Key Points
Amino acid residues comprising B-cell epitopes recognized by neutralizing anti-factor VIII antibodies (inhibitors) have been identified.
Amino acids contributing significant antigen–antibody binding avidity are candidates for mutagenesis in the design of less antigenic proteins.
Abstract
Neutralizing anti-factor VIII (FVIII) antibodies that develop in patients with hemophilia A and in murine hemophilia A models, clinically termed “inhibitors,” bind to several distinct surfaces on the FVIII-C2 domain. To map these epitopes at high resolution, 60 recombinant FVIII-C2 proteins were generated, each having a single surface-exposed residue mutated to alanine or a conservative substitution. The binding kinetics of these muteins to 11 monoclonal, inhibitory anti-FVIII-C2 antibodies were evaluated by surface plasmon resonance and the results compared with those obtained for wild-type FVIII-C2. Clusters of residues with significantly altered binding kinetics identified “functional” B-cell epitopes, defined as those residues contributing appreciable antigen–antibody avidity. These antibodies were previously shown to neutralize FVIII activity by interfering with proteolytic activation of FVIII by thrombin or factor Xa, or with its binding to phospholipid surfaces, von Willebrand factor, or other components of the intrinsic tenase complex. Fine mapping of epitopes by surface plasmon resonance also indicated surfaces through which FVIII interacts with proteins and phospholipids as it participates in coagulation. Mutations that significantly altered the dissociation times/half-lives identified functionally important interactions within antigen–antibody interfaces and suggested specific sequence modifications to generate novel, less antigenic FVIII proteins with possible therapeutic potential for treatment of inhibitor patients.
Introduction
The development of neutralizing anti-factor VIII (FVIII) antibodies is a serious complication that may be encountered when FVIII replacement therapy is administered to patients with hemophilia A (HA). It affects 25% to 30% of the treated HA population, with a peak occurrence after ∼14 FVIII infusions.1-3 Autoimmune responses to FVIII can also occur,4 and although this happens only rarely, the resulting bleeding phenotype can be severe. Inhibitors can be difficult and extremely expensive to manage clinically. Interestingly, porcine FVIII has been used effectively in the clinic as a “bypass” therapy; that is, a therapeutic protein that can evade neutralization by anti-FVIII antibodies in many allo- and autoimmune inhibitor patients.5-7 However, some patients have or could develop antibodies that neutralize porcine FVIII as well,8 because of antigenic cross-reactivity9 or because regions in which the porcine sequence differs from the human FVIII sequence stimulate effector T cells, leading to antibody production. Identification of the binding sites (B-cell epitopes) on FVIII that are recognized by inhibitors would allow rational design of novel therapeutic FVIII proteins that are more similar to human FVIII and, hence, likely to be less immunogenic.
The most common epitopes recognized by hemophilic inhibitors are on the FVIII A2 and C2 domains.10,11 The FVIII C2 domain (FVIII-C2) mediates numerous functions that are essential for the full procoagulant cofactor activity of FVIII, including membrane binding and assembly of the intrinsic tenase complex.12 The goal of the present study is to identify B-cell epitopes on FVIII-C2 that are recognized by neutralizing anti-FVIII antibodies. In an earlier study,13 competition enzyme-linked immunosorbent assay (ELISA) assays were employed to characterize 56 murine monoclonal antibodies (mAbs) that bound to FVIII-C2 and blocked FVIII procoagulant activity. Results of these assays indicated there were 3 distinct epitopes on this domain, types A, B, and C, as well as inhibitory antibodies that bound to partially overlapping epitopes AB and BC. A, B, and AB antibodies, termed “classical” anti-C2 antibodies, inhibit the assembly of the intrinsic tenase complex on negatively charged phospholipid membranes. C and BC antibodies, termed “nonclassical” anti-C2 antibodies, inhibit the proteolytic activation of FVIII to FVIIIa by thrombin and/or by activated factor X (FXa). To identify the specific amino acid residues comprising these 5 types of epitopes, 60 recombinant FVIII-C2 mutant proteins (muteins) plus the wild-type (WT) protein (WT-FVIII-C2) were generated using an Escherichia coli expression system, including 59 with an alanine substitution at a surface-exposed amino acid side chain plus the conservative substitution R2307Q. (The “legacy” numbering for FVIII residues is employed in this study for consistency with the earlier study.13 ) Surface plasmon resonance (SPR) experiments were carried out to measure binding kinetics of WT-FVIII-C2 and FVIII-C2 muteins to 10 representative mAbs from the series, characterized earlier by competition ELISA and functional assays, as well as to the human-derived monoclonal anti-FVIII antibody BO2C11.14
Methods
Antibodies
Ten murine mAbs were selected from 56 mAbs characterized earlier using ELISA assays13 as representative of type A, AB, B, BC, and C inhibitors. Murine anti-FVIII C2 domain mAbs ESH4 and ESH8 were from American Diagnostica, whereas mAbs 3E6 (GMA-8013), I54, I109, 1B5 (GMA-8008), 3D12, 3G6 (GMA-8014), 2-77 (GMA-8006), and 2-117 (GMA-8003) were prepared as described previously13 or were kindly provided by William Church (Green Mountain Antibodies). The human anti-FVIII mAb BO2C11 was kindly provided by Marc Jacquemin (Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium). Goat anti-mouse immunoglobulin G (IgG), Fc-γ (115-005-071) was from Jackson ImmunoResearch.
