Key Points
NET formation is required for neutrophil recruitment during sterile inflammation.
Platelet-induced NET formation requires stimulation of neutrophils by platelet chemokines and outside-in signaling via the integrin Mac-1.
Abstract
There is emerging evidence that neutrophil extracellular traps (NETs) play important roles in inflammatory processes. Here we report that neutrophils have to be simultaneously activated by integrin-mediated outside-in– and G-protein–coupled receptor (GPCR) signaling to induce NET formation in acute lung injury (ALI), which is associated with a high mortality rate in critically ill patients. NETs consist of decondensed chromatin decorated with granular and cytosolic proteins and they can trap extracellular pathogens. The prerequisite for NET formation is the activation of neutrophils and the release of their DNA. In a neutrophil- and platelet-dependent mouse model of ventilator-induced lung injury (VILI), NETs were found in the lung microvasculature, and circulating NET components increased in the plasma. In this model, blocking integrin-mediated outside-in or either GPCR-signaling or heteromerization of platelet chemokines decreased NET formation and lung injury. Targeting NET components by DNAse1 application or neutrophil elastase–deficient mice protected mice from ALI, whereas DNase1−/−/Trap1m/m mice had an aggravated ALI, suggesting that NETs directly influence the severity of ALI. These data suggest that NETs form in the lungs during VILI, contribute to the disease process, and thus may be a promising new direction for the treatment of ALI.
Introduction
Platelets are essential for primary hemostasis, but they also play an important role in inflammation and injury1-4 such as rheumatoid arthritis5 and ALI.6-8 We have shown that the murine acid–induced ALI model is critically dependent on platelets.6 During inflammatory conditions, platelets interact with neutrophils, thus promoting neutrophil recruitment into inflammatory tissue.4 Neutrophil-platelet interactions are mainly mediated by P-selectin glycoprotein ligand 1 (PSGL-1), which is expressed on neutrophils and binds to platelet P-selectin.9 Firm neutrophil adhesion to platelets is mediated by the β2-integrin macrophage antigen-1 (Mac-1, CD11b/CD18), which binds platelet GPIbα, and the simultaneous binding of fibrinogen to platelet GPIIb/GPIIIa and CD11b/CD18 on neutrophils. These interactions lead to the activation of neutrophils by integrin-mediated outside-in signaling.10,11 In addition, platelets deposit the chemokines CXCL4 and CCL5 at sites of inflammation, which promote adhesion of leukocytes.12 Depletion of platelets or disruption of platelet-neutrophil interactions reduced neutrophil recruitment and vascular permeability in models of inflammation.6,8,13
Activated neutrophils can release neutrophil extracellular traps (NETs) that are composed of DNA fibers decorated with histones and granule proteins.14,15 NETs exhibit antimicrobial functions by trapping and killing extracellular pathogens in blood and tissues during infection.16 However, when produced at the wrong time or in the wrong amount, NETs can also have a negative effect on the host. It has been shown that NETs contribute to the pathology of several inflammatory diseases.17-19 It has been recently demonstrated that intimate platelet-neutrophil interactions induce the formation of neutrophil extracellular traps under inflammatory conditions.7,20 However, the molecular mechanisms by which neutrophil extracellular traps are formed during acute lung injury (ALI) are poorly understood.
ALI is a life-threatening disease that can develop in the course of different clinical conditions such as acid aspiration, pneumonia, or prolonged mechanical ventilation, and contributes significantly to critical illness.21,22 Recent epidemiological studies demonstrated that the incidence of ALI is very high and, despite all innovations in intensive care medicine, the mortality of this disease remains as high as 40%.23 The pathogenesis of ALI is characterized by an influx of a protein-rich edema fluid into the interstitial and intraalveolar spaces as a consequence of increased permeability of the alveolar-capillary barrier21,22 in conjunction with excessive invasion of inflammatory cells—particularly neutrophils.6,24,25 Despite extensive research into the pathogenesis of ALI and many clinical trials testing new medications, there is no effective therapeutic agent to treat patients with this syndrome. More research is desperately required to discover novel pathways that can be targeted with new treatment options.
In the present study, we investigated the molecular mechanisms of NET formation in a murine model of ventilator-induced lung injury (VILI) and in vitro in a cell culture system. Although the clinical guidelines recommend lung protective ventilation with low tidal volumes, current clinical practice often deviates from these recommendations.26,27 Therefore this model of ALI is still clinically relevant.
Our in vivo and in vitro data show that neutrophils only release NETs when they are simultaneously activated by integrin-mediated outside-in– and G-protein–coupled receptor (GPCR) signaling. Blocking one of these pathways reduced NET formation in vivo and diminished the severity of ALI. By treating wild-type (WT) mice with DNAse1, and using DNAse1−/−/Trap1m/m mice, we demonstrate that NETs might directly affect neutrophil recruitment and vascular permeability. Finally, preventing NET formation results in marked lung protection in experimental ALI, suggesting that therapeutic inhibition of NET formation may provide a novel therapeutic approach to ALI that should be explored in clinical trials.
Methods
Reagents and animals
Unlike otherwise stated, all reagents were obtained from Sigma-Aldrich (Taufkirchen, Germany). We used 8- to 12-week-old C57BL/6 WT mice (Charles River Laboratories, Wilmington, MA) and DNAse1−/− mice,28 Itgb2−/− mice,29 Tyrobp−/−/Fcer1g−/− mice,30 and neutrophil elastase–deficient mice.31 In addition, the previously described DNAse1−/− mice28 were used as control for the experiments using DNAse1 treatment of WT mice. The availability of the complete mouse genome had however indicated that these mice contain an additional mutation within the tumor necrosis factor receptor–associated protein 1 (Trap1)/heat shock protein 75 (Hsp75) gene32 (ie, possess a combined DNase1−/−/Trap1m/m genotype). The Trap1 gene consists of 18 exons and its reading frame, which codes for 706 amino acids and is located at the opposite DNA strand to the DNAse1 gene. Furthermore, both genes overlap within their 3′-UTR. Because of the exchange of the entire DNAse1 gene by a Neo-resistance cassette, the 3′-end of the last Trap1 exon coding for 18 C-terminal amino acids and containing the 3′-UTR was inadvertently deleted and fused to the cassette. This was proven by sequencing the Trap1m cDNA isolated from DNAse1−/−/Trap1m/m mice. The fusion of the truncated Trap1 exon 18 to the Neo-cassette leads to an extended reading frame with addition of 48 unrelated amino acids. However, Trap1m gene expression was proven to be inefficient, resulting in an almost complete lack of Trap1m protein in addition to the lack of Trap1 itself. Mouse colonies were maintained under specific pathogen-free conditions. All animal experiments were approved by local government authorities and were in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Murine model of ventilator-induced lung injury
VILI was induced as reported previously with some modifications.33 Control mice were mechanically ventilated with an end-inspiratory pressure of 15 cm H2O and a positive end-expiratory pressure (PEEP) of 5 cm H2O at 140 bpm for 2 hours. Mice subjected to VILI group were ventilated with an end-inspiratory pressure of 45 cm H2O and a PEEP of 5 cm H2O at 40 bpm. After 2 hours, an arterial blood sample was withdrawn for blood gas analysis using an ABL800 (Radiometer; Bronshoy, Denmark). The lungs were lavaged 5 times with 0.7 mL physiological saline solution. Protein concentration in the bronchoalveolar lavage (BAL) fluid was measured using the BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). The number of neutrophils in the BAL was counted using Kimura staining. The recruitment of neutrophils into the intravascular and interstitial compartment of the lung was determined as described previously.6 For some experiments, platelet depletion in WT mice was achieved as described previously.34 In some experiments, mice were pretreated with 1 or 3 mg/kg of a blocking peptide against the formation of the CXCL4/CCL5-heteromer (Carolus Therapeutics, La Jolla, CA) or with blocking antibodies against LFA-1 (clone TIB-217), Mac1 (clone TIB-128), CXCL4 (R&D Systems, Minneapolis, MN), or CCL5 (eBioscience, San Diego, CA). For degradation of NETs in vivo, mice received 4000 KU of DNAse1 in some experiments.
Quantification of platelet-neutrophil interactions
The amount of platelet-neutrophil aggregates in murine blood was determined as described previously.6
Quantification of circulating NET structures
To measure the amount of circulating NET structures in mouse serum and in supernatant from in vitro NET formation assays, we performed a MPO-DNA enzyme-linked immunosorbent assay (ELISA) as described previously.7
Immunofluorescence staining of lung sections
Immunostaining of cryopreserved lung section was performed as previously described.7
In vitro NET formation
Mouse neutrophils were isolated from bone marrow as described previously.35 Isolated neutrophils were resuspendend in RPMI 1640 (PAN Biotech, Aidenbach, Germany) medium with 1% fetal calf serum. Citrate-anticoagulated whole blood was obtained from mice and platelets were isolated by low-speed centrifugation. Neutrophils (1 × 105) were incubated with platelets (1 × 106) pretreated with or without thrombin receptor activator peptide (TRAP; 50 µM) for 60 minutes at 37°C. Samples were fixed with 4% PFA for 10 minutes and incubated with MPO-antibody (Millipore, Billerica, MA), Histone H2A antibody (Santa Cruz Biotechnology), and CD41-antibody (clone MWReg30; Biolegend) at 4°C overnight, followed by incubation with secondary antibodies for 1 hour at room temperature. Nuclei counterstaining was performed with Hoechst-33342 for 5 minutes. Images were obtained with a confocal microscope (LSM780; Zeiss, Jena, Germany).
Intravital microscopy of the lung
The intravital microscopy of the lung was performed as described previously,8,36,37 with some modifications. After induction of anesthesia and placement of an arterial catheter in the carotid artery, mice were subjected to sham or VILI ventilation for 30 minutes. After right lateral thoracotomy, the lung was positioned under the window of a custom-built fixation device. A mild vacuum was applied to hold the lung in position during microscopy. Mice were injected with an Alexa488-conjugated Gr1-antibody (clone RB6-8C5) and PE-conjugated CD41-antibody (clone MWReg30; BD Biosciences) to visualize neutrophils and platelets, respectively.
Statistics
Statistical analysis was performed with SPSS (version 20.0; IBM, Inc., Armonk, NY) using one-way analysis of variance, Student-Newman-Keuls test, post-hoc correction, or Student t test where appropriate. All data are represented as means ± standard error of the mean. A P value < .05 was considered statistically significant.
See the supplemental material, available on the Blood Web site, for further information on the methods used.
Results
Platelet-dependent NET formation during VILI
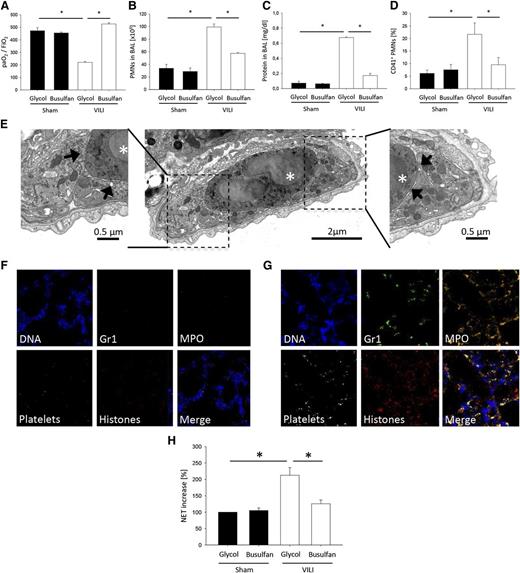
To investigate the role of platelets in VILI, we depleted platelets by injecting mice with busulfan, which reduced blood platelet counts by 88% without affecting the white blood cell differential count (supplemental Table 1). To investigate whether busulfan treatment causes preactivation of neutrophils, we analyzed the surface expression of CD11b (Mac-1) and CD62L (l-selectin) on circulating blood neutrophils from busulfan- and glycol-treated animals by flow cytometry. There was no difference regarding the expression of the cell surface molecules between the groups, suggesting that busulfan pre-treatment does not activate neutrophils (supplemental Table 1). Platelet depletion significantly prevented the deterioration of gas exchange after the induction of VILI (P < .05; Figure 1A). To investigate the polymorphonuclear neutrophil (PMN) recruitment pattern, including intravascular accumulation, transendothelial migration, and transepithelial migration, PMNs in lung homogenates were identified by flow cytometry.6,38 Two hours after inducing ALI, VILI mice showed a significant accumulation of PMNs in the intravascular, interstitial (supplemental Figure 1), and alveolar compartments (Figure 1B) compared with control mice (Figure 1B and supplemental Figure 1). Platelet depletion before the induction of ALI diminished the accumulation of PMNs in the intravascular, interstitial (supplemental Figure 1), and alveolar compartments (Figure 1B). Furthermore, platelet depletion reduced protein leakage as determined by BAL fluid protein levels (Figure 1C).
Ventilator-induced lung injury causes platelet-dependent NET formation. Glycol- and busulfan-treated WT mice were ventilated with sham or VILI settings. (A) The paO2/FiO2 ratio, (B) neutrophil recruitment into the alveoli, and (C) protein concentration in the BAL were analyzed after 2 hours (n = 4). (D) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). (E) Ultrathin cross-sectioned vessel/capillary imaged by transmission electron microscopy from lung tissue of WT mice 2 hours after induction of VILI ventilation showing a neutrophil (asterisk) surrounded by platelets. Inserts at higher magnification illustrate the close contact between platelets (arrows) and neutrophil. NET formation in lung sections was visualized by immunofluorescence staining. Exemplary images from (F) sham-ventilated and (G) VILI-ventilated animals. (H) Circulating NET structures were quantified by MPO-DNA-ELISA (n = 4). *P < .05.
Ventilator-induced lung injury causes platelet-dependent NET formation. Glycol- and busulfan-treated WT mice were ventilated with sham or VILI settings. (A) The paO2/FiO2 ratio, (B) neutrophil recruitment into the alveoli, and (C) protein concentration in the BAL were analyzed after 2 hours (n = 4). (D) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). (E) Ultrathin cross-sectioned vessel/capillary imaged by transmission electron microscopy from lung tissue of WT mice 2 hours after induction of VILI ventilation showing a neutrophil (asterisk) surrounded by platelets. Inserts at higher magnification illustrate the close contact between platelets (arrows) and neutrophil. NET formation in lung sections was visualized by immunofluorescence staining. Exemplary images from (F) sham-ventilated and (G) VILI-ventilated animals. (H) Circulating NET structures were quantified by MPO-DNA-ELISA (n = 4). *P < .05.
To better characterize our VILI model, we conducted additional experiments with animals subjected to 25 and 35 mm Hg of end-inspiratory pressures. Ventilation with end-inspiratory pressures of 35 mm Hg still produced a mild lung injury, whereas ventilation with end-inspiratory pressures of 25 mm Hg did not result in lung injury, as measured by gas exchange, neutrophil recruitment into the BAL, vascular permeability, and platelet-neutrophil aggregate formation (supplemental Figure 2A-D). We also measured the hemodynamic effects of VILI ventilation, which is accompanied by high tidal volumes. Ventilation with end-inspiratory pressures of 45 mm Hg did not significantly change the systolic blood pressure compared with the control group ventilated with end-inspiratory pressures of 15 mm Hg (supplemental Figure 2E).
Flow cytometric analysis of whole blood revealed the presence of platelet-neutrophil aggregates 30 minutes after inducing VILI, as detected by a significant increase in the percentage of PMNs (CD45+, Gr-1+, 7/4+) positive for the platelet-specific marker CD41 (Figure 1D). Depletion of platelets by busulfan led to a significant reduction of platelet-neutrophil aggregates in the blood 30 minutes after induction of ALI (Figure 1D). The presence of platelet-neutrophil aggregates after induction of VILI was also demonstrated by transmission electron microscopy (Figure 1E).
Because elimination of platelets decreases the severity of ALI,6,13 we hypothesized that the elimination of platelets would decrease NET formation by decreasing neutrophil activation. We found that NETs were present in abundance in the lung microcirculation of mice with VILI (Figure 1G) compared with control mice (Figure 1F), and platelet sequestration increased in areas of the lung in which NETs were present (Figure 1G). In addition, soluble NET components were higher in the peripheral blood after inducing VILI (Figure 1H). Platelet depletion before the induction of VILI decreased NET formation (Figure 1H). These data suggest that NET formation is dependent on platelets during VILI.
The simultaneous activation of neutrophils by chemokines and integrins is required for NET formation.
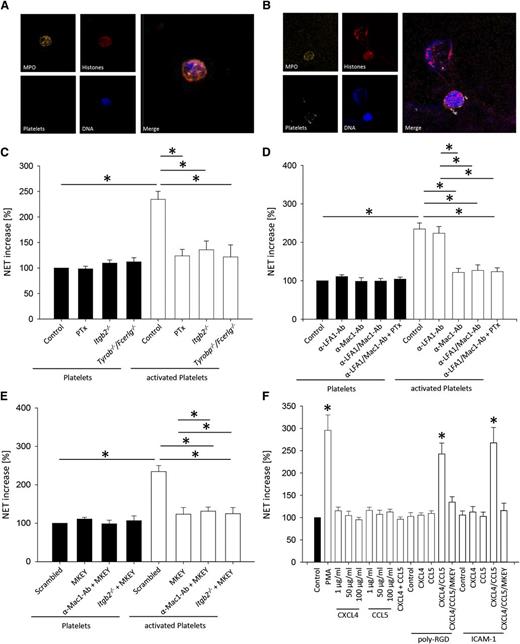
To elucidate the molecular mechanisms responsible for our in vivo results, we investigated the role of different signaling pathways activated by the interaction of platelets with neutrophils for NET formation. Incubating neutrophils with TRAP-stimulated platelets induced NET formation in vitro (Figure 2A-B) and increased soluble NET components in the supernatant (Figure 2C-F). However, an increase of soluble NET components was not detectable after incubating activated platelets with pertussis toxin (PTx)-pretreated WT neutrophils, Itgb2−/− (CD18-deficient) neutrophils, and Tyrobp−/−/Fcer1g−/− (DAP12- and FcRγ-deficient) neutrophils (Figure 2C). To investigate which β2-integrin on neutrophils is required for NET formation, we blocked the different β2-integrins by using monoclonal antibodies. Blocking LFA-1 did not decrease soluble NET components, but blocking Mac-1 significantly reduced the amount of soluble NET components (Figure 2D). The blockade of GPCR signaling by PTx in the absence of integrin-mediated outside-in signaling did not further decrease the amount of soluble NET formation (Figure 2D).
The simultaneous activation of neutrophils by chemokines and integrins is required for NET formation. In vitro NET formation was induced by incubating isolated neutrophils with TRAP-stimulated platelets or unstimulated platelets as control or incubation with different stimulating agents. NET formation was visualized by immunofluorescence staining. Exemplary images of NET formation by isolated neutrophil incubated with (A) unstimulated platelets and (B) TRAP-stimulated platelets. (C-F) NET structures in the supernatant were quantified by MPO-DNA-ELISA (n = 4). *P < .05.
The simultaneous activation of neutrophils by chemokines and integrins is required for NET formation. In vitro NET formation was induced by incubating isolated neutrophils with TRAP-stimulated platelets or unstimulated platelets as control or incubation with different stimulating agents. NET formation was visualized by immunofluorescence staining. Exemplary images of NET formation by isolated neutrophil incubated with (A) unstimulated platelets and (B) TRAP-stimulated platelets. (C-F) NET structures in the supernatant were quantified by MPO-DNA-ELISA (n = 4). *P < .05.
Heterodimerization of platelet-derived CCL5 and CXCL4 enhances their ability to activate and recruit inflammatory cells.12,39 To elucidate whether the heterodimerization of CCL5 and CXCL4 is involved in NET formation, we used the peptide antagonist MKEY.39 This antagonist was designed to specifically disrupt pro-inflammatory interactions of CCL5-CXCL4.39 The application of MKEY reduces leukocyte recruitment and consequently attenuates different inflammatory diseases.8,39 Incubating activated platelets with neutrophils in the presence of MKEY prevented NET formation (Figure 2E). Blocking the heterodimerization of CCL5 and CXCL4 in Itgb2−/− neutrophils or WT neutrophils pretreated with a blocking anti-Mac-1 antibody did not have an additional effect on NET formation (Figure 2E). These data suggest that the heterodimerization of CCL5 and CXCL4 is required for NET formation.
To directly show that GPCR- and integrin-signaling pathways have to be activated simultaneously and to exclude that other factors produced by platelets are responsible for NET formation, we directly stimulated neutrophils with chemokines or poly-RGD, or both substances. Stimulation of neutrophils with only chemokines or poly-RGD did not induce NET formation (Figure 2F). However, neutrophils produced NETs after stimulation with poly-RGD and chemokines (Figure 2F). Because poly-RGD binds to β1- and β2-integrins, we repeated this experiment with ICAM-1, which is known to engage only β2-integrins, to demonstrate that β2-integrins are required for NET formation. Stimulation of neutrophils with only chemokines or ICAM-1 did not induce NET formation (Figure 2F). However, neutrophils produced NETs after stimulation with ICAM-1 and chemokines (Figure 2F). To investigate whether outside-in signaling through β1-integrins may induce NET formation, we incubated isolated neutrophils on a surface coated with the β1-integrin substrate VCAM-1. Outside-in signaling via β1-integrins did not cause NET formation, and NET formation was also absent when isolated neutrophils were incubated on VCAM-1 in the presence of CXCL4 and CCL5 (data not shown).
It is known that NET formation requires the NADPH oxygenase, a central element of ROS production.40 To test the ROS dependency of platelet-induced NET formation in vitro, we inhibited the NADPH oxygenase using diphenyleneiodonium chloride (DPI). Blocking NADPH oxygenase significantly inhibited NET formation in vitro (supplemental Figure 4A) and in vivo (supplemental Figure 4B). Previous research has demonstrated a pivotal role for neutrophil-dependent ATP release in several inflammatory pathways, thus making an implication in NET formation likely.41 However, blocking PMN-dependent ATP-release with carbenoxolone or an inhibitory peptide (10 panx-1) did not affect platelet-induced NET formation (data not shown).
Disruption of CXCL4/CCL5 heteromer formation decreases the severity of VILI
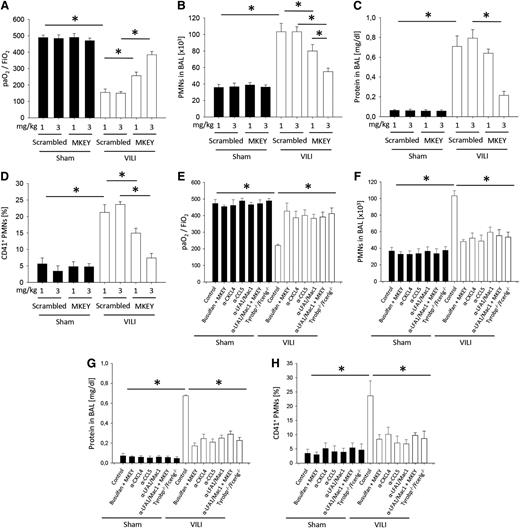
To investigate the therapeutic potential of disrupting CXCL4/CCL5 heteromer formation in VILI, we treated mice with different concentrations of MKEY before inducing VILI and analyzed lung gas exchange, PMN infiltration, edema formation, and the number of platelet-neutrophil aggregates in the blood (Figure 3A-D). As a negative control, we used a scrambled peptide, which had no effect on gas exchange, neutrophil recruitment, and vascular permeability compared with untreated animals (supplemental Figure 3). Treatment with MKEY significantly improved gas exchange (Figure 3A), reduced alveolar PMN counts (Figure 3B), prevented lung edema formation (Figure 3C), and reduced the number of platelet-neutrophil aggregates (Figure 3D) in the blood. The same results were seen in Tyrobp−/−/Fcer1g−/− mice and in WT mice pretreated with blocking antibodies against CXCL4 or CCL5, as well as blocking anti–LFA-1 and anti–Mac-1-antibodies after inducing VILI (Figure 3E-H).
Disruption of CXCL4/CCL5 heteromer formation decreases the severity of VILI. WT mice were treated with 1 or 3 mg/kg MKEY or scrambled control peptide 15 minutes before the initiation of mechanical ventilation with sham or VILI settings. (A) The paO2/FiO2 ratio, (B) neutrophil recruitment into the alveoli, and (C) protein concentration in the BAL were analyzed after 2 hours (n = 4). (D) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). Tyrobp−/−/Fcer1g−/− mice or WT mice were treated with busulfan, MKEY, blocking antibodies against CXCL4, CCL5, LFA1, Mac1, or a combination and (E) the paO2/FiO2 ratio, (F) neutrophil recruitment into the BAL, and (G) protein concentration in the BAL were analyzed after 2 hours (n = 4). (H) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). *P < .05.
Disruption of CXCL4/CCL5 heteromer formation decreases the severity of VILI. WT mice were treated with 1 or 3 mg/kg MKEY or scrambled control peptide 15 minutes before the initiation of mechanical ventilation with sham or VILI settings. (A) The paO2/FiO2 ratio, (B) neutrophil recruitment into the alveoli, and (C) protein concentration in the BAL were analyzed after 2 hours (n = 4). (D) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). Tyrobp−/−/Fcer1g−/− mice or WT mice were treated with busulfan, MKEY, blocking antibodies against CXCL4, CCL5, LFA1, Mac1, or a combination and (E) the paO2/FiO2 ratio, (F) neutrophil recruitment into the BAL, and (G) protein concentration in the BAL were analyzed after 2 hours (n = 4). (H) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). *P < .05.
Pretreating WT mice with blocking antibodies against chemokines (CXCL4 or CCL5) or β2-integrins (Mac-1 or LFA-1) improved gas exchange (Figure 3E), reduced the number of PMNs in the alveolar compartment (Figure 3F), reduced lung edema formation (Figure 3G), and decreased the number of platelet-neutrophil aggregates in the blood (Figure 3H).
To clarify whether NET formation as a result of synchronized neutrophil activation by chemokines and integrin-mediated outside-in signaling is a general mechanism, we investigated NET formation in another inflammatory model. For this purpose, we injected mice with lipopolysaccharide from Escherichia coli and analyzed NET formation and neutrophil recruitment in the liver and the release of circulating NET structures. Platelet depletion with busulfan caused a significantly reduced neutrophil recruitment into the liver and reduced NET formation compared with glycol-treated control animals. These data suggest that platelet-mediated NET formation is also required for neutrophil recruitment into the liver after lipopolysaccharide challenge (supplemental Figure 5). Similar to the observations in the lung, Tyrobp−/−/Fcerlg−/− mice, NE−/− mice, and WT mice pretreated with MKEY showed decreased NET formation in the liver, a reduced number of neutrophils recruited into the liver, and reduced soluble NET components in the plasma. In contrast, DNAse1−/−/Trap1m/m mice showed an aggravated phenotype with increased neutrophil recruitment into the liver and increased soluble NET components in the plasma (supplemental Figure 5).
Blocking integrin– or chemokine-induced signaling inhibits neutrophil adhesion and formation of platelet-neutrophil aggregates in the microcirculation of the lung after inducing VILI
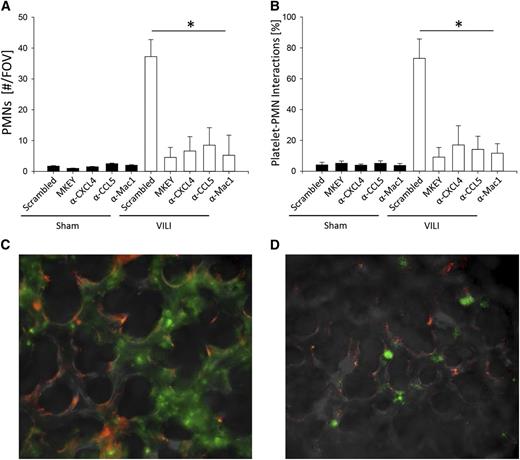
To directly visualize neutrophil adhesion and the interaction of neutrophils with platelets in the microcirculation of the lung, we used the intravital microscopy of the lung as described previously.8,36,37 Almost no adherent neutrophils (Figure 4A) and platelet-neutrophil aggregates were detectable in the microcirculation of the lung in control mice (Figure 4B). Thirty minutes after inducing VILI, the number of adherent neutrophils (Figure 4A) and platelet-neutrophil aggregates (Figure 4B) in the microcirculation of the lung significantly increased. Blocking CXCL4, CCL5, Mac-1, or the heterodimerization of CCL5 and CXCL4 by MKEY significantly reduced the number of adherent neutrophils (Figure 4A) and the formation of platelet-neutrophil aggregates (Figure 4B).
Blocking integrin- or chemokine-induced signaling inhibits neutrophil adhesion and formation of platelet-neutrophil aggregates in the microcirculation of the lung after inducing VILI. Mice were subjected to sham or VILI ventilation, and neutrophil accumulation in the lung was visualized by intravital microscopy of the middle right lung lobe by intravital microscopy after 30 minutes. (A) Number of accumulated neutrophils per field of view (n = 3). (B) Neutrophils interacting with platelets in the lung capillaries (n = 3). Exemplary images from VILI-ventilated WT mice pretreated with scrambled control peptide (C) or MKEY (D). Neutrophils, green; Platelets, red. *P < .05.
Blocking integrin- or chemokine-induced signaling inhibits neutrophil adhesion and formation of platelet-neutrophil aggregates in the microcirculation of the lung after inducing VILI. Mice were subjected to sham or VILI ventilation, and neutrophil accumulation in the lung was visualized by intravital microscopy of the middle right lung lobe by intravital microscopy after 30 minutes. (A) Number of accumulated neutrophils per field of view (n = 3). (B) Neutrophils interacting with platelets in the lung capillaries (n = 3). Exemplary images from VILI-ventilated WT mice pretreated with scrambled control peptide (C) or MKEY (D). Neutrophils, green; Platelets, red. *P < .05.
Modulation of NET formation by DNAse1 or neutrophil elastase influences neutrophil recruitment, vascular permeability, and severity of VILI
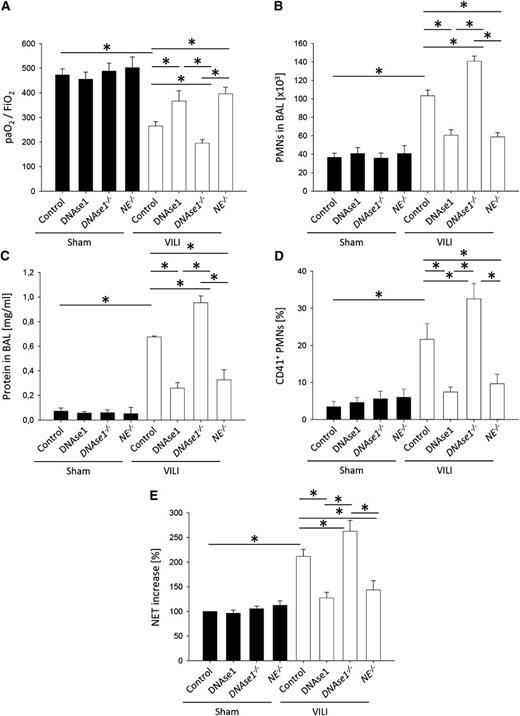
NET formation is modulated by DNAse1 and neutrophil elastase (NE).20,42 To demonstrate that the modulation of NET formation during ALI influences the severity of the disease, we used DNAse1−/−/Trap1m/m mice, neutrophil elastase-deficient mice, and treated WT mice with DNAse1. Under control conditions, control mice, WT mice treated with DNAse1, DNAse1−/−/Trap1m/m mice (included as controls for the DNAse1 injection experiments), and neutrophil elastase–deficient mice were similar regarding gas exchange, number of neutrophils in the BAL, edema formation, circulating platelet-neutrophil aggregates, and soluble NET components in the blood (Figure 5A-E). After inducing VILI, WT mice treated with DNAse1 and neutrophil elastase–deficient mice showed an improved gas exchange (Figure 5A), a reduced number of neutrophils in the BAL (Figure 5B), an abolished edema formation (Figure 5C), reduced number of circulating platelet-neutrophil aggregates (Figure 5D), and diminished soluble NET components in the blood (Figure 5E) compared with control mice. In contrast, DNAse1−/−/Trap1m/m mice had an impaired gas exchange (Figure 5A), an increased number of neutrophils in the BAL (Figure 5B), an elevated edema formation (Figure 5C), increased number of circulating platelet-neutrophil aggregates (Figure 5D), and elevated soluble NET components in the blood after inducing VILI (Figure 5E) compared with WT mice. These data demonstrate that modulating NET formation influences the severity of VILI.
Modulation of NET formation by DNAse1 or neutrophil elastase influences neutrophil recruitment, vascular permeability, and severity of VILI. WT mice with or without DNAse1 pretreatment and DNAse1−/−/Trap1m/m (DNAse1−/−) mice and neutrophil elastase–deficient (NE−/−) mice were ventilated with sham or VILI settings. (A) The paO2/FiO2 ratio, (B) neutrophil recruitment into the alveoli, and (C) protein concentration in the BAL were analyzed after 2 hours (n = 4). (D) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). (E) Circulating NET structures were quantified by MPO-DNA-ELISA (n = 4). *P < .05.
Modulation of NET formation by DNAse1 or neutrophil elastase influences neutrophil recruitment, vascular permeability, and severity of VILI. WT mice with or without DNAse1 pretreatment and DNAse1−/−/Trap1m/m (DNAse1−/−) mice and neutrophil elastase–deficient (NE−/−) mice were ventilated with sham or VILI settings. (A) The paO2/FiO2 ratio, (B) neutrophil recruitment into the alveoli, and (C) protein concentration in the BAL were analyzed after 2 hours (n = 4). (D) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). (E) Circulating NET structures were quantified by MPO-DNA-ELISA (n = 4). *P < .05.
The therapeutic application of MKEY decreases the severity of VILI
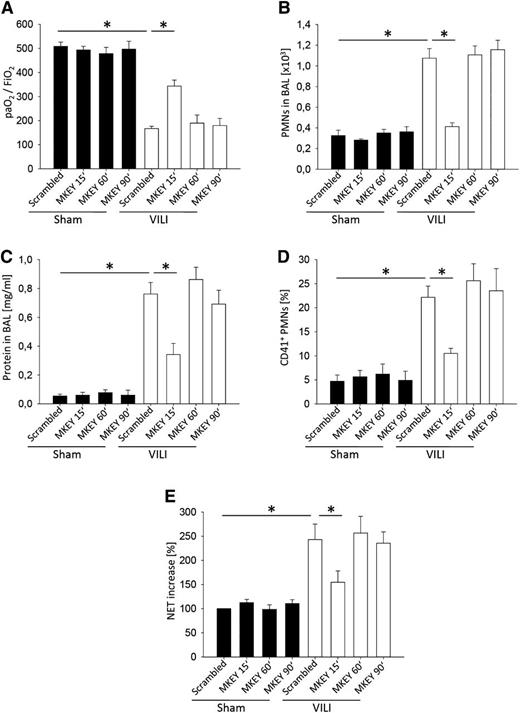
To investigate whether MKEY improves functional and morphological parameters of ALI, even when given after the insult, we injected MKEY (3 mg/kg) 15 minutes after the induction of VILI and measured gas exchange, neutrophil recruitment, protein concentration in the BAL, platelet-neutrophil aggregates, and the amount of soluble NET components in the blood. The application of MKEY in a therapeutic approach improved gas exchange (Figure 6A), and reduced neutrophil recruitment (Figure 6B), edema formation (Figure 6C), formation of platelet-neutrophil aggregates (Figure 6D), and the amount of soluble NET components in the blood (Figure 6E). Treatment of mice with MKEY 1 hour or 1.5 hours after induction of VILI ventilation did not significantly change neutrophil recruitment into the lung, vascular permeability, platelet-neutrophil aggregate formation, NET release, and gas exchange (Figure 6A-E).
The therapeutic application of MKEY decreases the severity of VILI. WT mice were treated with 3 mg/kg MKEY or scrambled control peptide 15, 60, or 90 minutes after the induction of mechanical ventilation with sham or VILI settings. (A) The paO2/FiO2 ratio, (B) neutrophil recruitment into the alveoli, and (C) protein concentration in the BAL were analyzed after 2 hours (n = 4). (D) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). (E) Circulating NET structures were quantified by MPO-DNA-ELISA (n = 4). *P < .05.
The therapeutic application of MKEY decreases the severity of VILI. WT mice were treated with 3 mg/kg MKEY or scrambled control peptide 15, 60, or 90 minutes after the induction of mechanical ventilation with sham or VILI settings. (A) The paO2/FiO2 ratio, (B) neutrophil recruitment into the alveoli, and (C) protein concentration in the BAL were analyzed after 2 hours (n = 4). (D) The formation of circulating platelet-neutrophil aggregates was measured by flow cytometry 30 minutes after induction of sham or VILI ventilation (n = 4). (E) Circulating NET structures were quantified by MPO-DNA-ELISA (n = 4). *P < .05.
Discussion
In this study, we have first identified that NET formation is platelet-dependent and that circulating NET components increase during VILI. Second, we found that the simultaneous stimulation of integrins and G-protein–coupled receptors on neutrophils is required for NET formation. Blocking one of the 2 pathways reduces NET formation, the amount of soluble NET components in the blood, and thus the development of VILI. Third, blocking heterodimerization of CCL5 and CXCL4 blocks NET formation and protects from VILI. Fourth, we demonstrated that modulating NETs by DNAse1 influences the severity of VILI. Therapeutic inhibition of platelet neutrophil aggregates and/or NET formation may provide a novel therapeutic approach to ALI that should be explored in clinical trials.
Neutrophils can combat pathogens by multiple means including phagocytosis and NET formation. Highly activated neutrophils form NETs, which can immobilize pathogens and facilitate subsequent phagocytosis of trapped microorganisms.15 Initially, NETs have been linked to infectious disease20,43 ; however, NETs are also found in noninfectious diseases.17,19 In these diseases, the presence of NETs may lead to tissue injury. Here we show that NETs are involved in the pathogenesis of VILI. Our data are in line with a recently published study showing that NETs are involved in the pathogenesis of transfusion-related acute lung injury.7,44
Platelets promote inflammation and injury through their interaction with neutrophils.2,4 During this cell-cell contact, platelets can activate neutrophils by engaging β2-integrins and they also deposit platelet-derived mediators, including the chemokines CXCL4, CXCL7, CXCL8, and CCL5. However, the molecular mechanisms by which platelets promote NETosis are still unknown, but we propose that the simultaneous activation of the GPCR- and integrin-triggered signaling pathways in neutrophils by activated platelets are required for NET formation. Furthermore, we excluded with our in vitro experiments that other factors produced by platelets (eg, lipid mediators) are required for NET formation. It has been shown that neutrophil-platelet aggregates have enhanced adhesive capacity, increase reactive oxygen species production, and phagocytic potential.45 The production of reactive oxygen species, which is tied closely to NET formation,46 is induced by the stimulation of either the GPCR- or the integrin-mediated outside-in signaling pathway in neutrophils. However, our experiments demonstrate that the production of NETs requires the activation of both signaling pathways. The double-activation mechanism could be a protection mechanism against an overwhelming NET formation.
Neutrophil recruitment and an increased vascular permeability are hallmarks of ALI.22 Local and systemic hypoxia during ALI activates the transcriptional factor HIF-1, leading to an activation and recruitment of myeloid cells. Hypoxia during inflammation may also contribute to NET formation.47,48 Recent publications suggest that platelet-induced NETs are also involved in the pathogenesis of ALI and may influence vascular permeability.7 Mechanistically, we identify platelet-derived CXCL4 and CCL5 as crucial mediators in forming NETs. Previous work has demonstrated that heterodimerization of platelet-derived CCL5 and CXCL4 enhances their ability to activate and recruit inflammatory cells.12,39 By using a synthetic peptide, termed MKEY, which specifically disrupts the synergistic interaction between CXCL4 and CCL5, we were able to show that this peptide reduces the formation of platelet-neutrophil aggregates and thus abrogates NET formation, neutrophil recruitment, and vascular permeability increase, and improves gas exchange. In line with models of vascular recruitment,39 these data imply that the CCL5 receptors CCR1 and CCR5, which support the CCL5-CXCL4 heteromer activity, are the GPCRs responsible for mediating NET formation together with integrins. In fact, a coengagement of integrins may have a critical synergistic effects previously observed.12 Previous studies demonstrated that the inhibition of the initial interaction of platelets with neutrophils by blocking P-selectin or GPIIb/IIIa6,13 reduces the severity of ALI. Although the architecture of the lung microcirculation is unique and neutrophil recruitment into the lung is influenced by several factors,49 the promotion of neutrophil emigration by platelets is probably a general mechanism because these aggregates are also seen in other inflammatory models.6,13,50
Platelet-induced NETs induce lung injury through neutrophil recruitment into the lung, as we showed in our in vivo VILI model, perhaps by trapping neutrophils and prolonging the exposure time of neutrophils to chemokines, leading to increased neutrophil activation and recruitment. Furthermore, by using neutrophil elastase–deficient mice and DNase1−/−/Trap1m/m mice, we directly demonstrated that modulation of NETs ameliorate VILI. However, evaluation of the aggravated VILI observed in DNase1−/−/Trap1m/m mice must take into account that these mice are not only deficient for DNAse1 but also display a lack of Trap1, which is a mitochondrial chaperon also known as Hsp75.32 For DNAse1, it is well known that it participates in extracellular DNA, chromatin, and also NET degradation.51,52 Indeed, the curing effect obtained by DNAse1 injection into WT mice clearly supports the hypothesis that a prolonged half-life of NETs leads to an elevated neutrophil response and an accumulation of circulating undegraded NET components during VILI, as observed in DNase1−/−/Trap1m/m mice. Nevertheless, a participation of Trap1, which among other functions is regarded as a cellular protector against ROS-mediated cell death,32 cannot be excluded. It is conceivable that because of the lack of Trap1 and the almost complete lack of Trap1m ROS released by activated neutrophils during VILI may lead to an enhanced alveolar cell death accompanied by chromatin release and subsequent further neutrophil activation in DNase1−/−/Trap1m/m mice. This would increase the enhancing effect of DNAse1 deficiency on VILI in these mice.
Currently there are no drugs to effectively manage pulmonary edema and PMN sequestration and to improve survival rates in ALI. We suggest that targeting NETs is a novel therapeutic approach to ALI. One treatment strategy is the inhibition of the initial NET formation by inhibiting the simultaneous activation of neutrophils by chemokines and integrins. However, this will be very difficult, because the NET formation occurs very early in the course of ALI.7 Another more feasible approach is to target already existing NETs. The treatment of mice with DNase1 15 minutes after inducing VILI was very effective because it reduced neutrophil recruitment and vascular permeability and also improved gas exchange. DNase1 is produced as a defense mechanism by bacteria53 and also naturally occurs in human blood.54 In patients with cystic fibrosis, the application of recombinant human DNAse1 is already an established and effective therapy, breaking down extracellular DNA that affects mucociliary clearance.55
In conclusion, NETs are involved in the pathogenesis of VILI, and inhibiting the simultaneous activation of neutrophils by the GPCR- and integrin engagement and thus NET formation or targeting NET components protects mice from lung injury. Therapeutic inhibition of NET formation may provide a novel therapeutic approach to ALI that should be explored in clinical trials.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dagmar Zeuschner and Karina Mildner for conducting the electron microscopy and Clifford Lowell (University of California-San Francisco) for providing the Tyrobp−/−/Fcer1g−/− mice, and Prof Hans Georg Mannherz for his helpful comments.
This work was supported by the German Research Foundation (ZA428/6-1, ZA428/8-1 [A.Z.], HE-6810/1-1 [J.H.], HU1618/1-2, WE1913/11-2 [C.W.] FOR809, SFB914-B08 [O.S., C.W.]), the European Research Council (AdG° 249949), The Netherlands Organization for Scientific Research (VIDI project 91712303 [O.S.]), and the Centre for Interdisciplinary Research (IZFK Münster, SEED 01/12 [J.R.]).
Authorship
Contribution: J.R. and J.M.H. designed and did most of the experiments, analyzed the results, and prepared the manuscript; M.N., Y.D., and O.S. provided reagents and mice and prepared the manuscript; C.W. provided reagents and funding and provided critical revision of the manuscript; M.N. provided reagents and wrote parts of the discussion; H.V.A. revised the manuscript; and A.Z. provided overall supervision, helped design all of the experiments, and prepared the manuscript
Conflict-of-interest disclosure: O.S. and C.W. are shareholders of Carolus Therapeutics, Inc. All other authors declare no competing financial interests.
Correspondence: Alexander Zarbock, University of Münster, Department of Anaesthesiology, Intensive Care and Pain Medicine, Albert-Schweitzer-Campus 1, Building A1, 48149 Münster, Germany; e-mail: zarbock@uni-muenster.de.
References
Author notes
J.R. and J.M.H. contributed equally to the study.