)-10, were observed to promote alternative macrophage polarization. Macrophage polarization was signal transducer and activator of transcription 3 dependent and was impaired by the Janus kinase inhibitor ruxolitinib. In conventional T cells, the production of T helper (Th)2-associated cytokines and IL-10, both of which promote alternative macrophage polarization, is regulated by the T-cell transcription factor GATA-binding protein 3 (GATA-3). Therefore, its role in the T-cell lymphomas was examined. GATA-3 expression was observed in 45% of PTCLs, not otherwise specified (PTCL, NOS) and was associated with distinct molecular features, including the production of Th2-associated cytokines. In addition, GATA-3 expression identified a subset of PTCL, NOS with distinct clinical features, including inferior progression-free and overall survival. Collectively, these data suggest that further understanding the cell of origin and lymphocyte ontogeny among the T-cell lymphomas may improve our understanding of the tumor microenvironment’s pathogenic role in these aggressive lymphomas.
Introduction
Lessons learned from large gene expression profiling and histopathological studies performed in the most common non-Hodgkin lymphomas have dramatically improved our understanding of these lymphomas. For example, lymphomas that are otherwise indistinguishable by histopathological examination originate from different subsets of normal lymphocytes (ie, the “cell of origin”), exploit distinct oncogenic pathways and transcriptional programs, and vary in their susceptibility to both conventional and novel therapies.1 In addition, nonmalignant (ie, “stromal”) cells within the tumor microenvironment promote lymphomagenesis by directly fostering the growth and survival of malignant lymphocytes, suppressing antitumor immunity, and driving angiogenesis.2-4 Subsets of nonmalignant lymphocytes may be distinguished by their immunoregulatory and functional properties, including differences in the ability to promote the recruitment, expansion, and functional polarization of both lymphoid- and myeloid-derived cells. Many of the functions associated with these disparate lymphocyte subsets are determined by master transcriptional regulators that drive lymphocyte differentiation.5 Therefore, the transcriptional programs that determine lymphocyte ontogeny (and the cell of origin) likely regulate the tumor microenvironment. Although the cell of origin and regulation of the tumor microenvironment are apparently related and interdependent, the association between these 2 fundamental questions is poorly understood for most non-Hodgkin lymphomas, including those that are T cell–derived.
The heterogeneity of the T-cell lymphomas (TCLs) and poor understanding of their pathogenesis continue to impede their classification and the development of novel therapeutic strategies.6,7 In fact, the cell of origin for the most common TCL remains elusive and is therefore designated as peripheral TCL not otherwise specified (PTCL, NOS).7,8 The association between the cell of origin and the tumor microenvironment is best exemplified by angioimmunoblastic TCL (AITL), an aggressive PTCL that originates from clonally expanded follicular helper T cells (Tfh).9 Conventional Tfh cells regulate B cells and follicular dendritic cells within the germinal center and are required for both the generation of a humoral immune response and germinal center formation. The Tfh-associated cytokines (eg, CXCL13 and interleukin [IL]-21) and cell surface receptors (eg, CXCR5 and inducible T-cell costimulator) that regulate B cells and follicular dendritic cells within the germinal center are coordinated by the master transcriptional repressor Bcl-6.10 In addition to sharing a common immunophenotype with Tfh cells, AITL and Tfh cells share gene expression profiling similarities.9 Therefore, the ontogeny of malignant T cells in AITL explains the histologic (eg, germinal center B-cell and follicular dendritic cell expansion) and clinical (eg, polyclonal hypergammaglobulinemia and autoimmunity) findings characteristic of this PTCL.
In contrast to AITL, characterized by an expanded meshwork of lymphoma-associated follicular dendritic cells, most T-cell lymphoproliferative disorders are infiltrated by an abundance of myeloid dendritic cells and lymphoma-associated macrophages.11 These myeloid-derived cells (MDCs) within the tumor microenvironment promote the growth and survival of malignant T cells, confer resistance to chemotherapy, and promote the suppression of host antitumor immunity.11-13 These MDC-associated functions are regulated by cytokines present locally within the tumor microenvironment. For example, we have previously shown that IL-10, produced by malignant T cells, inhibits the maturation of lymphoma-associated dendritic cells.11 The functional plasticity observed following macrophage activation is similarly regulated by cytokines, including IL-10, that are abundant within the microenvironment.14 Although interferon-γ (IFN-γ) and Toll-like receptor agonists promote classical macrophage polarization, IL-4/IL-13 and IL-10 promote alternative polarization.14 In contrast to classically polarized macrophages, which are associated with antigen processing/presentation, killing of intracellular pathogens, and tumor/tissue damage, alternatively polarized macrophages regulate tumor cell growth/survival, suppress host immunity, and promote angiogenesis and remodeling of the extracellular matrix. Therefore, alternatively polarized macrophages, and the factors regulating their biology, not only influence many aspects of tumor biology but represent a novel therapeutic target.4,14 Although we have previously shown that monocytes are actively recruited by malignant T cells and their progeny are abundant constituents of the tumor microenvironment, the polarization state of TCL-associated macrophages is poorly understood.11
Here we demonstrate that alternatively polarized macrophages are abundant in T-cell lymphoproliferative disorders. In conventional T cells, the transcription factor GATA-binding protein 3 (GATA-3), in addition to its established role in T helper (Th)2 differentiation, promotes IL-4/IL-13 transcription and epigenetically regulates the IL-10 locus.15,16 As these cytokines promote alternative macrophage polarization, GATA-3 expression in the TCLs was examined, where it was shown in shRNA-mediated knockdown experiments to regulate both IL-10 and Th2-associated cytokine production. Importantly, GATA-3 expression was observed in a subset of PTCL, NOS with distinct molecular and clinicopathological features, including a significantly inferior progression-free and overall survival.
