In this issue of Blood, Rossaint et al provide new mechanistic insights into how activated platelets signal to polymorphonuclear leukocytes (PMNs), trigger neutrophil extracellular trap (NET) formation, and contribute to inflammatory tissue damage.1
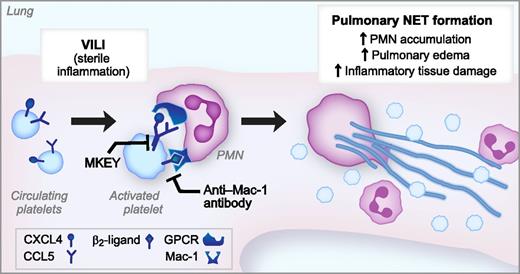
Activated platelets trigger injurious NET formation in a model of VILI. In a murine model of VILI, high-pressure mechanical ventilation activates platelets, leading to heterodimerization of CXCL4 and CCL5 as well as expression of a β2-integrin ligand. Simultaneous binding of the CXCL4/CCL5 heterodimer and β2-integrin ligand to the GPCR and Mac-1, respectively, trigger pulmonary NET formation, leading to NET-mediated lung injury. MKEY, a peptide that blocks the CXCL4/CCL5 heterodimerization, and an anti-Mac-1 blocking antibody inhibit NET formation and significantly ameliorate lung injury in this model of sterile inflammation.
Activated platelets trigger injurious NET formation in a model of VILI. In a murine model of VILI, high-pressure mechanical ventilation activates platelets, leading to heterodimerization of CXCL4 and CCL5 as well as expression of a β2-integrin ligand. Simultaneous binding of the CXCL4/CCL5 heterodimer and β2-integrin ligand to the GPCR and Mac-1, respectively, trigger pulmonary NET formation, leading to NET-mediated lung injury. MKEY, a peptide that blocks the CXCL4/CCL5 heterodimerization, and an anti-Mac-1 blocking antibody inhibit NET formation and significantly ameliorate lung injury in this model of sterile inflammation.
PMNs (neutrophils) form NETs in response to microbial tissue invasion and tissue damage.2,3 NETs are extracellular lattices of decondensed chromatin decorated with antimicrobial factors that trap and kill bacteria and fungi. Discovery of this potent and essential activity of neutrophils has reinvigorated the study of innate immunity and led to significant new discoveries regarding the mechanisms regulating NET formation and their importance in preventing overwhelming infections. Yet, a darker side of NET formation exists, with dysregulated NET formation now suggested as a pathogenic mechanism in autoimmune disorders and other inflammatory syndromes.4 The current and salient question regarding NET formation is therefore: Do NETs heal or harm? The clear answer is: Yes!
In this issue of Blood, Rossaint and colleagues provide new insights into the mechanism through which activated platelets signal to PMNs and trigger NET formation.1 The key new findings are that dysregulated NET formation participates in the pathogenesis associated with sterile inflammatory syndromes such as ventilator-induced lung injury (VILI) and that simultaneous engagement of G-protein coupled receptors (GPCR) and Mac-1 by the platelet-derived CCL5/CXCL4 heterodimer and a β2-integrin ligand, respectively, is required for robust NET formation in this model. The investigators used a physiologically relevant in vivo model of VILI and elegant in vitro experiments with human platelets and PMNs to make these discoveries, and their observations extend our information base on the biology of NET formation and the complexity of their activities in preclinical models.
Mac-1 (αMβ2, CD11b/CD18) is a member of the β2 or leukocyte integrin family that is critical for host defense but can also contribute to inflammatory injury via its adhesion and signaling functions.5 In these experiments, blocking either Mac-1 or GPCR signaling was not sufficient to inhibit NET formation by PMNs, suggesting, once again, the importance of regulating this potent component of innate immunity.6 The authors then interrupted both of these signaling pathways from platelets to PMNs and found significant amelioration of the severity of VILI in their in vivo model system. The peptide MKEY, which blocks the heterodimerization of CCL5 with CXCL4, and an anti–Mac-1 blocking antibody both decreased VILI with significant decreases in pulmonary NET formation, PMN accumulation, edema, and inflammatory tissue damage (see figure).
These observations are important for 3 reasons. First, these findings confirm preliminary data in other models suggesting that NET formation in response to sterile tissue injury without a component of acute or chronic infection can cause harm.4 Indeed, this represents the exact scenario in which NET formation might be disadvantageous and perpetuate inflammatory tissue damage through externalization of NET-associated degradative enzymes. Strategies, such as blocking GPCR and β2-integrin signaling, that are aimed at limiting NET formation in such scenarios warrant further exploration.
Second, these studies generate new mechanistic insights into the communication between activated platelets and PMNs and the complex regulatory network tasked with ensuring that NET formation occurs at the right time, in the right place, and with the appropriate magnitude. The platelet-derived CCL5/CXCL4 heterodimer and β2-integrin ligand signaling now represent new targets for study in the areas of dysregulated NET formation in inflammation, autoimmunity, and thrombosis. Furthermore, while disruption of these outside-in, platelet-to-PMN signaling pathways may suggest future therapeutic strategies for syndromes of sterile inflammation, MKEY and Mac-1 blocking antibodies represent reagents of immediate benefit in determining how dysregulated NET formation contributes to pathogenesis of other inflammatory syndromes such as sepsis, pneumonia, autoimmune diseases, and disorders of thrombosis. In vivo model systems for all of these disease states exist where MKEY could be tested in preclinical models to determine whether NET inhibition “heals” or “harms.” As a corollary, while signaling to NET formation through interactions of activated platelets with neutrophils is not the only way that NET formation is triggered, it may be a particularly important mechanism in vascular injury syndromes.7
Finally, at face value, the suggestion that inhibition of NET formation in the setting of acute infection might decrease inflammatory tissue damage without increasing the microbial load and allowing infection to spread unchecked seems dubious. Indeed, initial in vivo experiments using DNase to dismantle NETs in models of polymicrobial sepsis resulted in hypersusceptibility to infection.8,9 Nevertheless, an emerging body of evidence suggests that strategies aimed at inhibiting NET formation in the setting of severe sepsis or systemic inflammatory response syndrome may improve outcomes and increase survival.4 The studies by Rossaint et al, which establish the peptide MKEY and anti-Mac-1 antibodies as molecules capable of arresting NET formation by interrupting outside-in signaling from platelets to PMNs, represent a first step toward the development of the “ideal” inhibitor of NET formation: a neutrophil-specific, NET-inhibitory agent that preserves PMN respiratory burst, phagocytosis, intracellular bacterial killing, and other antimicrobial functions.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal