Abstract
Multicentric Castleman's disease (MCD) describes a heterogeneous group of disorders involving proliferation of morphologically benign lymphocytes due to excessive proinflammatory hypercytokinemia, most notably of interleukin-6. Patients demonstrate intense episodes of systemic inflammatory symptoms, polyclonal lymphocyte and plasma cell proliferation, autoimmune manifestations, and organ system impairment. Human herpes virus-8 (HHV-8) drives the hypercytokinemia in all HIV-positive patients and some HIV-negative patients. There is also a group of HIV-negative and HHV-8-negative patients with unknown etiology and pathophysiology, which we propose referring to as idiopathic MCD (iMCD). Here, we synthesize what is known about iMCD pathogenesis, present a new subclassification system, and propose a model of iMCD pathogenesis. MCD should be subdivided into HHV-8-associated MCD and HHV-8-negative MCD or iMCD. The lymphocyte proliferation, histopathology, and systemic features in iMCD are secondary to hypercytokinemia, which can occur with several other diseases. We propose that 1 or more of the following 3 candidate processes may drive iMCD hypercytokinemia: systemic inflammatory disease mechanisms via autoantibodies or inflammatory gene mutations, paraneoplastic syndrome mechanisms via ectopic cytokine secretion, and/or a non-HHV-8 virus. Urgent priorities include elucidating the process driving iMCD hypercytokinemia, identifying the hypercytokine-secreting cell, developing consensus criteria for diagnosis, and building a patient registry to track cases.
Introduction
Multicentric Castleman's disease (MCD) is a rare and poorly understood disorder that straddles the intersections of hematology, oncology, rheumatology, and virology and can be fatal if improperly treated. MCD describes a heterogeneous group of disorders with various etiologies that demonstrate episodic systemic inflammatory symptoms, reactive proliferation of morphologically benign lymphocytes, and multiple organ system impairment as a result of excessive interleukin-6 (IL-6) and other proinflammatory cytokines.1-3 The population incidence has not been established; a recent study estimates 4353 new cases of “Castleman disease” per year in the United States, which we approximate to include 1000 cases of MCD.4
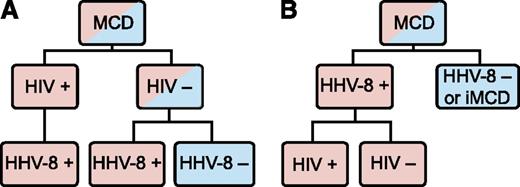
Human herpes virus-8 (HHV-8) is the well-established cause of the hypercytokinemia (highly elevated levels of cytokines) in all HIV-positive MCD patients and in some HIV-negative patients.5 Among these HHV-8-associated MCD cases, HIV infection or another cause of immunodeficiency enables HHV-8 to escape from host immune control, lytically replicate in lymph node plasmablasts, and signal the release of viral IL-6, human IL-6, and several other proinflammatory proteins.6,7 These proinflammatory proteins induce B-cell and plasma cell proliferation, vascular endothelial growth factor (VEGF) secretion and angiogenesis, and an acute phase reaction.8 There is also a group of HIV-negative and HHV-8-negative MCD patients whose disease we propose referring to as idiopathic MCD (iMCD). The etiology of iMCD proinflammatory hypercytokinemia is not known and may be viral, inflammatory, or neoplastic.5,6 MCD has been historically classified based on HIV status, but this has caused confusion because HHV-8 drives all HIV-positive MCD cases and some HIV-negative MCD cases. HIV infection merely results in immunodeficiency, which can occur through multiple other etiologies, making the patient more vulnerable to HHV-8 replication and concomitant viral-dependent hypercytokinemia. HHV-8 was detected in the lymph node or blood in >90 of the 999 HIV-negative MCD cases identified in a recent systematic literature search. HHV-8 was tested for, but not detected, in ∼600 of those MCD cases (D.C.F., C.S.N., and Amy Y. Liu, unpublished data, 2014). Building on a recent paper by Dossier et al indicating that all HHV-8-associated MCD cases may be considered as a single clinicopathological entity regardless of HIV status, we recommend classifying MCD based on HHV-8 status and believe this will decrease heterogeneity in future studies (Figure 1).6
MCD subclassification. (A) The traditional classification of MCD has been distinguished by HIV status. However, HHV-8 is responsible for cases of both HIV-positive and HIV-negative MCD. Therefore, HIV-based classification does not adequately distinguish the driving factor of MCD pathogenesis. (B) The newly proposed classification distinguishes MCD based on HHV-8-status, which is more accurate based on pathogenesis and response to treatments.
MCD subclassification. (A) The traditional classification of MCD has been distinguished by HIV status. However, HHV-8 is responsible for cases of both HIV-positive and HIV-negative MCD. Therefore, HIV-based classification does not adequately distinguish the driving factor of MCD pathogenesis. (B) The newly proposed classification distinguishes MCD based on HHV-8-status, which is more accurate based on pathogenesis and response to treatments.
Although no survival data currently exist for iMCD, a 2011 systematic literature review identified a 45.7% 3-year disease-free survival rate among 84 HIV-negative, HHV-8 unknown MCD case reports, and 45.4% of these patients died of MCD prior to publication of their respective case reports.9 The 10-year overall survival rate was ∼40% in a 2012 series of 60 HHV-8 unknown MCD cases, up from 13% in a 1993 series of 38 patients.10,11 Further progress in long-term outcome is anticipated with the advent of antibodies targeting the IL-6 signaling cascade, such as tocilizumab and siltuximab.12,13 However, these antibodies require long-term administration and are not effective in all patients. We conducted this comprehensive review to facilitate the continued discovery of iMCD pathogenesis and novel therapeutic targets. Herein, we define the entity of iMCD and describe its pathologic and clinical features. Further, we present the state of knowledge regarding the pathogenesis of iMCD and propose several mechanisms that may be responsible for iMCD hypercytokinemia. Finally, we discuss the rationale for current and future therapies for iMCD and briefly outline important next steps for research.
Pathological and clinical features
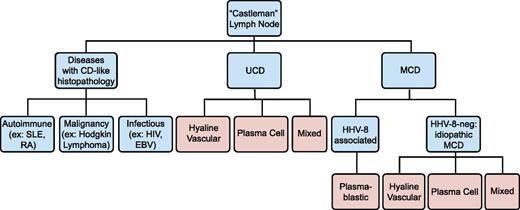
No official criteria currently exist for iMCD diagnosis, and no disease-specific histopathologic features or biomarkers have been identified. Diagnosis is made when Castleman lymph node histopathologic features and clinical correlates are observed and all other infectious, autoimmune, and neoplastic diseases known to demonstrate these features have been systematically excluded (Table 1; Figure 2).14 We propose that iMCD cases should have (1) negative HIV serology and (2) no replicating HHV-8 virus detectable by polymerase chain reaction in the peripheral blood or negative staining for HHV-8 latent nuclear antigen in lymph node tissue. HHV-8 latent nuclear antigen staining and HHV-8 by polymerase chain reaction are positive in nearly 100% of HHV-8-associated cases during acute episodes.15,16
Diseases that can demonstrate iMCD-like histopathology and diseases reported to cooccur with iMCD
| Neoplastic . | Inflammatory . | Infectious/toxin ingestion . |
|---|---|---|
| iMCD-like histopathology | ||
| Non-Hodgkin lymphoma | Systemic lupus erythematous | Epstein-Barr virus |
| Cutaneous lymphoma | Rheumatoid arthritis | HIV |
| Hodgkin lymphoma | Sjogren syndrome | Hydrochoride ingestion |
| Cardiac myxoma | Relapsing polychondritis | |
| Multiple myeloma | Systemic IGG4 plasmacytic syndrome | |
| Clear cell meningioma | Systemic/cutaneous plasmacytosis | |
| Choroid meningioma | ||
| Giant cell carcinoma of lung | ||
| Calcifying fibrous pseudotumor | ||
| Inflammatory myofibroblastic tumor | ||
| Cooccur with iMCD | ||
| POEMS syndrome | Adult onset Still’s disease | Human herpes virus 6 |
| Paraneoplastic pemphigus | Systemic juvenile idiopathic arthritis | Hepatitis b virus |
| Melanoma | Sarcoidosis | Toxoplasma |
| Angioimmunoblastic T-cell lymphoma | Amyloidosis | Mycobacterium tuberculosis |
| Indolent T-lymphoblastic proliferation | Pure red cell aplasia | Cytomegalovirus |
| Inflammatory hepatocellular adenoma | Acquired factor VIII deficiency | Toxoplasma |
| Myasthenia gravis | ||
| Familial mediterranean fever | ||
| Glomerulonephritides |
| Neoplastic . | Inflammatory . | Infectious/toxin ingestion . |
|---|---|---|
| iMCD-like histopathology | ||
| Non-Hodgkin lymphoma | Systemic lupus erythematous | Epstein-Barr virus |
| Cutaneous lymphoma | Rheumatoid arthritis | HIV |
| Hodgkin lymphoma | Sjogren syndrome | Hydrochoride ingestion |
| Cardiac myxoma | Relapsing polychondritis | |
| Multiple myeloma | Systemic IGG4 plasmacytic syndrome | |
| Clear cell meningioma | Systemic/cutaneous plasmacytosis | |
| Choroid meningioma | ||
| Giant cell carcinoma of lung | ||
| Calcifying fibrous pseudotumor | ||
| Inflammatory myofibroblastic tumor | ||
| Cooccur with iMCD | ||
| POEMS syndrome | Adult onset Still’s disease | Human herpes virus 6 |
| Paraneoplastic pemphigus | Systemic juvenile idiopathic arthritis | Hepatitis b virus |
| Melanoma | Sarcoidosis | Toxoplasma |
| Angioimmunoblastic T-cell lymphoma | Amyloidosis | Mycobacterium tuberculosis |
| Indolent T-lymphoblastic proliferation | Pure red cell aplasia | Cytomegalovirus |
| Inflammatory hepatocellular adenoma | Acquired factor VIII deficiency | Toxoplasma |
| Myasthenia gravis | ||
| Familial mediterranean fever | ||
| Glomerulonephritides |
Diseases with Castleman-like lymph node histopathological features. Angiofollicular lymph node hyperplasia, or Castleman-like histopathological features are seen in unicentric and multicentric Castleman disease. These changes are nonspecific and can also be seen with other autoimmune diseases, malignancies, and infections. Disease states are listed in blue, and histopathological findings are listed in pink.
Diseases with Castleman-like lymph node histopathological features. Angiofollicular lymph node hyperplasia, or Castleman-like histopathological features are seen in unicentric and multicentric Castleman disease. These changes are nonspecific and can also be seen with other autoimmune diseases, malignancies, and infections. Disease states are listed in blue, and histopathological findings are listed in pink.