Methods
Patient samples and cell lines
A cohort of cutaneous TCL (CTCL) and PTCL patients from the Mayo Clinic, Rochester, MN (between 1994 and 2009) and the University of Michigan Comprehensive Cancer Center, Ann Arbor, MI (between 1985 and 2011) were included in this study with the approval of the respective Institutional Review Board. This study was conducted in accordance with US federal regulations and the Declaration of Helsinki. The PTCL/CTCL cell lines and their source are shown in supplemental Table 1 on the Blood Web site. The cell lines stably expressing short hairpin RNAs (shRNAs) targeting GATA-3 (supplemental Table 2) were generated by lentiviral infection and puromycin selection. The cell lines were maintained in RPMI 1640 supplemented with 10% calf serum in 37°C, 5% CO2. Peripheral blood mononuclear cells were obtained from healthy donors.
Gene expression analysis
The mRNA expression profiling was determined by nCounter technology (Nanostring Technologies) (supplemental Table 3) and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) (supplemental Table 4). Protein expression was determined by western blot, enzyme-linked immunosorbent assay, flow cytometry, and immunochemistry.
Alternatively microphage polarization
The function of alternatively polarized macrophage was examined by mixed lymphocyte reaction with a carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay by flow cytometry.11 Alternatively, polarized microphages in PTCL, NOS specimens and normal tissues were visualized by double immunofluorescence staining of phosphorylated signal transducer and activator of transcription 3 (pSTAT3) and CD163, with nuclei stained with 4′6 diamidino-2-phenylindole.
Statistical analysis
Patient clinical data were analyzed using JMP 8 software (SAS, Cary, NC). Progression-free and overall survival were estimated using the Kaplan-Meier method and 2-tailed log-rank test.17 GATA-3 expression was dichotomized, and the Cox proportional hazards model was used to evaluate its ability to predict overall survival/progression-free survival and to adjust for the prognostic index for PTCL, NOS (PIT).18,19 Comparisons among groups were evaluated using a Student t test, and P < .05 was determined to be statistically significant.
The methods are described more fully in the supplemental Methods.
Results
Malignant T cells promote alternative macrophage polarization in an IL-10–dependent manner
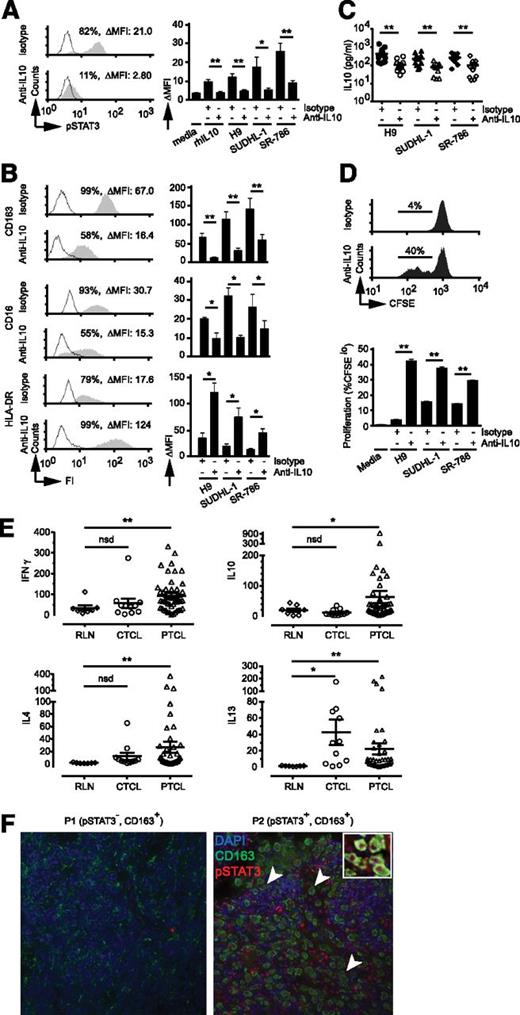
In contrast to classically polarized macrophages, which are dependent on STAT1 phosphorylation, alternative macrophage polarization in response to IL-4/IL-13 or IL-10 is dependent on STAT6 or STAT3 phosphorylation, respectively.14 Therefore, we examined the activation state of these relevant STATs in monocytes exposed to conditioned media generated from patient-derived TCL lines. STAT3 phosphorylation in response to TCL-conditioned media (TCL-CM) was observed for many cell lines tested (Figure 1A). In contrast, STAT1 phosphorylation was not observed, and STAT6 phosphorylation was noted in monocytes exposed to MyLa-conditioned media only (and was IL-13 dependent; data not shown). IL-10 neutralization in TCL-CM and monocyte cocultures abrogated STAT3 phosphorylation (Figure 1A). IL-10 levels were low (or undetectable) in the conditioned media derived from those TCL lines failing to induce STAT3 phosphorylation (data not shown). Conversely, IL-6 neutralization, which also signals via STAT3, failed to inhibit STAT3 phosphorylation (data not shown). We next examined the expression of selected cell surface proteins with an established role in distinguishing classically (ie, IFN-γ) and alternatively (ie, IL-4/IL-13 or IL-10) polarized macrophages, including CD16, CD163, and human leukocyte antigen DR (HLA-DR) (supplemental Figure 1).14,20 We observed that monocyte-derived macrophages generated in the presence of TCL-CM more closely resembled IL-10 polarized macrophages (compare supplemental Figure 1 with Figure 1B; data not shown), and IL-10 neutralization significantly impaired CD16 and CD163 expression while increasing HLA-DR expression (Figure 1B). Functionally, alternatively polarized macrophages are an important source of IL-10 within the tumor microenvironment and are impaired in their ability to stimulate T-cell proliferation.14 Therefore, these functional attributes of alternatively polarized macrophages were examined in TCL-polarized macrophages (Figure 1C-D). In these experiments, IL-10 production was significantly diminished (Figure 1C), and the capacity to induce T-cell proliferation was dramatically enhanced (Figure 1D) on IL-10 neutralization. Collectively, these results demonstrate that TCLs promote alternative macrophage polarization in an IL-10–dependent manner in vitro, and both CD163 and phosphorylated STAT3 may aid in their identification in clinical TCL specimens.