FVIII-C2 proteins and SPR measurements
FVIII-C2 proteins were expressed in E coli and purified and analyzed by SPR, as described in the supplemental Methods, available at the Blood Web site. Briefly, SPR measurements were carried out on a Biacore T100 instrument (GE Healthcare Life Sciences) under standard conditions (25°C and 1 atm). Goat anti-mouse IgG specifically directed toward the Fc-γ fragment was immobilized covalently on all channels of a CM5 chip by amine derivatization. Murine anti-FVIII mAb stock solutions were injected over the sensor in 3 flow channels, whereas the fourth channel served as a reference. BO2C11-Ag-binding fragment (Fab) was immobilized covalently by amine derivatization. Single-cycle kinetics experiments15 were carried out in which WT or mutant FVIII-C2 proteins were injected serially over the biosensor surfaces at increasing concentrations without regenerating the biosensor surface after each injection, followed by a 30- to 60-minute buffer injection to measure dissociation rates. The association (ka) and dissociation (kd) rate constants for binding of WT-FVIII-C2 were measured during each set of SPR runs, and the resulting kd values were used to compute the kd(mutein)/kd(WT) ratios for that set of muteins. FVIII-C2 muteins with a kd more than 2.0 times the kd for WT-FVIII-C2 were considered candidates for B-cell epitope residues. For each of the mAbs, the rate constants for the binding of WT-FVIII-C2 were determined by averaging the results obtained from at least 3 SPR runs. Dissociation rate constants (kd), rather than affinities, were chosen as the most relevant metric for identifying “functional B-cell epitopes” because the residence time (1/kd for a bimolecular interaction) of an antibody–antigen complex indicates its maximum potential lifetime in the circulation. Analysis of residence times is widely used in lead optimization studies of potential inhibitory drug targets.16-19
Visualization of B-cell epitopes
After the SPR data were collected, the crystal structures of FVIII-C220 and B-domain-deleted FVIII21,22 were visualized using the graphics program PyMOL23 to localize the sites showing altered binding kinetics to the mAbs analyzed herein. The cutoff kd value was chosen empirically to minimize the number of potential epitope residues located distal from the primary clusters of candidate residues for this series of mAbs.
Results
FVIII-C2 proteins
WT-FVIII-C2 and 60 FVIII-C2 muteins were purified to more than 95% homogeneity (supplemental Figure 1). Six additional FVIII-C2 muteins (S2193A, K2227A, V2232A, K2236A, K2279A, and K2281A) were not expressed in a soluble form and were therefore not analyzed. Dynamic light scattering analyses of the purified proteins, carried out for aliquots of each protein preparation both before and after freezing at −80°C, showed a single peak at the expected size for monomeric FVIII-C2 (not shown). Some of the mutant protein preparations showed evidence of higher molecular weight aggregates; these were not analyzed further and were discarded. Multiple aliquots of each well-behaved FVIII-C2 protein preparation were stored frozen to avoid multiple freeze–thaw cycles that could endanger protein structural integrity.
Altered binding kinetics resulting from amino acid substitutions in FVIII-C2
The amino acid substitutions affected kd values (relative to the values for WT-FVIII-C2) more than ka values in almost all cases. Therefore, a cutoff value based on the ratio of measured kd values of the mutant vs WT protein was chosen to indicate whether the WT residues at these positions should be considered as potential contributors to functional B-cell epitopes recognized by the corresponding antibodies. Setting this cutoff value at kd > 2.0× the measured kd for WT-FVIII-C2 resulted in the identification of 5 to 18 residues as candidates for the B-cell epitopes recognized by the 10 murine and 1 human (BO2C11) mAbs.24 Because all of the mAbs were attached on the biosensor surface, the antigen–antibody binding interactions could be modeled as 1:1 interactions (with each Fab region available to bind a single FVIII-C2 protein).
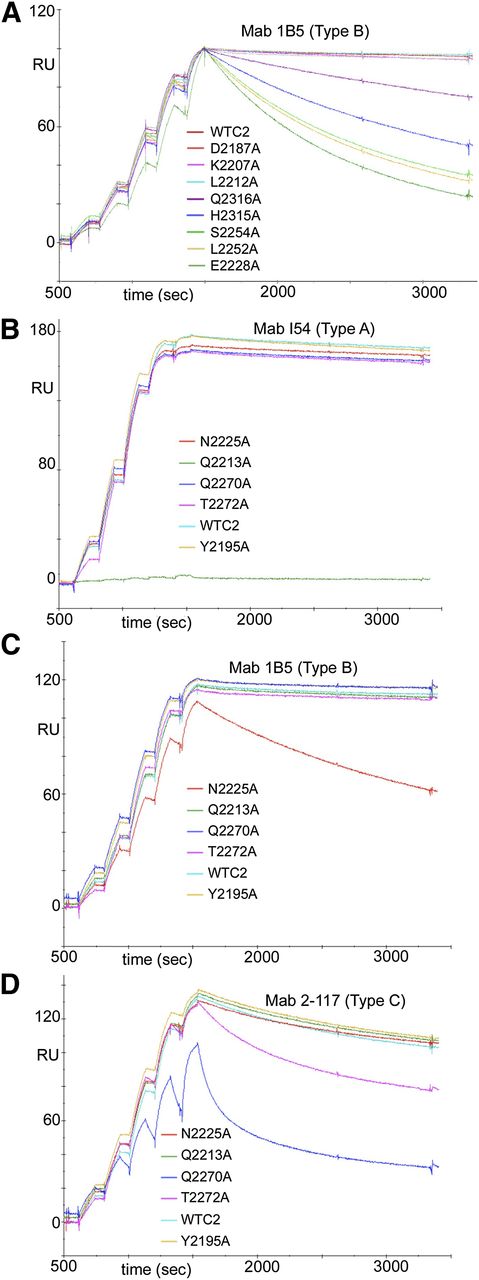