Castleman disease is named for Benjamin Castleman, who first described the characteristic histopathological findings of angiofollicular lymph node hyperplasia in a localized lymph node region in 1954.17 In 1974, Gaba et al described the first cases of this histopathology in multiple lymph nodes.18 Castleman disease has since been separated into unicentric (UCD) and MCD, although some authors only report “Castleman disease,” which should be avoided. Although not the subject of this review, UCD is often asymptomatic and has a high cure rate with surgical excision of the enlarged lymph node.19 MCD histopathological features can be divided into 4 variants: hyaline-vascular (HV), plasma cell (PC), mixed, and plasmablastic. HV is characterized by widened mantle zones composed of concentric rings of small lymphocytes in an “onion skin” pattern around small atrophic germinal centers with penetrating hyalinized vessels and dysplastic follicular dendritic cells (FDCs).1 In PC, the germinal centers are hyperplastic rather than atrophic, the interfollicular region contains sheets of plasma cells and vascular proliferations, the FDC network is normal, and there is preserved lymph node architecture.20 The mixed variant displays features of both HV and PC. The reliability and significance of these variants is unclear, as there are reports of transitions between HV and PC variants on subsequent biopsies, as well as the simultaneous presence of both types at separate sites within the same patient.21 Three recent studies reported 17% to 49% HV, 46% to 77% PC, and 4% to 20% mixed pathology among 198 HIV-negative MCD cases.9,10,12 Of note, these histopathological features are nonspecific, reactive to hypercytokinemia from any source in the body, and can be found in several infectious, rheumatologic, and neoplastic diseases (Table 1).3,22-24 Thus, iMCD lymph nodes should not be considered tumors. The plasmablastic variant is only found in HHV-8-associated MCD and will not be discussed.
Immunoglobulin and T-cell receptor gene studies indicate that the lymphocyte proliferation in iMCD is polyclonal and thus reactive to hypercytokinemia.25 The discovery of monoclonal lymphocytes should raise the possibility of an alternative malignancy.26 Rare cases of cytogenetic aberrations and light chain restriction have been described, including 1 report describing a translocation involving the IL-6 gene.20,27 Chang et al recently identified monoclonality in 19 of 25 HV-UCD cases, 2 of 2 PC-UCD, 3 of 3 HV-iMCD cases, and 1 of 1 PC-iMCD cases in a study of 31 female patients. Immunoglobulin and T-cell receptor genes were in germ-line status in 3 of 4 iMCD cases, indicating that stromal cells must be the monoclonal cells in those cases. The authors concluded that most cases of HV-UCD and HV-MCD are monoclonal proliferations, most likely of stromal actin-positive myoid cells and FDCs.28 Further investigations of stromal cell monoclonality in iMCD are needed.
iMCD can occur at any age, but it typically affects individuals in the fourth and fifth decades of life, and it is more frequent in men than women.10,19 The clinical spectrum ranges from waxing and waning mild lymphadenopathy with B-symptoms to more severe cases involving intense inflammation, hepatosplenomegaly, vascular leak syndrome with anasarca, pleural effusions, and ascites, organ failure, and even death.10,29 Acute episodes can display significant clinical overlap with acute viral illnesses and autoimmune diseases.3 Skin involvement in the form of cherry hemangiomata often occurs.30 Clinical differences can be observed between ethnic groups. Patients of Asian descent often display large violaceous skin lesions and interstitial pneumonitis,13,31 and Polynesian patients demonstrate a mild clinical course despite significant biochemical derangements.32
Laboratory findings commonly include anemia, elevated erythrocyte sedimentation rate, C-reactive protein, IL-6, VEGF, and fibrinogen; positive anti-nuclear antibody, anti-erythrocyte autoantibodies, and anti-platelet antibodies; and proteinuria, hypoalbuminemia, polyclonal marrow plasmacytosis, polyclonal hypergammaglobulinemia, and thrombocytosis or thrombocytopenia.14 Kawabata et al recently described a group of iMCD patients in Japan with thrombocytopenia, ascites, reticulin fibrosis, renal dysfunction, and organomegaly (TAFRO syndrome), which demonstrate milder lymphadenopathy, mixed or HV histopathology, severe thrombocytopenia, normal or mildly elevated levels of IL-6 and VEGF, the presence of autoantibodies, severe anasarca, and normal immunoglobulin levels.33 Polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome, a paraneoplastic syndrome involving VEGF and other cytokine secretion by malignant plasma cells, can demonstrate significant clinical and histopathological overlap with iMCD, such as volume overload, renal disease, and cherry hemangiomata. Thirty-seven percent to 60% of POEMS cases have elements of Castleman disease histopathology, and many patients with CD may also demonstrate POEMS features, including IgA monoclonal gammopathy of undetermined significance.14,34,35 We suspect that the frequent association between POEMS and iMCD may occur, because the malignant, POEMS-driving plasma cells secrete cytokines that also cause iMCD-like reactive lymph node changes, rather than separate diseases cooccurring. There are also a host of other neoplastic, infectious, and autoimmune diseases reported to cooccur with iMCD, for which it is difficult to differentiate or determine a causative relationship from limited case reports (Table 1).3
Pathogenesis
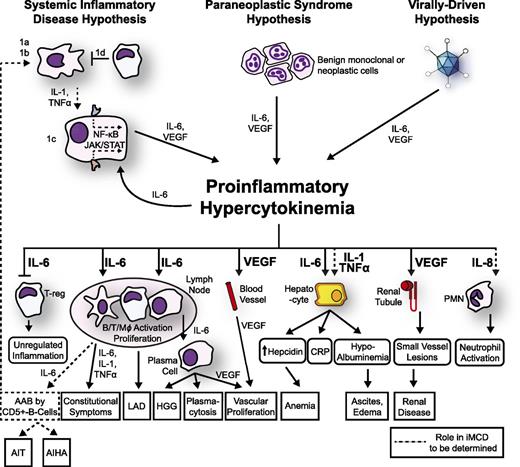
The mechanisms responsible for iMCD pathogenesis are poorly understood and may differ between cohorts of patients. In the following paragraphs, we summarize the most important observations regarding iMCD pathogenesis and present a model (Figure 3).
Proposed model of iMCD pathogenesis. Three hypothesized mechanisms may be responsible for iMCD proinflammatory hypercytokinemia. (1) The systemic inflammatory disease hypothesis involves (1a) autoantibodies triggering proinflammatory cytokine release by antigen-presenting cells that induce the hypercytokine-secreting cell to release IL-6; (1b/c) an error in kinase or inhibitory signaling in an antigen presenting cell or other hypercytokine-secreting cell causing IL-6 secretion, or (1d) a defect in the regulation of activated inflammatory cells. Systemic inflammation is perpetuated by positive feedback of IL-6 and possibly further autoantibody stimulation. (2) The paraneoplastic syndrome hypothesis involves a somatic mutation in benign or malignant cells inside or outside of the lymph node that causes constitutive proinflammatory cytokine release. (3) The virally driven hypothesis involves a non-HHV-8 virus (ex: EBV, HHV-6) signaling proinflammatory cytokines. Regardless of the etiology, the proinflammatory hypercytokinemia is the common pathway that results in the subsequent clinical and histopathological features of iMCD. AAB, autoantibodies; AIHA, autoimmune hemolytic anemia; AIT, autoimmune thrombocytopenia; LAD, lymphadenopathy; PMN, polymorphic neutrophil.
Proposed model of iMCD pathogenesis. Three hypothesized mechanisms may be responsible for iMCD proinflammatory hypercytokinemia. (1) The systemic inflammatory disease hypothesis involves (1a) autoantibodies triggering proinflammatory cytokine release by antigen-presenting cells that induce the hypercytokine-secreting cell to release IL-6; (1b/c) an error in kinase or inhibitory signaling in an antigen presenting cell or other hypercytokine-secreting cell causing IL-6 secretion, or (1d) a defect in the regulation of activated inflammatory cells. Systemic inflammation is perpetuated by positive feedback of IL-6 and possibly further autoantibody stimulation. (2) The paraneoplastic syndrome hypothesis involves a somatic mutation in benign or malignant cells inside or outside of the lymph node that causes constitutive proinflammatory cytokine release. (3) The virally driven hypothesis involves a non-HHV-8 virus (ex: EBV, HHV-6) signaling proinflammatory cytokines. Regardless of the etiology, the proinflammatory hypercytokinemia is the common pathway that results in the subsequent clinical and histopathological features of iMCD. AAB, autoantibodies; AIHA, autoimmune hemolytic anemia; AIT, autoimmune thrombocytopenia; LAD, lymphadenopathy; PMN, polymorphic neutrophil.
Candidate etiological triggers
Candidate etiological triggers from limited numbers of case reports and studies include a somatic chromosomal abnormality in non-neoplastic cells (eg, translocation in the IL-6 gene), germ-line genetic mutation in an inflammatory gene (eg, IL-6 promoter polymorphism), ectopic IL-6 release by malignant tumor cells, autoantibody antigenic stimulation, and infectious agents.3,27,36,37 Epstein-Barr virus (EBV), HHV-6, hepatitis B virus, cytomegalovirus, toxoplasma, and Mycobacterium tuberculosis have each been reported to cooccur in ≥1 case of iMCD, but most iMCD cases do not report an associated infectious agent.38-43 These infections may be pathologic, coincidental, or secondary to iMCD-related immune dysfunction.
Key iMCD cytokines
Human and animal studies have demonstrated IL-6’s role as a common mediator of iMCD symptomatology, histopathology, and pathogenesis. Symptoms typically wax and wane in accordance with serum IL-6 levels, which are often highly elevated in iMCD.14 Interruption of the IL-6 signaling cascade with anti-IL-6 or anti-IL-6 receptor monoclonal antibodies (mAb) often ameliorates iMCD symptoms and can lead to lymph node involution.13,44 Furthermore, human IL-6 transgenic mice and mice infected with an IL-6-expressing recombinant retrovirus develop an iMCD-like syndrome, which also improves with anti-IL-6 receptor mAb administration45-47 (Table 2, murine models of MCD). Patients with IL-6 secreting hematologic and solid malignancies develop iMCD-like histopathology and symptomatology, which resolve with IL-6 blockade or tumor excision.48,49 Finally, the administration of pharmacologic doses of recombinant IL-6 to humans can lead to an iMCD-like syndrome.50 These findings also demonstrate that iMCD histopathology and symptomatology are secondary to hypercytokinemia.50
Murine models of MCD
| Citation . | Murine model . | Key findings . |
|---|---|---|
| Brandt et al 199045 | Congenitally anemic W/Wv mice reconstituted with bone marrow cells were transduced with a retroviral vector that introduced the coding sequences of murine IL-6 into mouse hematopoietic cells. | Mice demonstrated MCD features, including anemia, transient granulocytosis, hypoalbuminemia, plasma cell infiltration, and polyclonal hypergammaglobulinemia, with marked splenomegaly and peripheral lymphadenopathy |
| Screpanti et al 199547 | C/EBPβ knockout mice were generated by gene targeting through homologous recombination at the endogenous C/EBPβ locus. | C/EBPβ−/− mice develop a syndrome similar to mice overexpressing IL-6 and very similar to human MCD, with splenomegaly, lymphadenopathy, and enhanced hematopoiesis. There was also dysfunction of humoral, innate and cellular immunity, including defective activation of splenic macrophages, impaired IL-12 production, increased susceptibility to Candida albicans, and T-helper cell dysfunction. |