IL-10–dependent macrophage polarization and macrophage-polarizing cytokines in TCLs. (A) Monocytes were cultured in media alone or in media supplemented with either IL-10 (20 ng/mL) or conditioned-media (50% v/v) obtained from the TCL cells indicated. An isotype control or neutralizing IL-10 monoclonal antibody was included, as indicated, and STAT3 phosphorylation (Y705) determined by flow cytometry. (Left) Representative histograms (open, isotype control; gray, pSTAT3). (Right) Change in mean fluorescent intensity (ΔMFI) of pSTAT3 from triplicate wells (±standard error). (B) Monocyte-derived macrophages (MDMs) were generated and polarized in TCL-conditioned media with an isotype control or IL-10 neutralizing antibody. CD163, CD16, and HLA-DR expression (gray histogram) were determined (isotype control, open histogram). MDMs generated in the presence of H9-conditioned media are shown in (left) representative histograms and (right) change in mean fluorescent intensity (ΔMFI) from triplicate wells (±standard error). (C) MDMs were generated and functionally polarized in TCL-conditioned media (TCL-CM) supplemented with an isotype control (closed markers) or IL-10 neutralizing monoclonal antibody (open markers) in triplicate in a flat-bottom 96-well plate. Adherent macrophages were gently washed with fresh media, and lipopolysaccharide was added. IL-10 production was determined 24 hours later. The mean (±standard error) from 10 individual normal donors is shown (note logarithmic scale). (D) MDMs were polarized with TCL-CM in triplicate wells of a 96-well plate. CFSE-labeled allogeneic CD3+ T cells were added, and T-cell proliferation determined by CFSE dilution. (Upper) Representative histograms using MDMs generated in H9-CM. (Lower) Mean proliferation (% CFSElo) (±standard error). All experiments shown are representative of ≥3 similarly performed experiments. (E) The abundance of cytokine transcripts (IFN-γ, IL-10, IL-4, IL-13) were quantified by Nanostring nCounter technology from FFPE reactive lymph node (n = 7), CTCL (n = 11), and 48 PTCL (PTCL, NOS, n = 31; AITL, n = 10, ALCL, n = 7) specimens. (F) Immunofluorescent double staining for CD163 and pSTAT3 (Y705) with nuclei stained by 4′6 diamidino-2-phenylindole was performed in PTCL, NOS specimens (n = 80, representative examples shown). *P < .05 and **P < .01 in unpaired 2-sided Student t test.
IL-10–dependent macrophage polarization and macrophage-polarizing cytokines in TCLs. (A) Monocytes were cultured in media alone or in media supplemented with either IL-10 (20 ng/mL) or conditioned-media (50% v/v) obtained from the TCL cells indicated. An isotype control or neutralizing IL-10 monoclonal antibody was included, as indicated, and STAT3 phosphorylation (Y705) determined by flow cytometry. (Left) Representative histograms (open, isotype control; gray, pSTAT3). (Right) Change in mean fluorescent intensity (ΔMFI) of pSTAT3 from triplicate wells (±standard error). (B) Monocyte-derived macrophages (MDMs) were generated and polarized in TCL-conditioned media with an isotype control or IL-10 neutralizing antibody. CD163, CD16, and HLA-DR expression (gray histogram) were determined (isotype control, open histogram). MDMs generated in the presence of H9-conditioned media are shown in (left) representative histograms and (right) change in mean fluorescent intensity (ΔMFI) from triplicate wells (±standard error). (C) MDMs were generated and functionally polarized in TCL-conditioned media (TCL-CM) supplemented with an isotype control (closed markers) or IL-10 neutralizing monoclonal antibody (open markers) in triplicate in a flat-bottom 96-well plate. Adherent macrophages were gently washed with fresh media, and lipopolysaccharide was added. IL-10 production was determined 24 hours later. The mean (±standard error) from 10 individual normal donors is shown (note logarithmic scale). (D) MDMs were polarized with TCL-CM in triplicate wells of a 96-well plate. CFSE-labeled allogeneic CD3+ T cells were added, and T-cell proliferation determined by CFSE dilution. (Upper) Representative histograms using MDMs generated in H9-CM. (Lower) Mean proliferation (% CFSElo) (±standard error). All experiments shown are representative of ≥3 similarly performed experiments. (E) The abundance of cytokine transcripts (IFN-γ, IL-10, IL-4, IL-13) were quantified by Nanostring nCounter technology from FFPE reactive lymph node (n = 7), CTCL (n = 11), and 48 PTCL (PTCL, NOS, n = 31; AITL, n = 10, ALCL, n = 7) specimens. (F) Immunofluorescent double staining for CD163 and pSTAT3 (Y705) with nuclei stained by 4′6 diamidino-2-phenylindole was performed in PTCL, NOS specimens (n = 80, representative examples shown). *P < .05 and **P < .01 in unpaired 2-sided Student t test.
To investigate the abundance of macrophage-polarizing cytokines within the tumor microenvironment in clinical specimens, RNA was isolated from formalin-fixed, paraffin-embedded (FFPE) cutaneous (n = 11) and peripheral TCL (n = 48) specimens. Nanostring nCounter technology, a well-validated mRNA expression profiling technique suitable for FFPE tissues,21 was used to determine the abundance of IL-10, the Th2-associated cytokines IL-4 and IL-13, and IFN-γ transcripts. These cytokine transcripts were more abundant in both cutaneous and peripheral TCL specimens compared with reactive lymph nodes (Figure 1E).