Representative sensorgrams are shown in Figure 1. Almost all of the FVIII-C2 muteins showed binding kinetics to some or all of the mAbs that were highly similar to the binding of WT-FVIII-C2 (see summary of results in Table 1 and kinetic constants in supplemental Table 2), indicating the substitutions did not cause global protein misfolding. Thirty-eight of the 60 muteins tested showed altered binding kinetics restricted to a subset of the mAbs. FVIII-C2-F2200A showed altered kinetics in binding to type AB and type B mAbs (I109, BO2C11, 1B5, and 3D12), with a kd > 2.0× that of WT-FVIII-C2. FVIII-C2-R2220A showed altered binding to all mAbs except type A antibodies 3E6 and I54 and type BC antibody 3G6. The ratio (kd[mutein]/kd[WT]) for binding to the third type A mAb, ESH4, was 2.7, and with a more stringent cutoff value for this ratio, R2220 would not be identified as part of the epitope recognized by this antibody. Removal of the mostly buried R2220 side chain would be expected to destabilize the membrane-binding region of FVIII-C2. Therefore, its effect on binding kinetics to ESH4 was judged to be a conformational effect, and it was not assigned to the epitope for this mAb. The kinetic data from analyzing several preparations of FVIII-C2-F2200A and FVIII-C2-R2220A were more variable and of poorer quality than that of WT-FVIII-C2 and the other muteins, indicating that these substitutions altered the protein stability. Nevertheless, the fact that neighboring residues were also pinpointed as parts of epitopes recognized by type AB and B antibodies supported their assignment to these epitopes. Qualitatively, several other FVIII-C2 muteins showed increased antibody–antigen dissociation rates relative to WT-FVIII-C2, although accurate kinetic constants could not be obtained. These results are indicated in Table 1 as qualitative fast dissociation (QFD).
Representative superimposed sensorgrams showing single-cycle kinetics experiments in which WT-FVIII-C2 and FVIII-C2 muteins were injected at 5 increasing concentrations over biosensor flow channels with captured murine anti-FVIII mAbs, as indicated. Residues were flagged as potential contributors to the epitope if the kd for the FVIII-C2 mutein was >2.0× the kd for the WT protein. (A) Alanine substitutions at residues E2228, L2252, S2254, H2315, and Q2316 met this criterion in this SPR run with mAb 1B5 (which is a type B inhibitor). Separate SPR runs identified residues F2196, T2197, N2198, F2200, T2202, R2220, Q2222, N2225, and K2239 (subsequently identified as an outlier) as also possibly contributing to the epitope recognized by mAb IB5. (B) WT-FVIII-C2 and FVIII-C2 muteins Y2195A, Q2213A, N2225A, Q2270A, and T2272A were injected over the flow channel containing mAb I54 (which is a type A inhibitor). The substitution Q2213A abrogated binding to this mAb, indicating that Q2213 forms part of the epitope recognized by I54. (C) WT-FVIII-C2 and FVIII-C2 muteins Y2195A, Q2213A, N2225A, Q2270A, and T2272A were injected over the flow channel in a second SPR run to analyze interactions of FVIII-C2 muteins with mAb 1B5. Altered binding kinetics indicated that residue N2225 forms part of the epitope recognized by 1B5. (D) WT-FVIII-C2 and FVIII-C2 muteins Y2195A, Q2213A, N2225A, Q2270A, and T2272A were injected over the flow channel containing mAb 2-117 (which is a type C inhibitor). Altered binding kinetics indicated that residues Q2270 and T2272 form part of the epitope recognized by 2-117.
Representative superimposed sensorgrams showing single-cycle kinetics experiments in which WT-FVIII-C2 and FVIII-C2 muteins were injected at 5 increasing concentrations over biosensor flow channels with captured murine anti-FVIII mAbs, as indicated. Residues were flagged as potential contributors to the epitope if the kd for the FVIII-C2 mutein was >2.0× the kd for the WT protein. (A) Alanine substitutions at residues E2228, L2252, S2254, H2315, and Q2316 met this criterion in this SPR run with mAb 1B5 (which is a type B inhibitor). Separate SPR runs identified residues F2196, T2197, N2198, F2200, T2202, R2220, Q2222, N2225, and K2239 (subsequently identified as an outlier) as also possibly contributing to the epitope recognized by mAb IB5. (B) WT-FVIII-C2 and FVIII-C2 muteins Y2195A, Q2213A, N2225A, Q2270A, and T2272A were injected over the flow channel containing mAb I54 (which is a type A inhibitor). The substitution Q2213A abrogated binding to this mAb, indicating that Q2213 forms part of the epitope recognized by I54. (C) WT-FVIII-C2 and FVIII-C2 muteins Y2195A, Q2213A, N2225A, Q2270A, and T2272A were injected over the flow channel in a second SPR run to analyze interactions of FVIII-C2 muteins with mAb 1B5. Altered binding kinetics indicated that residue N2225 forms part of the epitope recognized by 1B5. (D) WT-FVIII-C2 and FVIII-C2 muteins Y2195A, Q2213A, N2225A, Q2270A, and T2272A were injected over the flow channel containing mAb 2-117 (which is a type C inhibitor). Altered binding kinetics indicated that residues Q2270 and T2272 form part of the epitope recognized by 2-117.
Criteria for assigning residues to B-cell epitopes
| No. . | Substitution . | A . | AB . | B . | BC . | C . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESH4 . | 3E6 . | I54 . | I109 . | BO2C11 . | 1B5 . | 3D12 . | 3G6 . | 2-77 . | 2-117 . | ESH8 . | ||
| 1 | E2181A | QFD | FD | FD | ||||||||
| 2 | D2187A | NB | QFD | QFD | ||||||||
| 3 | Y2195A | FD | ||||||||||
| 4 | F2196A | FD | FD | FD | FD | |||||||
| 5 | T2197A | FD | FD | |||||||||
| 6 | N2198A | FD | FD | FD | FD | |||||||
| 7 | M2199A | FD | FD | |||||||||
| 8 | F2200A | QFD | FD | QFD | QFD | |||||||
| 9 | T2202A | FD | FD | FD | FD | |||||||
| 10 | S2206A | FD | FD | |||||||||
| 11 | K2207A | NB | FD | FD | ||||||||
| 12 | H2211A | FD | FD | FD | ||||||||
| 13 | L2212A | QFD | QFD | QFD | ||||||||
| 14 | Q2213A | NB | NB | NB | ||||||||
| 15 | R2215A | FD | ||||||||||
| 16 | R2220A | FD | NB | NB | QFD | NB | QFD | FD | FD | |||
| 17 | Q2222A | NB | ||||||||||
| 18 | N2224A | FD | ||||||||||
| 19 | N2225A | FD | QFD | QFD | QFD | |||||||
| 20 | E2228A | FD | FD | FD | FD | |||||||
| 21 | K2239A | FD | FD | |||||||||
| 22 | K2249A | FD | ||||||||||
| 23 | S2250A | FD | FD | |||||||||
| 24 | L2251A | FD | FD | |||||||||
| 25 | L2252A | FD | FD | FD | ||||||||
| 26 | T2253A | FD | FD | |||||||||
| 27 | S2254A | FD | FD | FD | ||||||||
| 28 | H2269A | FD | FD | |||||||||
| 29 | Q2270A | FD | FD | FD | ||||||||
| 30 | T2272A | FD | FD | |||||||||
| 31 | L2273A | FD | FD | FD | FD | |||||||
| 32 | V2280A | FD | FD | |||||||||
| 33 | V2282A | FD | FD | |||||||||
| 34 | R2307Q | QFD | NB | FD | ||||||||
| 35 | H2309A | QFD | QFD | QFD | QFD | QFD | ||||||
| 36 | Q2311A | FD | ||||||||||
| 37 | H2315A | FD | FD | |||||||||
| 38 | Q2316A | FD | FD | FD | ||||||||
| No. . | Substitution . | A . | AB . | B . | BC . | C . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESH4 . | 3E6 . | I54 . | I109 . | BO2C11 . | 1B5 . | 3D12 . | 3G6 . | 2-77 . | 2-117 . | ESH8 . | ||
| 1 | E2181A | QFD | FD | FD | ||||||||
| 2 | D2187A | NB | QFD | QFD | ||||||||
| 3 | Y2195A | FD | ||||||||||
| 4 | F2196A | FD | FD | FD | FD | |||||||
| 5 | T2197A | FD | FD | |||||||||
| 6 | N2198A | FD | FD | FD | FD | |||||||
| 7 | M2199A | FD | FD | |||||||||
| 8 | F2200A | QFD | FD | QFD | QFD | |||||||
| 9 | T2202A | FD | FD | FD | FD | |||||||
| 10 | S2206A | FD | FD | |||||||||
| 11 | K2207A | NB | FD | FD | ||||||||
| 12 | H2211A | FD | FD | FD | ||||||||
| 13 | L2212A | QFD | QFD | QFD | ||||||||
| 14 | Q2213A | NB | NB | NB | ||||||||
| 15 | R2215A | FD | ||||||||||
| 16 | R2220A | FD | NB | NB | QFD | NB | QFD | FD | FD | |||
| 17 | Q2222A | NB | ||||||||||
| 18 | N2224A | FD | ||||||||||
| 19 | N2225A | FD | QFD | QFD | QFD | |||||||
| 20 | E2228A | FD | FD | FD | FD | |||||||
| 21 | K2239A | FD | FD | |||||||||
| 22 | K2249A | FD | ||||||||||
| 23 | S2250A | FD | FD | |||||||||
| 24 | L2251A | FD | FD | |||||||||
| 25 | L2252A | FD | FD | FD | ||||||||
| 26 | T2253A | FD | FD | |||||||||
| 27 | S2254A | FD | FD | FD | ||||||||
| 28 | H2269A | FD | FD | |||||||||
| 29 | Q2270A | FD | FD | FD | ||||||||
| 30 | T2272A | FD | FD | |||||||||
| 31 | L2273A | FD | FD | FD | FD | |||||||
| 32 | V2280A | FD | FD | |||||||||
| 33 | V2282A | FD | FD | |||||||||
| 34 | R2307Q | QFD | NB | FD | ||||||||
| 35 | H2309A | QFD | QFD | QFD | QFD | QFD | ||||||
| 36 | Q2311A | FD | ||||||||||
| 37 | H2315A | FD | FD | |||||||||
| 38 | Q2316A | FD | FD | FD | ||||||||
FD, fast dissociation.
The kd(mutein) was >2.0× the kd for WT-FVIII-C2 binding to this mAb. In these cases, the kd could be estimated (by visual inspection) as more than 2.0 times the kd for WT-FVIII-C2, but the quality of the sensorgram was insufficient to fit the data to theoretical binding curves, as required to determine accurate kinetic constants for these interactions. NB, nonbinding. These amino acid substitutions completely abrogated binding to the antibody. Boldface indicate alanine substitutions that resulted in kd(mutein) > 2.0× the kd for WT-FVIII-C2 binding to this mAb, but these residues were not conformationally contiguous with the other surface-exposed candidate residues identified using the same kinetic criterion. Therefore, these outlier residues were not assigned to the corresponding epitopes. These substitutions likely had a localized effect on the stability/conformation of the protein, rather than disrupting specific interactions between the native FVIII side chains and the mAb. Twenty-two of the 60 FVIII-C2 muteins tested did not show altered binding kinetics to any of the mAbs (supplemental Table 2), and therefore they were not assigned to any of the functional B-cell epitopes.
Identification of B-cell epitopes
The residues identified by the cutoff criterion of kd(mutein) >2.0× kd(WT), when visualized using PyMOL,23 formed distinct clusters indicating the FVIII-C2 surfaces recognized by each mAb (Figure 2). Once a cluster was localized to a specific surface region, the full set of FVIII-C2 muteins was not analyzed by SPR, as it was considered sufficient to concentrate on residues adjacent to the initial surface thus identified. Substitutions causing altered kinetics at positions that were conformationally noncontiguous with the SPR-identified clusters of potential B-cell epitope residues are noted herein as “outliers.” Specific characteristics of the binding interactions in each antibody–antigen complex are described herein, with the inhibitory mAbs identified as type A, AB, B, BC, or C, according to the criteria of Meeks et al.13
The B-cell epitopes indicated by the SPR experiments are visualized using space-filling depictions of the FVIII-C2 domain crystal structure in standard orientation, with the membrane-interacting loops pointing downward. The FVIII-C2 structure is also shown rotated 180° about the vertical axis for type AB and type B mAbs to visualize both sides of the molecule. The B-cell epitopes identified on the basis of altered binding kinetics are color-coded according to FVIII inhibitor type; that is, A (red/salmon), AB (orange/yellow), B (dark/light green), BC (dark/light blue), and C (dark/light magenta). The darker colors indicate residues for which amino acid substitutions increased the residence time by at least 10 times compared with that for WT-FVIII-C2 binding to this mAb. Substitutions abrogating binding were also colored darker. Substitutions for which accurate kd values could not be obtained were not colored darker because their effects on kinetics may have been in part a result of effects on protein stability. Several outlier residues identified as candidates using the cutoff criterion of kd(mutein) > 2.0 kd(WT) are not shown, as they were eliminated after visualization of the FVIII-C2 crystal structure.
The B-cell epitopes indicated by the SPR experiments are visualized using space-filling depictions of the FVIII-C2 domain crystal structure in standard orientation, with the membrane-interacting loops pointing downward. The FVIII-C2 structure is also shown rotated 180° about the vertical axis for type AB and type B mAbs to visualize both sides of the molecule. The B-cell epitopes identified on the basis of altered binding kinetics are color-coded according to FVIII inhibitor type; that is, A (red/salmon), AB (orange/yellow), B (dark/light green), BC (dark/light blue), and C (dark/light magenta). The darker colors indicate residues for which amino acid substitutions increased the residence time by at least 10 times compared with that for WT-FVIII-C2 binding to this mAb. Substitutions abrogating binding were also colored darker. Substitutions for which accurate kd values could not be obtained were not colored darker because their effects on kinetics may have been in part a result of effects on protein stability. Several outlier residues identified as candidates using the cutoff criterion of kd(mutein) > 2.0 kd(WT) are not shown, as they were eliminated after visualization of the FVIII-C2 crystal structure.
Type A inhibitors
Three type A inhibitors (ESH4, 3E6, and I54) were evaluated herein. SPR assays identified the following residues as possibly interacting with all 3 of these mAbs: D2187, K2207, H2211, L2212, and Q2213. These 3 epitopes identified kinetically were similar but not identical. Experiments with ESH4 also identified residues E2181, T2202, S2206, and R2220 as possibly contributing to this epitope, but R2220 was excluded subsequently as an outlier, as described earlier. Experiments with I54 also identified residues E2181 and S2206. These type A epitopes are immediately adjacent to the β turn at FVIII residues 2198 to 2201, which is 1 of the 2 “greasy feet” hydrophobic regions of FVIII that bind to phospholipid membranes.20,25
Type AB inhibitors
Two type AB inhibitors (I109 and BO2C11) were evaluated. SPR assays identified a cluster of residues as possibly interacting with both of these mAbs: F2196, N2198, F2200, and R2220. I109 also possibly interacted with T2202, S2250, L2251, L2252, T2253, S2254, and H2315, and BO2C11 also possibly interacted with E2181 (outlier), M2199, R2215, Q2270 (outlier), and Q2316. Thus, the type AB inhibitors were seen to bind either or both of these hydrophobic β turns.
Type B inhibitors
Two type B inhibitors (1B5 and 3D12) were evaluated. SPR assays identified the following residues as possibly interacting with both of them: F2196, N2198, F2200, T2202, R2220, N2225, E2228, L2252, S2254, and Q2316. Ala substitutions at residues T2197, Q2222, K2239 (outlier), and H2315 also affected binding to 1B5, whereas Ala substitutions at residues Y2195, M2199, N2224, K2249, S2250, L2251, T2253, and H2309 affected binding to 3D12. The type B epitopes include the 2 hydrophobic β turns as well as migrating further up the “back face” of the molecule to include the loop from N2224-E2228 and H2309. Being of type AB, the epitope of BO2C11 overlaps with the type B inhibitors, 1B5 and 3D12, at one of the hydrophobic β turns.
Type BC inhibitors
Two type BC inhibitors (3G6 and 2-77) were evaluated. SPR identified the following residues as possibly interacting with both of them: N2225, E2228, L2273, R2307, and H2309. Ala substitutions at residues T2197 (outlier) and Q2270 affected binding only to 3G6, whereas Ala substitutions at R2220 (outlier), K2239 (outlier), H2269, and V2280 affected binding only to 2-77.
Type C inhibitors
Two type C inhibitors (2-117 and ESH8) were evaluated. SPR identified the following residues as possibly interacting with both 2-117 and ESH8: R2220 (outlier), T2272, L2273, V2282, and H2309. Ala substitutions at residues H2269 and Q2270 affected binding only to 2-117, whereas Ala substitutions at residues V2280 and Q2311 affected only ESH8 binding. The conservative substitution R2307Q affected binding to mAb 2-117, but not to ESH8.
Modeling of epitopes in the FVIII structure
Figure 3A shows the BC and C epitope residues that comprise nonclassical inhibitor antibodies (eg, antibodies that prevent FVIII activation by thrombin and/or FXa). Figure 3B shows the location of the epitopes recognized by type A, AB, B, BC, and C antibodies.
Visualization of FVIII-C2 epitopes in the B-domain-deleted FVIII crystal structure. With the exception of type A inhibitors, the neutralizing mAbs analyzed here bound to an outside-facing surface of FVIII, where they would not be expected to interfere with the packing or orientation of FVIII domains. (A) The type BC and C epitopes recognized by nonclassical inhibitors are shown as space-filling purple spheres in the FVIII structure.21 The protein is oriented with the membrane-binding residues M2199, F2200, L2251, L2252, K2092, and F2093 pointing down. Also indicated by space-filling spheres are residues known to be at the interface between FVIIIa and activated factor IX in the intrinsic tenase complex: region i (FVIII residues 558-565) is colored light blue; region ii consists of residues near residue 712, which is colored gray; region iii (residues 1811-1818) is colored salmon. Note that the type BC epitope, which corresponds to a docking site for activated thrombin, is on the opposite side of FVIII to the FVIIIa-FIXa interface. (B) All of the residues identified as contributing to each of the 5 types of epitopes are shown as space-filling spheres in the FVIII crystal structure.21 (C) Venn diagram depictions of specific amino acid residues localized to the B-cell epitopes A, AB, B, BC, and C. Amino acid side chains that SPR assays, followed by visual inspection of the FVIII-C2 crystal structure, indicated contribute to functional B-cell epitopes in human FVIII. Amino acid residues in porcine FVIII that differ from the human FVIII sequence at positions corresponding to functional B-cell epitopes identified in this study are indicated in the second Venn diagram.
Visualization of FVIII-C2 epitopes in the B-domain-deleted FVIII crystal structure. With the exception of type A inhibitors, the neutralizing mAbs analyzed here bound to an outside-facing surface of FVIII, where they would not be expected to interfere with the packing or orientation of FVIII domains. (A) The type BC and C epitopes recognized by nonclassical inhibitors are shown as space-filling purple spheres in the FVIII structure.21 The protein is oriented with the membrane-binding residues M2199, F2200, L2251, L2252, K2092, and F2093 pointing down. Also indicated by space-filling spheres are residues known to be at the interface between FVIIIa and activated factor IX in the intrinsic tenase complex: region i (FVIII residues 558-565) is colored light blue; region ii consists of residues near residue 712, which is colored gray; region iii (residues 1811-1818) is colored salmon. Note that the type BC epitope, which corresponds to a docking site for activated thrombin, is on the opposite side of FVIII to the FVIIIa-FIXa interface. (B) All of the residues identified as contributing to each of the 5 types of epitopes are shown as space-filling spheres in the FVIII crystal structure.21 (C) Venn diagram depictions of specific amino acid residues localized to the B-cell epitopes A, AB, B, BC, and C. Amino acid side chains that SPR assays, followed by visual inspection of the FVIII-C2 crystal structure, indicated contribute to functional B-cell epitopes in human FVIII. Amino acid residues in porcine FVIII that differ from the human FVIII sequence at positions corresponding to functional B-cell epitopes identified in this study are indicated in the second Venn diagram.
Discussion
The SPR experiments identified 3 distinct clusters of surface-exposed side chains on FVIII-C2 that contributed significant binding avidity for type A, B, and C FVIII-neutralizing antibodies, plus 2 clusters containing residues that comprised overlap regions for mAb types AB and BC, respectively. SPR experiments were carried out for 3 type A and 2 each of types AB, B, BC, and C mAbs. The resulting assignments of epitope residues were consistent within each mAb type and were also consistent with the competition ELISA experiments13 and with peptide-based epitope mapping of FVIII-C2,26,27 as well as with ELISA assays evaluating binding of the mAbs to the following FVIII muteins: F2196L, K2227E, M2199I/F2200L, V2223A/K2227E, and M2199I/F2200L/L2251V/L2252F.13 A recent analysis of antibodies purified from a patient with an autoimmune response to FVIII (acquired HA) indicated that these included antibodies with epitopes similar to those recognized by ESH4 (type A) and ESH8 (type C).28 The bleeding phenotype of acquired HA is often more severe than that of congenital severe HA, possibly because these antibodies bind to a somewhat different set of immunodominant B-cell epitopes and block FVIII functionality more effectively.
The strategy chosen to identify specific residues as members of a B-cell epitope by SPR was to compare the experimental dissociation rate constant kd for a given FVIII-C2 mutein with the kd for WT-FVIII-C2 dissociating from the same antibody, noting which substitutions increased the kd to more than 2.0× that of WT-FVIII-C2. Once 1 or more muteins with this property were identified, the FVIII-C2 structure was visualized using PyMOL.23 Care was taken to analyze the FVIII-C2 muteins with substitutions in close proximity to the initial cluster of surface-exposed residues identified by their altered kd values, but it was not considered essential to analyze the entire series of muteins for each antibody. The precise mapping of this series of B-cell epitopes, combined with the earlier analysis of the biochemical events (intrinsic tenase assembly, proteolytic activation of FVIII, etc) that were blocked by each type of inhibitor,13 pinpointed specific surfaces on the FVIII protein that interact with its partners in promoting blood coagulation. The lists of residues comprising these epitopes (Table 1) are not comprehensive because not all surface residues were mutated and several substitutions affected protein stability, and so were not analyzed further. However, the coverage of the protein surface was sufficient to definitively identify distinct clusters of residues contributing to specific antigen–antibody binding avidities.
The type A, AB, and B inhibitors interfere with FVIII or FVIIIa binding to phosphatidylserine-containing phospholipid membranes.13 As expected, the epitopes recognized by the AB and B mAbs included the hydrophobic β hairpin turns, as well as residues H2315-Q2316, which are known to participate in membrane binding.25,29 ELISA experiments reported earlier13 showed that the epitope recognized by type AB inhibitor I109 includes residues M2199 and F2200, and the SPR results confirm that this mAb also binds to the second hairpin turn containing L2251 and L2252. Interestingly, the epitopes recognized by the type A inhibitors ESH4, I54, and 3E6 are poised just above these projecting hairpin turns, and their inclusion of charged residues (E2181, D2187, K2206, K2207) indicates that these inhibitors block electrostatic interactions that could otherwise form between the positively charged FVIII side chains and negatively charged membrane surfaces. The kinetics of FVIII neutralization by inhibitory antibodies have long been classified as type 1 or type 2.30,31 Type 1 inhibitors completely block FVIII activity at saturating concentrations, whereas type 2 inhibitors do not completely inhibit clotting, even at saturating levels. Mabs I54 and 3E6 are type 1 inhibitors, whereas ESH4 is a type 2 inhibitor. The 3 type A epitopes identified by SPR are highly similar (Figure 2). The different inhibition kinetics might be a result of binding to FVIII with different affinities/avidities or with a slightly different orientation that does not completely preclude FVIIIa assembly into the intrinsic tenase complex. The patient-derived inhibitory mAb BO2C11 is a type AB antibody that buries a large surface area on the FVIII C2 domain.14,24 A crystal structure of a BO2C11-Fab fragment to FVIII-C2 identified 15 FVIII side chains that contacted the antibody.24 However, SPR-based analysis of BO2C11 binding to a series of FVIII-C2 muteins has shown that fewer than half of these side chains contributed sufficient binding avidity to be considered part of this functional B-cell epitope.32 Similarly, we expect that the B-cell epitope residues identified herein comprise a subset of the actual contact areas between each inhibitory antibody and FVIII. This expectation has been confirmed, in part, by recent crystallographic studies illustrating the epitope recognized by another type BC inhibitor (mAb G99)33,34 and by a nuclear magnetic resonance/mass spectrometry study that identified several FVIII-C2 surfaces occluded by the binding of 4 neutralizing mAbs.35 SPR-based assignments of epitope residues presented herein are consistent with the regions identified using the same or similar mAbs and these other techniques. The advantage of the mutagenesis-plus-SPR methodology is that it accurately pinpoints specific amino acid side chains that contribute significant binding avidity, distinguishing them from “bystander” residues at the protein–protein interface and thus identifying promising targets for B-cell epitope modification.
Type C and BC inhibitors including ESH8, 2-117, 2-77, and 3G6 do not prevent FVIII binding to phospholipids or to von Willebrand factor (vWF). ESH8 slows the release of thrombin-activated FVIII from vWF,36 and a similar mode of inhibition has been reported for IgG from an inhibitor subject,37 demonstrating the physiological relevance of this inhibitory mechanism. ESH8 is inhibitory only in the presence of vWF. The epitope for ESH8 has been localized by immunoblotting to FVIII residues 2248 to 2285.38 ESH8 blocks FVIII activation by FXa,39 and patient-derived antibodies with a similar immunoblot profile have been shown to block FVIII activation by thrombin.40 Low-molecular-weight peptide decoys that mimic ESH8 epitopes have been used to map this antibody’s epitope, identifying FVIII regions 2231 to 2240 and 2267 to 2270.27,41 Recently, peptide array analysis localized this epitope to residues 2265 to 2280.41 The SPR results reported herein confirm that residues T2272, L2273, V2280, and V2282 comprise part of this epitope. The ESH8 epitope also includes residues H2309 and Q2311, which are distal in the linear amino acid sequence of FVIII but adjacent to the other epitope residues on the protein surface. This surface is on the opposite side of the C2 domain from the type A epitopes (Figures 2 and 3). The epitope recognized by the other type C mAb, 2-117, maps to a similar surface on FVIII-C2. Unlike ESH8, however, it includes residues H2269 and Q2270, which are on a loop adjacent to the ESH8 epitope, and R2307. Unlike ESH8, 2-117 is only weakly inhibitory.13 Possibly, its binding to this loop precludes stabilization of the FVIII-vWF complex and/or the facilitated removal of thrombin-cleaved FVIIIa that has been hypothesized for the other type C inhibitor, ESH8.36 The substitution R2220A affected binding to type C mAbs 2-117 and ESH8, increasing their kd constants by 3.0 and 4.5 times, respectively. R2220 is not conformationally contiguous with the other residues identified by SPR, and therefore it was identified as an outlier and not included as part of these functional epitopes. The epitopes recognized by type BC mAbs 2-77 and 3G6 overlap the type C epitopes but also include N2225 and E2228, which are part of the type B epitopes and are further down the “back” face of FVIII-C2 toward the membrane-binding surface. Type C and BC antibodies inhibit FVIII activation by thrombin and/or FXa, but most of those analyzed to date did not compete effectively for FVIII binding to vWF.13 These antibodies are considered nonclassical inhibitors because their identification pointed to a previously underappreciated and important role for the FVIII-C2 domain in proteolytic activation of FVIII. Their localization at a surface distinct from the other types of epitopes, and also on an outer surface of the FVIII protein (ie, not at an interdomain interface), is shown in Figure 3A-B.
The mapping of this series of FVIII-C2 domain epitopes will facilitate additional studies to model the domain orientations in FVIII (which may well differ among the solution, vWF-bound, and membrane-bound FVIII structures),21,22,42 as well as the interactions between FVIIIa and the other components of the intrinsic tenase complex. The high-resolution definition of these physiologically relevant and medically important epitopes also suggests specific sites at which the FVIII sequence could be modified to generate less antigenic FVIII variants. We propose that next-generation therapeutic FVIII proteins could include rationally designed variants of human FVIII that, similar to porcine FVIII, could provide at least short-term hemostatic support in patients with high-titer inhibitors.5,6 The C2 domain of porcine FVIII contains 32 residues that differ from the human FVIII sequence.43 Soluble FVIII-C2 proteins with alanine substitutions at 20 of these sites were characterized by SPR. The human residues at 14 of these sites were identified as contributing to B-cell epitopes recognized by neutralizing anti-FVIII antibodies (Figure 3C).
Both antigen–antibody affinities44,45 and residence times16,19 (which reflect avidity of binding) of these complexes are of interest in characterizing inhibitor responses. Amino acid substitutions at the antigen–antibody interfaces decreased some of the binding affinities (∆∆G values) by 0 to 18 kJ/mol (supplemental Table 2). Because kds are directly related to residence times and dissociation half-lives (half-life, ln 2/kd), and hence the expected duration of FVIII neutralization by inhibitor antibodies, functional B-cell epitopes were identified on the basis of the effects of amino acid substitutions on kd constants. This experimental approach may be used to target functional B-cell epitopes, including critical residues in antigenic loops in the FVIII A2 domain and in other regions of FVIII,46-52 in designing novel FVIII muteins that could provide useful bypass therapy options for inhibitor patients. Because their sequences would be closer to that of the FVIII used to treat the original bleeding disorder, the risk of provoking new T-cell responses and subsequent new inhibitors53-58 to such rationally designed therapeutic FVIII muteins might also be lowered, in comparison with porcine FVIII used as bypass therapy. We expect that sequence modifications to neutralize immunodominant B-cell and T-cell epitopes will eventually be a feature of therapeutic FVIII proteins targeted to patients with refractory inhibitor responses, as well as to patients with poor prognostic factors such as high-risk F8 gene mutations or a family history of inhibitors.59
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Emily Spence, Amjad Hussain, Rebecca Lefavor, and Shelley Nakaya Fletcher for generating FVIII-C2 muteins.
This work was supported by unrestricted research funds from Bayer (K.P.P.) and CSL Behring (K.P.P.), a Pfizer ASPIRE hemophilia award (K.P.P.), grants from the National Institutes of Health, Heart, Lung and Blood Institute (1RC2-HL101851) (K.P.P.); (U54-HL112309, K08-HL102262) (S.L.M.); and (U54-HL112309, R01-HL082609, and R01-HL040921) (P.L.), and Hemophilia of Georgia, Inc (S.L.M., P.L.).
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
Authorship
Contribution: P.-C.T.N., K.B.L., R.A.E., J.T.S., and J.C.L. carried out the experiments; K.B.L., P.-C.T.N., J.T.S., R.A.E., J.C.L., and K.P.P. designed the experiments; J.F.H., S.L.M., and P.L. performed mAb isolation and characterization; and P.-C.T.N., K.B.L., R.A.E., J.T.S., J.C.L., and K.P.P. analyzed the data and wrote the manuscript, which was reviewed and approved by all authors.
Conflict-of-interest disclosure: K.P.P. and K.B.L. are inventors on a patent (International Patent Application No. PCT/US2012/61/553 660) that describes characterization of B-cell epitopes using SPR. J.T.S. is an employee of GE Healthcare. K.P.P. has received unrestricted research funding from Bayer, Pfizer, and CSL Behring and honoraria from Bayer, Pfizer, and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: Kathleen P. Pratt, Department of Medicine (MED) A3075, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Rd, Bethesda, MD 20814; e-mail: kathleen.pratt@usuhs.edu.