| Katsume et al 200246 | Transgenic mice carrying human IL-6 cDNA fused with a murine major histocompatibility class I promoter were administered with an anti-IL-6 receptor antibody. | The untreated transgenic mice developed plasmacytosis, mesangial proliferative glomerulonephritis, leukocytosis, thrombocytosis, anemia, and other laboratory abnormalities. Treatment prevented all symptoms and extended the lifetime of most mice. |
| Citation . | Murine model . | Key findings . |
|---|---|---|
| Brandt et al 199045 | Congenitally anemic W/Wv mice reconstituted with bone marrow cells were transduced with a retroviral vector that introduced the coding sequences of murine IL-6 into mouse hematopoietic cells. | Mice demonstrated MCD features, including anemia, transient granulocytosis, hypoalbuminemia, plasma cell infiltration, and polyclonal hypergammaglobulinemia, with marked splenomegaly and peripheral lymphadenopathy |
| Screpanti et al 199547 | C/EBPβ knockout mice were generated by gene targeting through homologous recombination at the endogenous C/EBPβ locus. | C/EBPβ−/− mice develop a syndrome similar to mice overexpressing IL-6 and very similar to human MCD, with splenomegaly, lymphadenopathy, and enhanced hematopoiesis. There was also dysfunction of humoral, innate and cellular immunity, including defective activation of splenic macrophages, impaired IL-12 production, increased susceptibility to Candida albicans, and T-helper cell dysfunction. |
| Katsume et al 200246 | Transgenic mice carrying human IL-6 cDNA fused with a murine major histocompatibility class I promoter were administered with an anti-IL-6 receptor antibody. | The untreated transgenic mice developed plasmacytosis, mesangial proliferative glomerulonephritis, leukocytosis, thrombocytosis, anemia, and other laboratory abnormalities. Treatment prevented all symptoms and extended the lifetime of most mice. |
IL-6 is a multifunctional cytokine produced by various cells, including mesothelial cells, monocytes, macrophages, lymphocytes, fibroblasts, endothelial cells, mesangial cells, and a variety of tumor cells. IL-6 induces plasmacytosis and hypergammaglobulinemia, VEGF secretion, thrombocytosis, acute inflammatory protein production in the liver, and activation of macrophages and T cells.8 IL-6 may also be responsible for autoimmune phenomena in iMCD, such as hemolytic anemia, by inducing expansion of autoantibody-producing, CD5-positive B lymphocytes.51,52
Several other cytokines may play important roles in iMCD pathogenesis as well. There are iMCD cases with low or moderately elevated systemic levels of IL-6, and others who do not respond adequately to anti-IL-6 therapy, suggesting that other pathways and cytokines may contribute to pathogenesis.53 Considering the redundancy of cytokine roles, it is certainly plausible that the hypersecretion of similar cytokines could result in a related clinical phenotype.
VEGF, IL-1, and tumor necrosis factor α (TNF-α) are also frequently elevated in iMCD.54-56 VEGF, which promotes cell survival, angiogenesis, and vascular permeability, is often elevated in parallel with iMCD symptoms.56 Elevated systemic VEGF levels typically decrease in iMCD cases following anti-IL-6 treatment, suggesting that VEGF release is downstream of IL-6. However, VEGF’s role has not been fully elucidated in iMCD, and it may be upstream of IL-6 in some patients as it is in the related disease POEMS syndrome.57
IL-1, which is upstream of IL-6 in the proinflammatory cascade and leads to IL-6 production through nuclear factor (NF)-κB signaling, was expressed at elevated levels in 5 cases of HHV-8 unknown MCD.54,58,59 Additionally, a patient who did not respond to anti-IL-6 therapy had a complete response to anti-IL-1 therapy in 1 case report.60 IL-1 and IL-6 may both be overexpressed in iMCD because of a common aberrant upstream regulator, such as NF-κB, or up-regulated IL-1, in turn inducing IL-6.61
Other cofactors reportedly elevated in MCD include epidermal growth factor receptor, cyclooxygenase-2, IL-8, IL-5, macrophage colony stimulating factor, soluble IL-2 receptor, B-lymphocyte stimulator, IL-2, and serum soluble Fas ligand.2,55,62-68 Comprehensive cytokine panels and proteomic studies are needed to further elucidate the cytokine network.
Candidate activated pathways
It is unclear which pathways are responsible for iMCD hypercytokinemia. Candidate pathways include transcription factors upstream and downstream of the IL-6 gene, such as NF-κB, CCAAT/enhancer binding protein β (C/EBPβ), and Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3), and aberrantly activated kinases known to cause hypercytokinemia in related diseases. NF-κB is the primary transcription factor for several of the cytokines commonly elevated in iMCD, and it has been proposed as one of the important dysregulated pathways in iMCD pathogenesis.61 Mutations in the gene responsible for turning off NF-κB transcription result in autoinflammatory disorders that demonstrate episodic bouts of intense inflammation.69 Also, knocking out C/EBPβ in mice causes a lymphoproliferative disease nearly identical to iMCD, and symptoms can be reversed with IL-6 blockade.70 The elevated IL-6 secretion in these mice, which was not anticipated, was hypothesized to occur because of unopposed STAT3 signaling or preferential expansion of type 2 helper (TH2) pools.70 A candidate aberrantly activated kinase is the anaplastic lymphoma kinase (ALK) gene, which drives the growth of inflammatory myofibroblastic tumors and causes a Castleman-like syndrome following a translocation event.49 Further studies using gene expression profiling and genome-wide discovery studies using next-generation sequencing are needed to reveal the activated pathways that drive iMCD hypercytokinemia.
Genetic background
Although iMCD does not follow classical Mendelian inheritance patterns and iMCD typically presents at older ages than in monogenic diseases, a few recent studies and observations suggest that the underlying genetic background may contribute to iMCD pathogenesis. We have recently observed 2 familial cases of iMCD and are currently searching for a common genetic aberration (F.v.R., unpublished data, September 13, 2013). Additionally, Stone et al identified an increased frequency of 2 single nucleotide polymorphisms in the IL-6 receptor among 58 iMCD patients compared with 50 controls. They also found higher levels of soluble IL-6 receptor among individuals with these polymorphisms.37 Polymorphisms within cytokine genes can influence cytokine expression and are associated with other inflammatory diseases, such as systemic-onset juvenile chronic arthritis.71 Additionally, a case of Castleman disease was heterozygous for a Mediterranean fever (MEFV) gene mutation, which promotes the acute phase response and the development of severe vasculitis, periodic fevers, and autoimmune disorders.72,73 (Table 3, genetic aberrations reported to be associated with iMCD and iMCD-like diseases). Genome-wide studies and next-generation sequencing are needed to elucidate the existence of a predisposing genetic background in patients with iMCD.
Genetic aberrations reported to be associated with iMCD and iMCD-like diseases
| Citation . | Gene . | Key findings . |
|---|---|---|
| Nakamura et al 199327 | IL-6 (presumed by authors) | Somatic translocation [46,XY,t(7;14)(p22;q22)] at the IL-6 gene locus (7p21-22) in lymph node cells in 1 case report |
| Gleason et al 200849 | ALK | Somatic ALK-mutated tumors secrete cytokines and cause MCD-like features |
| Kone-Paut et al 200973 | MEFV | Heterozygous for a germ-line MEFV gene mutation in one CD case |
| Stone et al 201337 | IL-6 receptor | SNP present in 49% of 58 iMCD cases vs 33% of 50 controls (P = .0175) |
| Citation . | Gene . | Key findings . |
|---|---|---|
| Nakamura et al 199327 | IL-6 (presumed by authors) | Somatic translocation [46,XY,t(7;14)(p22;q22)] at the IL-6 gene locus (7p21-22) in lymph node cells in 1 case report |
| Gleason et al 200849 | ALK | Somatic ALK-mutated tumors secrete cytokines and cause MCD-like features |
| Kone-Paut et al 200973 | MEFV | Heterozygous for a germ-line MEFV gene mutation in one CD case |
| Stone et al 201337 | IL-6 receptor | SNP present in 49% of 58 iMCD cases vs 33% of 50 controls (P = .0175) |
Hypercytokine-secreting cell
The cell type responsible for initiating iMCD hypercytokinemia by secreting IL-6 and other proinflammatory cytokines has not been elucidated and may differ between patients and histological subtypes. These “hypercytokine-secreting cells” could exist anywhere in the body. Candidate cell types include macrophages, FDCs, fibroblasts, B cells, T cells, plasma cells, and cytokine-producing tumor cells. The frequent classification of iMCD as a lymphoproliferative disorder has caused confusion within the medical community, because it suggests that there are cytogenetically abnormal lymphocytes, which has not been shown and may lead to improper management.
B cells and plasma cells are typically implicated as the pathologic overproducers of IL-6 in the literature.61,74 Yoshizaki et al’s seminal paper is often cited, in which IL-6 expression came from blastoid B cells in the germinal center in 1 UCD lymph node and 1 HIV-negative, HHV-8 unknown MCD lymph node.75 However, subsequent studies have not consistently demonstrated IL-6 expression by B cells and plasma cells. Leger-Ravet et al and Ishiyama et al reported IL-1β and IL-6 expression by monocytes, macrophages, FDCs, lymphocytes, and endothelial cells, but not plasma cells in HIV-negative, HHV-8 unknown MCD lymph nodes.59,64 A recent study of HIV-negative, HHV-8 unknown MCD lymph nodes found IL-6 expression by plasma cells, endothelial cells, macrophages, and FDCs.76 Chang et al’s recent finding of monoclonality in all 4 iMCD cases (3 HV and 1 PC) suggests that stromal cells, such as myoid cells and follicular dendritic cells, may be the pathologic cell in iMCD.28 Activated T cells may play an important role as well, as soluble IL-2 receptor levels are often elevated and cyclosporine A is an effective monotherapy in a small number of cases.2,77 Further studies involving in situ hybridization for IL-6 mRNA and investigations of stromal cell monoclonality in iMCD are needed.
Unifying model of iMCD pathogenesis
Considering the heterogeneity of clinical, histological, and laboratory presentations of iMCD, we propose that iMCD represents a common end point that can be reached through multiple processes, each involving immune dysregulation and a common pathway of elevated proinflammatory cytokine release. We hypothesize that 1 or more of the following 3 candidate processes are responsible for driving iMCD hypercytokinemia: (1) autoimmune/autoinflammatory mechanisms via autoantibody antigenic stimulation or a germ-line genetic aberration in innate immune regulation (systemic inflammatory disease hypothesis), (2) ectopic cytokine secretion by malignant or benign tumor cells within the lymph nodes or extranodal (paraneoplastic syndrome hypothesis), and (3) viral signaling by a non-HHV-8 virus (virally driven hypothesis). We describe these hypothesized mechanisms below and present a model (Figure 3).
Systemic inflammatory disease hypothesis
There are 2 mechanisms that may drive iMCD hypercytokinemia under the systemic inflammatory disease hypothesis. Autoantibodies stimulate antigen-presenting cells (eg, FDCs and macrophages) in the lymph node to release proinflammatory cytokines (eg, IL-6, IL-1, and/or TNF-α). Then, the cytokine signals via a transcription factor in an as-yet-undetermined hypercytokine-secreting cell resulting in proinflammatory hypercytokinemia, downstream iMCD symptoms, and perpetuation of proinflammatory signaling. Alternatively, autoinflammatory mechanisms involving a germ-line aberration in a gene of innate immunity (eg, IL-6 promoter polymorphism, mutation in NF-κB regulatory gene, or inability of natural killer cells or cytotoxic T cells cells to lyse activated inflammatory cells78 ) may drive iMCD hypercytokinemia. The following lines of evidence support the systemic inflammatory disease hypothesis:
Autoimmune diseases can demonstrate histopathology identical to iMCD. Nearly all lymph nodes of rheumatoid arthritis patients and 15% to 30% of systemic lupus erythematous (SLE) lymph nodes display MCD-like histopathology.3,22-24
IMCD involves intense episodes of inflammation that are clinically similar to autoimmune and autoinflammatory diseases, such as SLE, adult-onset Still’s disease, periodic fever syndromes, and hemophagocytic syndromes.3,78
Immunosuppressive therapies and monotherapy with anti-cytokine antibodies can bring about sustained remissions.2,12
Several connective tissue diseases, such as rheumatoid arthritis, Sjogren syndrome, SLE, and myasthenia gravis have been reported to be diagnosed concurrently with iMCD.3 The clinical and laboratory features leading to these diagnoses may have been driven by iMCD.
Autoimmune manifestations and autoantibodies are frequently found in iMCD when no other autoimmune disease is diagnosed.14
Germ-line mutations in 2 important inflammatory genes have been reported in iMCD.37,73
Further investigation is needed to determine if at least a cohort of iMCD cases are driven by systemic inflammatory disease mechanisms. The discovery of autoreactive T cells and pathogenic autoantibodies would support iMCD being an autoimmune disorder. Priorities for investigators include determining whether iMCD autoantibodies trigger the IL-6 hypersecretion or are secondary to iMCD immune dysregulation and also searching for aberrations in cells responsible for regulating inflammation.
Paraneoplastic syndrome hypothesis
The paraneoplastic syndrome hypothesis posits that a somatic mutation causes malignant or benign tumor cells inside or outside of the lymph node to release iMCD cytokines. A small population of monoclonal stromal cells in multiple lymph nodes or in a sentinel node could be the hypercytokine-secreting cells that trigger an enormous reactive response, such as has been observed in Hodgkin lymphoma and recently in 4 cases of iMCD.28 The following 4 lines of evidence support the paraneoplastic syndrome hypothesis:
Monoclonality was observed in most HV-UCD cases and 4 of 4 recently tested iMCD cases (3 HV-iMCD and 1 PC-iMCD). These cases demonstrated polyclonal lymphocytes, so the monoclonal cells were assumed to be stromal.28 Monoclonality is an early step in the neoplastic process, and the subsequent development of stromal tumors have been frequently reported in HV-UCD.1 The somatic mutations responsible for monoclonality could certainly also cause the cytokine secretion that drives iMCD.
POEMS syndrome, a paraneoplastic syndrome that can demonstrate significant clinical and histopathological overlap with iMCD, is driven predominently by VEGF secretion by malignant plasma cells. IMCD pathology and symptoms may be due to cytokine release by as-yet-undetermined monoclonal cells, such as monoclonal plasma cells in POEMS.
Solid malignancies can cause iMCD symptoms and histopathology in neighboring lymph nodes through IL-6 release that resolves with the excision of these tumors.36 These solid tumors may be present, but undetected at the time of diagnosis, in more cases of iMCD.
Hematologic malignancies are diagnosed at an increased frequency within 2 years of iMCD diagnosis.79 These malignant cells may have been present all along and actually secreted the IL-6 that was responsible for the preceding iMCD symptoms and diagnosis. For instance, 1 reported case of iMCD was subsequently found to have Hodgkin lymphoma. Review of the initial histopathology identified Reed-Sternberg cells, which were also found to secrete IL-6. The iMCD-like features disappeared with anti-IL-6 receptor blockade.48
Alternative explanations for the frequent association between iMCD and malignancy include the possibility that iMCD is a prelymphoma that eventually transforms, excessive cytokine release promotes malignant transformation, iMCD treatments cause or increase susceptibility to secondary malignancies, or an unidentified virus may be responsible for both the iMCD and the malignancy.80,81 We suggest that a search for an underlying malignancy be conducted in all newly diagnosed iMCD patients. Additionally, further investigation is needed to search for monoclonal cells in iMCD lymph nodes.
Virally driven hypothesis
The virally driven hypothesis proposes that a virus other than HHV-8 is responsible for driving iMCD in a cohort of patients. The virus may directly signal for viral IL-6 or human IL-6, such as in HHV-8-associated MCD, or it may cause immune dysregulation that results in subsequent disease. The following 4 lines of evidence support the virally driven hypothesis:
The acute onset of intense symptoms that can be seen in a subset of patients is characteristic of a viral process.
The periods of waning disease activity and intermittent flares fits well with a latent viral infection that occasionally switches to lytic cycle.
iMCD demonstrates clinical and histopathological overlap with HHV-8-associated MCD.6 Furthermore, there is redundancy among lymphotrophic gammaherpesviruses regarding host cell and inflammatory proteins.82
EBV and a few other candidate viruses have been reported to occur with iMCD even though systematic viral serologies and sequencing studies have not been performed to date.38-43
Comprehensive viral serologies and deep sequencing studies are needed to identify a known or undiscovered virus that may drive iMCD.
Treatment
Three treatment strategies have been used based on our understanding of iMCD: anti-inflammatory and immunosuppressive therapies, cytotoxic elimination of cells responsible for hypercytokinemia, and blockade of IL-6 signaling with mAbs. Corticosteroids can improve symptoms during acute exacerbations of iMCD, but most patients relapse during steroid tapering.83,84 Immunosuppressive therapies, particularly cyclosporine A, are being used more frequently as some physicians are treating iMCD more like a systemic inflammatory disease.85,86 Rituximab, which is a frequent first- or second-line therapy in iMCD, is only partially effective and typically does not provide long-term disease control.87-89 Cytotoxic lymphoma-based chemotherapies (eg, cyclophosphamide, doxorubicin, vincristine, and prednisone) induce responses in a large portion of the most severely ill iMCD patients by eliminating a large portion of hypercytokine-secreting cells, but relapses are common and side effects are significant.90,91
Over the last decade, treatments directly targeting IL-6 have been used. Tocilizumab, an anti-IL-6 receptor mAb that is approved to treat MCD in Japan, has demonstrated effectiveness at inducing and maintaining remission.13 Siltuximab, an anti-IL-6 mAb that has been submitted for approval to the US Food and Drug Administration and European Medicines Agency, demonstrated durable tumor and symptomatic response at a significantly higher rate compared with placebo in the first randomized phase 2 study in MCD (34% vs 0%; P = .0012).92,93 Both mAbs have shown clinical activity in iMCD and are potential candidates for frontline therapy. However, they require lifelong administration and are not effective in all patients. Anti-IL-6 and anti-CD-20 therapies may not be effective in all cases of iMCD, because hypercytokinemia and B-cell proliferation are 2 common products seen in iMCD but not necessarily the drivers of the underlying pathogenesis. Additional variability may be due to disease heterogeneity, misclassification or misdiagnosis, and timing of therapy.
Recently, therapeutic approaches targeting pathways upstream of IL-6 have been reported in iMCD, and these deserve further exploration, particularly for anti-IL-6 refractory patients. Bortezomib, a selective proteasome inhibitor that preferentially targets plasma cells, has lowered IL-6 levels and induced remission in 4 cases of iMCD.94-96 Bortezomib may work in iMCD via direct inhibition of NF-κB by degrading the IκB kinase.97 Thalidomide, an immunomodulator that inhibits TNF-α, IL-1, IL-6, IL-12, and VEGF and stimulates T cells potentially via cereblon inhibition, has demonstrated effectiveness at inducing remission, decreasing IL-6 levels, and lowering C-reactive protein in iMCD.66,98,99 Anakinra, an IL-1 receptor antagonist, has also been reported to induce remission in a pediatric case of iMCD and in a patient who did not respond to anti-IL-6 therapy.60,100 Further elucidation of iMCD pathogenesis is urgently needed to provide additional candidate targeted therapies. The multiple therapies used, variation in scientific rigor (eg, sample size, reporting bias), and heterogeneity of reported outcomes, as well as length of follow-up, make detailed analyses of therapies in iMCD very complicated. Therefore, a synthesis of treatments used in iMCD with response and survival outcomes will be reported in a separate paper (D.C.F., C.S.N., and Amy Y. Liu, unpublished data, 2014).
Next steps
Much progress has been made in patient outcomes over the last 20 years, but confusion in the literature as outlined in this article has slowed our understanding of iMCD pathogenesis. Here, we synthesize what is known about iMCD pathogenesis, present a new subclassification system, and propose a model of iMCD pathogenesis. Investigators must fill the current gaps in our understanding of iMCD through the following urgent steps: elucidating the mechanisms responsible for the hypercytokinemia in iMCD, searching for monoclonal stromal cells or other pathological hypercytokine-cytokine secreting cell, searching for viral sequences, and identifying disease-specific biomarkers.
The Castleman Disease Collaborative Network (CDCN) was developed in August 2012 by a group of physicians and researchers to accelerate research and facilitate collaboration among the global research community. The CDCN has coordinated the largest-to-date meeting of MCD researchers and physicians, developed a database of >200 treating physicians and researchers worldwide, launched a platform for private communication and online discussion among iMCD physicians and researchers, assembled a 23-member Scientific Advisory Board of experts from around the globe, and developed an initial scientific agenda. More information about the CDCN and a forum for iMCD physicians and researchers to communicate with one another can be accessed at www.castlemannetwork.org.
Important next steps for the field and priorities for the CDCN include developing an international consensus criterion on iMCD diagnosis, creating a patient registry with a virtual tissue repository, offering research funding, collecting patient quality-of-life data, and facilitating greater collaboration with rheumatologists, immunologists, and virologists. With these steps in motion and continued engagement from the global iMCD physician and researcher community, the potential for progress in our understanding and treatment of iMCD is great.
Acknowledgments
The authors thank Drs Arthur Rubenstein, Vera Krymskaya, and Ed Behrens for review and insight. They also wish to thank the members of the CDCN Scientific Advisory Board.
This work was supported in part by funding from the Charles and Clydene Scharlau Chair for Hematological Malignancies Research.
Authorship
Contribution: D.C.F performed the review, analyzed the results of the review, and wrote the manuscript; C.S.N. created the figures and wrote the manuscript; and F.v.R. analyzed the results of the review and wrote the manuscript.
Conflict-of-interest disclosure: D.C.F. is on an advisory board for Janssen Pharmaceuticals. F.v.R. receives research funding from Janssen Pharmaceuticals. The remaining author declares no competing financial interests.
Correspondence: David C. Fajgenbaum, Raymond and Ruth Perelman School of Medicine, University of Pennsylvania, Smilow Center for Translational Research, 3400 Civic Center Blvd, Bldg 421, Room 12-122, Philadelphia, PA 19104-5160; e-mail: david.fajgenbaum.wg15@wharton.upenn.edu.
References
Author notes
D.C.F., F.v.R., and C.S.N. contributed equally to this paper.