Therefore, polarized macrophages were visualized by double immunofluorescence in TCL specimens using CD163 and phosphorylated STAT3 as markers. IL-10 polarized macrophages (nuclear pSTAT3+/ surface CD163+) were not identified in normal tissues (supplemental Figure 2) but were abundant constituents of the tumor microenvironment in 41% of the TCLs examined (n = 80; representative examples shown in Figure 1F). In parallel, we failed to observe nuclear STAT1 (n = 84) or STAT6 (n = 81) phosphorylation in CD163-expressing macrophages in TCL specimens (data not shown). As alternatively polarized macrophages promote carcinogenesis and tumor progression, the development of therapeutic strategies impairing their formation in the TCLs is warranted.
Ruxolitinib prevents alternative macrophage polarization
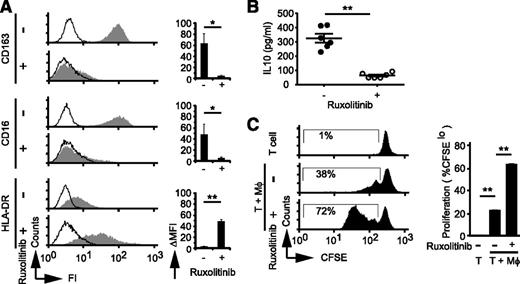
STAT3 phosphorylation in response to IL-10 is dependent on the Janus kinases (JAKs) JAK1 and TYK2, both of which are inhibited at nanomolar (and clinically achievable) concentrations by the JAK2 inhibitor ruxolitinib. Therefore, we sought to determine whether ruxolitinib may prevent IL-10–induced macrophage polarization. As expected, ruxolitinib inhibits IL-10–induced STAT3 phosphorylation (supplemental Figure 3A). More importantly, macrophages polarized in the presence of ruxolitinib lost the immunophenotype characteristics of IL-10–polarized macrophages and were CD16−/loCD163−/loHLA-DRhi (Figure 2A). Furthermore, IL-10 production was significantly impaired in macrophages polarized in the presence of ruxolitinib (Figure 2B), whereas their ability to stimulate T-cell proliferation was significantly increased (Figure 2C). Ruxolitinib similarly impaired STAT6 phosphorylation in response to IL-4/IL-13 and inhibited IL-4/IL-13–dependent alternative macrophage polarization (supplemental Figure 3B-D). An alternative approach to therapeutically target macrophage polarization may emerge with improved understanding of the mechanism driving IL-10 and IL-4/IL-13 production in malignant T cells. Therefore, in a separate initiative, we sought to identify factors that regulate cytokine production in these lymphomas.
Ruxolitinib inhibits IL-10–dependent macrophage polarization. (A) MDMs were generated and polarized with IL-10 as before. Cultures were supplemented with vehicle control (dimethylsulfoxide) or ruxolitinib (1 μM), and CD163, CD16, and HLA-DR expression was determined. (B-C) Polarized macrophages generated in the presence or absence of ruxolitinib (Rux) were washed with fresh media. (B) Media supplemented with lipopolysaccharide (100 ng/mL) were added, and cell-free supernatants were collected 24 hours later for determination of IL-10 production by cytometric bead array assay. The mean (±standard error) from 6 individual normal donors is shown. (C) In parallel, CFSE-labeled T cells were added in triplicate, and T-cell proliferation was determined by CFSE dilution. *P < .05 and **P < .01 in unpaired 2-sided Student t test.
Ruxolitinib inhibits IL-10–dependent macrophage polarization. (A) MDMs were generated and polarized with IL-10 as before. Cultures were supplemented with vehicle control (dimethylsulfoxide) or ruxolitinib (1 μM), and CD163, CD16, and HLA-DR expression was determined. (B-C) Polarized macrophages generated in the presence or absence of ruxolitinib (Rux) were washed with fresh media. (B) Media supplemented with lipopolysaccharide (100 ng/mL) were added, and cell-free supernatants were collected 24 hours later for determination of IL-10 production by cytometric bead array assay. The mean (±standard error) from 6 individual normal donors is shown. (C) In parallel, CFSE-labeled T cells were added in triplicate, and T-cell proliferation was determined by CFSE dilution. *P < .05 and **P < .01 in unpaired 2-sided Student t test.
GATA-3 expression in TCLs
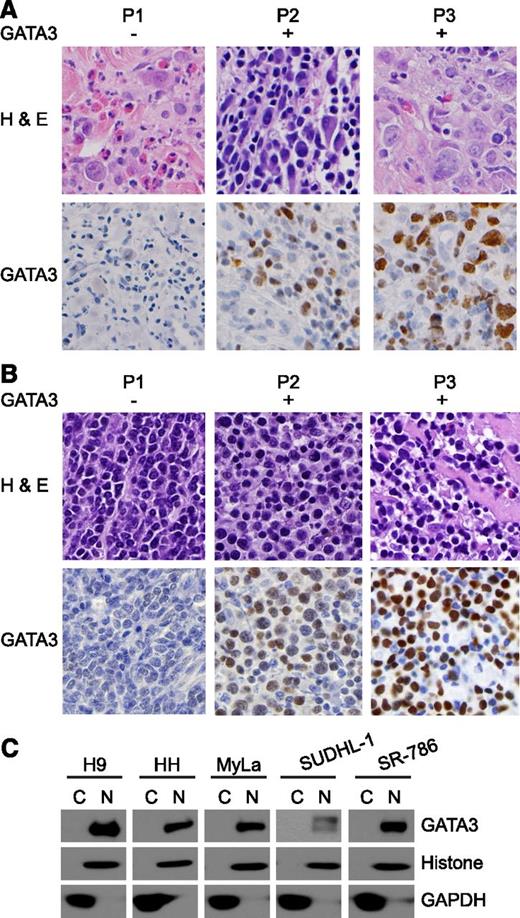
Both IL-10 and IL-4/IL-13 play a preeminent role in alternative macrophage polarization and are epigenetically or transcriptionally regulated by the transcription factor GATA-3.15,16 Therefore, we examined GATA-3 expression by immunohistochemistry in TCL specimens (Figure 3A-B). GATA-3 expression was observed in 67% of cutaneous TCLs (n = 43) and in 45% of the most common subtype of peripheral TCL, PTCL, NOS (n = 128), but was less prevalent in the other TCL subtypes we examined (supplemental Table 5). Among the patient-derived TCL lines examined, GATA-3 was widely expressed, with only a single exception (ie, Karpas 299; supplemental Figures 4 and 6). On closer inspection, GATA-3 expression was confined to the nuclei (Figure 3A-C), suggesting that it may be an important epigenetic and transcriptional regulator in T-cell lymphoproliferative disorders.
GATA-3 expression in T-cell lymphoproliferative disorders. GATA-3 expression was determined by immunohistochemistry in (A) CTCL and (B) PTCL, NOS clinical specimens. Three representative cases (P1-P3) are shown. (C) Cytoplasmic (C) and nuclear (N) fractions were obtained from the patient-derived TCL lines shown, and GATA-3 expression was determined by western blot.
GATA-3 expression in T-cell lymphoproliferative disorders. GATA-3 expression was determined by immunohistochemistry in (A) CTCL and (B) PTCL, NOS clinical specimens. Three representative cases (P1-P3) are shown. (C) Cytoplasmic (C) and nuclear (N) fractions were obtained from the patient-derived TCL lines shown, and GATA-3 expression was determined by western blot.
GATA-3 expression is associated with IL-10 and Th2-associated cytokine production
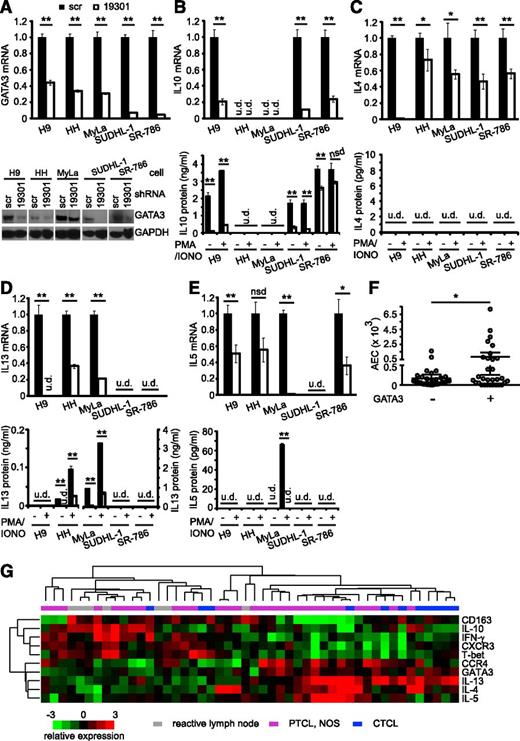
To address the possibility that GATA-3 regulates IL-10 and Th2-associated cytokines, GATA-3 was knocked down in the GATA-3+ TCL cells following lentiviral transduction with a GATA-3 targeting or nonspecific (scramble) shRNA. We screened multiple GATA-3 targeting shRNA for the ability to knock down GATA-3 expression in H9 cells (as these cells highly express GATA-3), and observed modest GATA-3 knockdown with 2 of the shRNAs screened and substantial knockdown with shRNA 19301 (supplemental Figure 5). Therefore, shRNA 19301 was carried forward for further analysis in our panel of GATA-3+ TCL cell lines, and varying degrees of GATA-3 knockdown were observed in the cell lines examined (Figure 4A; supplemental Figure 6). To determine the extent to which GATA-3 promotes IL-10 production in these cells, we quantified IL-10 transcripts and protein (Figure 4B) following GATA-3 knockdown. A significant reduction in IL-10 production was observed in cells transduced with a GATA-3 targeting shRNA. Of note, IL-10 production was similarly reduced in H9 cells transduced with additional GATA-3 shRNA (supplemental Figure 5). As potential differences in cell proliferation during the in vitro culture could confound this analysis (although such a difference was not observed), IL-10 production was also examined by intracellular cytokine staining, whereby GATA-3 knockdown was similarly associated with diminished IL-10 production (supplemental Figure 7). However, an association between GATA-3 expression and the prevalence or density of lymphoma-associated macrophages, including those that were pSTAT3+/CD163+, was not observed in the PTCL, NOS cases examined (data not shown).
GATA-3–dependent cytokine production in TCLs. (A) GATA-3 expression was determined by (upper) qRT-PCR and (lower) western blot in TCL lines lentivirally transduced with a nonspecific (“scramble”) or GATA-3–specific (“19301”) shRNA. (B) IL-10, (C) IL-4, (D) IL-13, and (E) IL-5 production following GATA-3 knockdown was examined by (upper) qRT-PCR or (lower) enzyme-linked immunosorbent assay. For determination of cytokine production, cells were cultured at the same density in triplicate in 96-well plates in media alone or stimulated with phorbol 12-myristate 13-acetate (50 ng/mL) and ionomycin (iono, 500 ng/mL), and cell free supernatants were collected after a 12- to 24-hour incubation. (F) The absolute eosinophil count at diagnosis is shown in PTCL, NOS patients stratified by GATA-3 expression. (G) Unsupervised clustering of PTCL, NOS (n = 31), CTCL (n = 11), and reactive lymph nodes (RLNs; n = 7) on a panel of Th1-associated (T-bet, IFN-γ, CXCR3) and Th2-associated (GATA-3, IL-4, IL-5, IL-13) transcripts. *P < .05 and P < .01 in unpaired 2-sided Student t test.
GATA-3–dependent cytokine production in TCLs. (A) GATA-3 expression was determined by (upper) qRT-PCR and (lower) western blot in TCL lines lentivirally transduced with a nonspecific (“scramble”) or GATA-3–specific (“19301”) shRNA. (B) IL-10, (C) IL-4, (D) IL-13, and (E) IL-5 production following GATA-3 knockdown was examined by (upper) qRT-PCR or (lower) enzyme-linked immunosorbent assay. For determination of cytokine production, cells were cultured at the same density in triplicate in 96-well plates in media alone or stimulated with phorbol 12-myristate 13-acetate (50 ng/mL) and ionomycin (iono, 500 ng/mL), and cell free supernatants were collected after a 12- to 24-hour incubation. (F) The absolute eosinophil count at diagnosis is shown in PTCL, NOS patients stratified by GATA-3 expression. (G) Unsupervised clustering of PTCL, NOS (n = 31), CTCL (n = 11), and reactive lymph nodes (RLNs; n = 7) on a panel of Th1-associated (T-bet, IFN-γ, CXCR3) and Th2-associated (GATA-3, IL-4, IL-5, IL-13) transcripts. *P < .05 and P < .01 in unpaired 2-sided Student t test.
In addition to its role in epigenetically regulating the IL-10 locus, GATA-3 is also the master transcriptional regulator of Th2 differentiation. Multiple lines of evidence suggest that a subset of TCLs may represent the clonal expansion of Th2 cells. First, patients with both cutaneous and peripheral TCLs may present with a concurrent peripheral blood hypereosinophilia. As Th2-associated cytokines (eg, IL-5) are required for eosinophil expansion, hypereosinophilia is a harbinger of Th2-biased immunity. Conversely, lymphocytic variant hypereosinophilic syndrome is associated with the clonal expansion of Th2 cells that is not clinically apparent initially but may eventually evolve into a TCL.22,23 Finally, the association of GATA-3 transcripts and GATA-3–dependent cytokines in CTCL has led to the long-held belief that a subset of cutaneous TCLs is Th2-derived.24 Therefore, we next examined the relationship between GATA-3 expression and the Th2-associated cytokines IL-4, IL-13, and IL-5. In cells that were rendered GATA-3 deficient by shRNA-mediated knockdown, the transcription and protein production (Figure 4C-E) of these Th2-associated cytokines was significantly reduced. As IL-5 is required for eosinophil expansion, we examined the relationship between GATA-3 expression in a cohort of PTCL, NOS patients and the absolute eosinophil count at diagnosis. Although most GATA-3+ PTCL, NOS were associated with a normal eosinophil count, a direct correlation between GATA-3 expression and the development of hypereosinophilia was observed (Figure 4F). Collectively, these data demonstrate that GATA-3 regulates both IL-10 and Th2-associated cytokine production by malignant T cells and may suggest that its expression identifies a distinct subset of T-cell lymphoproliferative disorders. Therefore, we analyzed the expression of IL-10 and both Th1 (IFN-γ, T-bet, CXCR3)- and Th2 (IL-4, IL-5, IL-13, CCR4, GATA-3)-associated transcripts by unsupervised hierarchical clustering in CTCL (n = 11) and PTCL, NOS specimens (n = 31) (Figure 4G). Among PTCL, NOS cases, 2 dominant subsets were identified. GATA-3– and Th2-associated transcripts were abundant in a subset that more closely resembled the CTCL cases we examined and could be distinguished from a subset that was enriched for T-bet, Th1-associated transcripts, and IL-10. To determine whether GATA-3 expression is associated with any distinguishing clinical characteristics, we analyzed GATA-3 expression by immunohistochemistry in a cohort of PTCL, NOS patients.
GATA-3 expression identifies a high-risk subset of PTCL, NOS
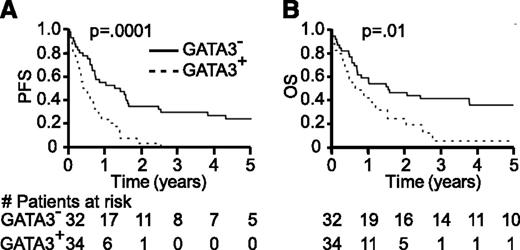
GATA-3 expression was dichotomized in a cohort of 66 PTCL, NOS patients (Table 1; supplemental Table 6). In this cohort, GATA-3 expression was associated with significantly inferior progression-free and overall survival (Figure 5). The median progression-free and overall survival observed in GATA-3+ PTCL, NOS was 5 (95% confidence interval [CI], 4-8 months) and 8 months (95% CI, 5-14 months), respectively. In contrast, the median progression-free and overall survival in GATA-3− PTCL, NOS was 1.3 (95% CI, 0.6-1.6) and 1.6 years (95% CI, 0.7-6.9), respectively. GATA-3 expression was associated with both advanced age and Ann Arbor stage III/IV disease (supplemental Table 6). Therefore, we analyzed GATA-3 expression as a prognostic factor for survival on both univariate and multivariate analyses, adjusting for pertinent risk factors, including patient age and tumor stage, included in the PIT.18 On multivariate analysis (Table 2), GATA-3 expression was associated with inferior progression-free and overall survival when adjusting for high-risk features. To determine whether GATA-3 expression identifies a group of high-risk patients among those who would otherwise be risk-stratified as low-intermediate risk by the PIT, high-risk PIT patients were excluded from analysis. In the subset of patients classified as low-intermediate risk by the PIT (n = 48), GATA-3 expression was associated with significantly inferior median progression-free (8 vs 19 months, P = .0006) and overall survival (14 months vs 3.8 years, P = .03). Therefore, GATA-3 expression is able to identify high-risk patients who would otherwise be classified as low-intermediate risk by the PIT.
Patient characteristics (n = 66)
| Characteristics . | No. of patients . | Percentage . |
|---|---|---|
| Age, years | ||
| Median (range) | 64 (28-90) | |
| Gender, male | 34 | 52 |
| Ann Arbor stage | ||
| I | 9 | 14 |
| II | 2 | 3 |
| III | 9 | 14 |
| IV | 46 | 70 |
| LDH > normal | 39 | 59 |
| >1 extranodal site | 25 | 38 |
| Bone marrow involvement | 26 | 39 |
| ECOG PS > 1 | 28 | 42 |
| Treatment with CHOP | 37 | 64 |
| Characteristics . | No. of patients . | Percentage . |
|---|---|---|
| Age, years | ||
| Median (range) | 64 (28-90) | |
| Gender, male | 34 | 52 |
| Ann Arbor stage | ||
| I | 9 | 14 |
| II | 2 | 3 |
| III | 9 | 14 |
| IV | 46 | 70 |
| LDH > normal | 39 | 59 |
| >1 extranodal site | 25 | 38 |
| Bone marrow involvement | 26 | 39 |
| ECOG PS > 1 | 28 | 42 |
| Treatment with CHOP | 37 | 64 |
CHOP, cyclophosphamide, vincristine, adriamycin, prednisone; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PS, performance status.
GATA-3 expression identifies a subset of PTCL, NOS with inferior survival. Kaplan-Meier estimates of progression-free and overall survival are shown for PTCL, NOS patients stratified by GATA-3 expression (dashed line, GATA-3 positive; solid line, GATA-3 negative).
GATA-3 expression identifies a subset of PTCL, NOS with inferior survival. Kaplan-Meier estimates of progression-free and overall survival are shown for PTCL, NOS patients stratified by GATA-3 expression (dashed line, GATA-3 positive; solid line, GATA-3 negative).
Univariate and multivariate analyses for progression-free and overall survival
| Prognostic factors . | Progression-free survival . | Overall survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| GATA-3 positive: | 2.7 (1.6-4.9) | .0004 | 2.8 (1.6-5.0) | .0005 | 2.2 (1.2-4.0) | .01 | 2.0 (1.1-3.7) | .02 |
| PIT high risk (≥3 poor risk features) | 2.6 (1.5-4.5) | .0007 | 2.6 (1.5-4.5) | .0008 | 3.5 (1.9-6.3) | <.0001 | 3.3 (1.8-6.0) | .0001 |
| Prognostic factors . | Progression-free survival . | Overall survival . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| GATA-3 positive: | 2.7 (1.6-4.9) | .0004 | 2.8 (1.6-5.0) | .0005 | 2.2 (1.2-4.0) | .01 | 2.0 (1.1-3.7) | .02 |
| PIT high risk (≥3 poor risk features) | 2.6 (1.5-4.5) | .0007 | 2.6 (1.5-4.5) | .0008 | 3.5 (1.9-6.3) | <.0001 | 3.3 (1.8-6.0) | .0001 |
The PIT is comprised of 4 adverse prognostic factors: age >60 years, elevated LDH, bone marrow involvement, and stage III/IV disease. PIT, prognostic index in PTCL-U.
Taken together, these data demonstrate that GATA-3–dependent cytokines are exploited to regulate the tumor microenvironment in a subset of high-risk TCLs with distinct clinicopathological features.
Discussion
On antigenic stimulation, naïve CD4+ T cells orchestrate both cellular and humoral immunity by differentiating into Th subsets that are characterized by the production of distinct, and frequently subset-specific, cytokines. Studies performed in murine models using knockout and transgenic technology demonstrate that each Th subset expresses a lineage-specific transcription factor that serves as a master regulator of Th-cell differentiation.5 Unfortunately, the cell of origin has remained obscure for most subtypes of PTCL, with the notable exception of AITL9 and an overlapping follicular variant of PTCL-NOS.25 However, the results of the present study suggest that analysis of Th-subset specific transcription factors, like GATA-3, may have merit and unveil previously unrecognized subtypes of TCLs with similar cytokine- and gene-expression profiles and a common cell of origin. Importantly, the finding that GATA-3 expression is associated with distinct clinical and molecular features in a subset of PTCL, NOS has been independently confirmed in a larger cohort of patients included in the International T-cell Lymphoma Project.26
The epigenetic and transcriptional regulation of most cytokines is complex and incompletely understood. Therefore, it seems likely that GATA-3 expression, while necessary, may not be sufficient for Th2-associated cytokine production by malignant T cells. Furthermore, stromal elements, including lymphoma-associated macrophages, also shape the cytokine milieu within the tumor microenvironment. Interestingly, IL-10 production was at least partially GATA-3 dependent in vitro, but unsupervised hierarchical clustering of PTCL, NOS cases demonstrated that IL-10 transcripts were enriched in a distinct subset of cases associated with T-bet and IFN-γ expression. Furthermore, the abundance of IL-10 transcripts was directly associated with the presence of pSTAT3+/CD163+ macrophages (127.8 [95% CI, 7.34-248.2] vs 27.2 [95% CI, −71.2 to 125.5], mean normalized IL-10 count/300 ng total RNA) in the cases examined, but did not reach statistical significance (P = .19). Therefore, IL-10 expression in these cases may attenuate the influence of IFN-γ within the tumor microenvironment and may explain why an association between lymphoma-associated macrophage density, including those that are pSTAT3+/CD163+ and possibly IL-10 polarized, and GATA-3 expression was not observed (data not shown). The association observed between GATA-3 expression, Th2-associated cytokine production, and hypereosinophilia supports the conclusion that GATA-3 identifies a subset of Th2-derived (or Th2-like) TCLs. This association has been previously reported in the lymphocytic variant of the hypereosinophilic syndrome, which frequently harbor a clonal population of Th cells that secrete Th2-associated cytokines, including IL-4 and IL-5.22,27,28 Gene expression profiling analysis demonstrates that clonal T cells obtained from these patients highly express GATA-3 and other Th2-associated transcripts and are Th2-derived.23 Although GATA-3 may be considered the sine qua non of Th2 differentiation, its mere expression may not be specific for Th2-derived lymphomas or signify a specific cell of origin. For example, multiple signaling pathways and transcription factors, including Notch, STAT6, nuclear factor-κB, and mTOR, transcriptionally or post-transcriptionally regulate GATA-3 and have been implicated in T-cell lymphomagenesis.29-32 The aberrant activation of these pathways may culminate in GATA-3 expression. Alternatively, genetic/epigenetic alterations, including gains involving chromosome 10p, as has been observed in a subset of CTCL patients,24 may result in GATA-3 expression. Furthermore, consideration of a single transcription factor alone may be insufficient to implicate a particular cell of origin, as T-cell differentiation is likely associated with considerably more plasticity than previously appreciated. For example, Hegazy et al recently demonstrated that the adoptive transfer of Th2 cells into virally infected mice led to the generation of GATA-3+T-bet+ “Th2+1” cells.33 Similarly, GATA-3 is required for the development of fully functional FoxP3+ regulatory T cells.34 Therefore, GATA-3 may be coexpressed with additional transcription factors, like T-bet or FoxP3, that were once thought to be mutually exclusive. In a preliminary effort to address these possibilities, we examined T-bet and FoxP3 expression in a subset of PTCL, NOS cases and rarely observed GATA-3, T-bet or FoxP3 coexpression (data not shown).35 Conversely, GATA-3 and Bcl-6 expression may be mutually exclusive, as Bcl-6, a transcriptional repressor expressed by clonally expanded Tfh cells in AITL, represses GATA-3 expression.36 Therefore, future studies will be needed to define the prevalence and implications of GATA-3 expression in a larger cohort of specific PTCL subtypes. Detection of GATA-3 expression by immunohistochemistry may be insufficiently sensitive or specific to identify T-cell lymphoproliferative disorders with a shared cell of origin or molecular signature, and the optimal cutoff defining positivity will require further study and independent validation. Interrogation of additional GATA-3–dependent gene products (including Th2-associated markers) in future studies may aid in the development of algorithms (akin to those used in diffuse large B-cell lymphoma) suitable for the identification of these lymphomas.
Disease progression within 1 year of diagnosis was observed in the vast majority of patients with GATA-3+ PTCL, NOS, most of whom ultimately succumbed to disease, despite therapy with an anthracycline-based regimen (usually cyclophosphamide, vincristine, adriamycin, prednisone). The rapid rate of disease progression observed suggests that GATA-3 expression may represent a predictive biomarker for primary refractory disease. This could be explained by GATA-3–dependent cell autonomous effects. For example, GATA-3 promotes the growth/survival of Th2 cells37 and more recently has been implicated in the survival of CD8+ T cells.38,39 However, this may be cell context dependent, as GATA-3, despite its role in the development of T-cell progenitors, is not required for their survival.40 In addition, GATA-3–positive lymphomas may be associated with a unique genetic landscape, as recurrent mutations or chromosomal rearrangements may be restricted to specific TCL subtypes with a shared cell of origin.41-45 An alternative explanation (and the 2 are not mutually exclusive) may attribute the aggressive biology of these lymphomas to non–cell autonomous effects via the production of GATA-3–dependent cytokines, like IL-10 and IL-4/IL-13, that foster the development of a microenvironment that is conducive to lymphoma growth/survival. Alternatively polarized macrophages, for example, regulate tumor cell senescence, extracellular matrix remodeling and tumor cell invasion/metastasis, and angiogenesis/lymphangiogenesis, all of which promote tumor growth.14 This view may be supported by the observation that IL-10 overproduction,46,47 and an abundance of lymphoma-associated macrophages, including markers associated with alternative polarization (eg, CD163),48 are adverse prognostic factors in many lymphomas, including those that are T cell–derived.49 Furthermore, we have previously shown that lymphoma-associated macrophages promote the growth and engraftment of malignant T cells in a xenograft model.11 The observation that ruxolitinib was able to prevent alternative polarization of lymphoma-associated macrophages is significant, as several JAK2 inhibitors are in various stages of clinical development and appear promising in many lymphoproliferative disorders in preclinical studies. Both IL-10 and the Th2-associated cytokines examined here are likely important in shaping the tumor microenvironment. However, it would be naïve to think that their influence is limited to lymphoma-associated macrophages. As STAT3 is critically important in the development of pro-tumoral myeloid-derived cells within the tumor microenvironment, the data we present suggest that the JAK2 inhibitors warrant closer scrutiny as immunomodulatory agents in PTCL.
In conclusion, GATA-3 expression is able to identify a subset of T-cell lymphoproliferative disorders with distinct clinicopathological features, including aggressive disease biology and a dismal prognosis. These findings provide a strong rationale for future and confirmatory studies investigating the role of relevant transcriptional regulators with a well-defined role in T-cell ontogeny and suggest that a key to understanding the tumor microenvironment in the T-cell lymphoproliferative disorders may be found in their cell of origin.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Doug Engel for helpful discussions and thoughtful review of this manuscript.
This work was supported in part by grants from the Leukemia and Lymphoma Society, Leukemia Research Foundation, and National Cancer Institute (K08CA172215) (R.A.W.).
Authorship
Contribution: R.A.W. conceived the study, designed and performed research, analyzed data, and wrote the manuscript; T.W. assisted with study design, performed research, analyzed data, and assisted with manuscript preparation; A.L.F., D.A.W., Y.L., A.P., D.T., S.C.Z., L.E.W., T.M.L., N.G.B., A.C.H., and M.S.L. performed research and assisted with data analysis; R.B. and K.R. abstracted clinical data, assisted with database management, and assisted with the interpretation of clinical data; T.M.H., M.R.P., and T.E.W. provided patients and assisted with data analysis; S.M.A. provided patients, assisted with study design, and data analysis; and all study authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ryan A. Wilcox, University of Michigan Comprehensive Cancer Center, 4310 Cancer Center, 1500 East Medical Center Dr, Ann Arbor, MI 48109-5936; e-mail: rywilcox@med.umich.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal