Abstract
TAM receptors (Tyro3, Axl, and Mer) belong to a family of receptor tyrosine kinases that have important effects on hemostasis and inflammation. Also, they affect cell proliferation, survival, adhesion, and migration. TAM receptors can be activated by the vitamin K–dependent proteins Gas6 and protein S. Protein S is more commonly known as an important cofactor for protein C as well as a direct inhibitor of multiple coagulation factors. To our knowledge, the functions of Gas6 are limited to TAM receptor activation. When activated, the TAM receptors have effects on primary hemostasis and coagulation and display an anti-inflammatory or a proinflammatory effect, depending on cell type. To comprehend the effects that the TAM receptors and their ligands have on hemostasis and inflammation, we compare studies that report the different phenotypes displayed by mice with deficiencies in the genes of this receptor family and its ligands (protein S+/−, Gas6−/−, TAM−/−, and variations of these). In this manner, we aim to display which features are attributable to the different ligands. Because of the effects TAM receptors have on hemostasis, inflammation, and cancer growth, their modulation could make interesting therapeutic targets in thromboembolic disease, atherosclerosis, sepsis, autoimmune disease, and cancer.
Introduction
In recent years, views on hemostasis and inflammation have shifted from a concept of 2 independent areas of biology toward 2 closely related processes. It is now appreciated that molecules that affect hemostasis often have an effect on inflammation and vice versa. In this review, we explore this dual roles of Gas6, protein S, and TAM receptors. TAM receptors are 1 of 20 subfamilies of receptor tyrosine kinases.1 First cloned in 1991, they were considered orphan receptors until 1995.2 In that year, their ligands, protein S and growth arrest–specific gene 6 (Gas6), were identified.3-5 Members of the TAM receptor family are Tyro3 (also called Brt, Dtk, Etk-2, Rek, Rse, Sky, and Tif), Axl (also called Ark, Tyro7, and Ufo), and Mer (also called c-Eyk, Mertk, Nyk, and Tyro12). TAM receptors comprise 2 immunoglobulin-like and 2 fibronectin type III repeats in their extracellular domains in tandem. This is connected to a single-pass transmembrane domain and a cytoplasmic protein tyrosine kinase (Figure 1A). Upon ligand binding, the receptor dimerizes and the tyrosine kinase becomes activated.6 In recent years, several signaling functions of TAM receptors have been described, such as stimulation of cell growth and proliferation, inhibition of apoptosis,7,8 mediation of efferocytosis,9 stimulation of hemostasis,10 and modulation of inflammation.11 In this review, we will focus on the functions of the TAM receptors pertaining to hemostasis and inflammation. When activated by Gas6, TAM receptors stimulate hemostasis by facilitating platelet stabilization.10 The other ligand, protein S, has a TAM-independent inhibitory effect on hemostasis.12-14 Activation of the TAM receptors was found to inhibit Toll-like receptor (TLR) signaling, to induce phagocytosis, and to stimulate natural killer cell development, leading to speculations about a role in preventing autoimmunity.
The structure of the Tyro3/Axl/Mer receptor. (A) The N-terminal starts with 2 Ig-like domains, followed by 2 fibronectin type 3 domains, followed by a single-pass transmembrane domain and a protein tyrosine kinase at the C-terminal. (B) The structure of the TAM ligands protein S and Gas6. The N-terminal contains a GLA domain, followed by a thrombin-sensitive region (TSR), followed by 4 EGF-like domains, followed by a C-terminal (SHBG-like domain, consisting of 2 LG repeats. EGF, epidermal growth factor; Ig, immunoglobulin; LG, laminin G; SHBG, sex hormone–binding globulin.
The structure of the Tyro3/Axl/Mer receptor. (A) The N-terminal starts with 2 Ig-like domains, followed by 2 fibronectin type 3 domains, followed by a single-pass transmembrane domain and a protein tyrosine kinase at the C-terminal. (B) The structure of the TAM ligands protein S and Gas6. The N-terminal contains a GLA domain, followed by a thrombin-sensitive region (TSR), followed by 4 EGF-like domains, followed by a C-terminal (SHBG-like domain, consisting of 2 LG repeats. EGF, epidermal growth factor; Ig, immunoglobulin; LG, laminin G; SHBG, sex hormone–binding globulin.
This review delineates the known functional similarities and differences between protein S, Gas6, and the TAM receptors. The recently described knockout mice for protein S, Gas6, and the individual TAM receptors have strongly contributed to the new insights in this field. By comparing the phenotypes of the different knockout mice, we discuss the functions of protein S that are attributable to TAM receptor activation and those functions that are the effect of protein S alone.
Protein S
Protein S is a vitamin K–dependent protein encoded by the PROS1 gene in humans and by Pros1 in mice. Unlike genes encoding for most vitamin K–dependent factors, the PROS1 gene is also expressed in other tissues than the liver: transcription of PROS1 can be found in the kidney, lungs, or gonads. Protein S is produced by a variety of cell types (eg, hepatocytes, endothelial cells, megakaryocytes, osteoblasts).15 It contains an amino terminal γ carboxyglutamic acid (GLA) domain, followed by a thrombin-sensitive loop region and 4 epidermal growth factor–like domains ending with the carboxy-terminal (C-terminal), consisting of 2 laminin G repeats that together comprise the sex hormone–binding globulin domain (Figure 1B).16 The C-terminal region is sufficient for TAM receptor binding and phosphorylation.17
Protein S circulates in plasma at a concentration of 346 nmol/L18 and serves as an anticoagulant by working as a nonenzymatic cofactor for activated protein C in the breakdown of coagulation factors (F) Va and FVIIIa.12 It is further capable of binding FXa and FVa directly in that it can autonomously inhibit coagulation.13,14,19-21 Factor Xa is also inhibited by protein S through acting as a cofactor for tissue factor pathway inhibitor.22 In humans, it exists in a free active form (30% to 40%) and in an (almost) inactive form bound to C4b-binding protein (60% to 70%).23 It is therefore plausible that in human protein S is apt to affect the complement system.24 In mice, however, protein S exists only in its free form because the murine C4b–binding protein lacks the β-chain that is essential for binding protein S.25 The functional consequences of this difference remain unknown.
Heterozygous deficiency of PROS1 is associated with an elevated risk for developing thrombosis,26,27 whereas homozygous deficiency is incompatible with life or leads to neonatal purpura fulminans in rare cases.28
Last, protein S has been identified as a ligand for the TAM receptors in addition to Gas6. It has been shown capable of binding Tyro3 (eg, in osteoclasts).29 In retinal pigment, epithelium protein S has been described to be equally important and interchangeable with Gas6 in vivo as a Mer ligand.30 Affinity between protein S and Axl, however, has never been shown. Protein S binding to Tyro3 and Mer shows a high degree of species specificity. Peculiar is that human protein S shows only weak or no affinity for the different human TAM receptors, whereas bovine protein S displays good affinity to human Tyro3 (reviewed by Hafizi31 ).
Gas6
Gas6 is a 75-kDa vitamin K–dependent protein first discovered under conditions of growth arrest in embryonic mouse NIH 3T3 fibroblasts.32,33 It has high structural homology (∼42%) with protein S and the modular composition is the same (as described previously and shown in Figure 1B). Unlike in protein S, the thrombin-sensitive region in Gas6 (a disulfide-bridged thumb loop) does not seem to be susceptible to cleavage by the action of serine proteases. The concentration of Gas6 is around 20-50 ng/mL (0.25 nmol/L) in plasma, and elevated to about 110 ng/mL in severe sepsis patients.34,35 These levels are much lower than those of the other vitamin K–dependent proteins of plasma. Another difference with other vitamin K–dependent proteins is that Gas6 is barely produced in the liver, but instead in the heart, kidneys, and lungs. Important tissues where Gas6 is expressed are endothelial cells,33 vascular smooth muscle cells,7 and bone marrow.36 Gas6 has been shown to be present in murine platelets,37,38 but this presence in humans has been debated. Evidence does suggest that human platelets will aggregate upon TAM activation by Gas6.39
There have been no reports in literature of cases with homo- or heterozygous deficiency of the GAS6 gene. Certain haplotypes of the GAS6 gene seem to have a protective role in the development of stroke.40
Gas6 binds the TAM receptors with different affinities: Axl ≥ Tyro3 >> Mer.5 Functions of Gas6 seem to be limited to those caused by activation of the TAM receptors. These functions are dealt with in the next paragraphs.
TAM receptors
Expression of the individual TAM receptors can be found in many cell types, but the patterns vary. Tyro3 is mostly found in the central nervous system, kidneys, ovaries, and testes.41,42 Axl is nearly ubiquitously expressed in most human cells originating from hematopoietic, epithelial, and mesenchymal sources.43 Mer is predominantly expressed in ovaries, testes, prostate, lungs, and kidneys and to a lesser extent in the thymus, spleen, liver, small intestine, colon, and placenta.44,45
Important cell types in which TAM receptors are active are, for example, antigen-presenting cells,46 monocytes, and natural killer cells in the immune system 44 ; osteoclasts in bone28 ; Sertoli cells in the testis 47 ; endothelial cells and vascular smooth muscle cells in the vasculature 48 ; and pigmental epithelium cells in the retina.49 In contrast, they are not expressed in granulocytes or blood lymphocytes.50 In tumor cells, TAM receptors are often upregulated (reviewed by Linger51 ).
Along with this wide expression of TAM receptors, many functions can be described. This review will not discuss all of these functions, but will focus on hemostasis and inflammation.
With respect to hemostasis, all 3 TAM receptors are located on platelets and mediate thrombogenesis and platelet stabilization. Platelet stabilization occurs after integrin activation, granule secretion, and platelet aggregation through platelet-to-platelet contact. Without this mechanism, platelet plugs disaggregate prematurely (reviewed by Prevost52 ). Important downstream mechanisms in platelets include increased granule secretion, activation of PI3K, and phosphorylation of β3 integrin, leading to an increase in outside-in signaling via the αIIbβ3 integrin (Figure 2).10,39 Also, vascular Gas6 upregulates tissue factor in vascular cells when vessel injury occurs, leading to activation of the extrinsic coagulation pathway and thrombus formation.53 Gas6 is released from mouse platelets,37,38 but human platelets do not seem to contain Gas6.34,35 Besides, only expression of the Mer receptor on human platelets has been shown.54 Still, Gas6 levels in plasma were higher in patients with venous thromboembolic disease as compared with healthy volunteers.55 Also, genetic evidence shows an association between certain single nucleotide polymorphisms in the GAS6 gene and stroke,56 making involvement of TAM receptor in human, and not only murine, hemostasis likely.
TAM-mediated platelet stabilization and leukocyte adhesion. ADP and Gas6 increase expression of αIIbβ3 integrin via PI3K/Akt. After binding to fibrinogen, granule secretion is elevated by outside-in signaling. TAM receptor phosphorylation also leads to increased expression of P-selectin, which binds to PSGL-1 on leukocytes, and increased expression of adhesion molecules ICAM-1 and VCAM-1 by endothelial cells, also stimulating sequestration of leukocytes. Gas6 upregulates tissue factor in endothelial cells upon vessel injury (not depicted), leading to activation of the extrinsic coagulation pathway. ADP, adenosine diphosphate; PI3K, phosphatidylinositol 3-kinases; PSGL-1, P-selectin glycoprotein ligand-1.
TAM-mediated platelet stabilization and leukocyte adhesion. ADP and Gas6 increase expression of αIIbβ3 integrin via PI3K/Akt. After binding to fibrinogen, granule secretion is elevated by outside-in signaling. TAM receptor phosphorylation also leads to increased expression of P-selectin, which binds to PSGL-1 on leukocytes, and increased expression of adhesion molecules ICAM-1 and VCAM-1 by endothelial cells, also stimulating sequestration of leukocytes. Gas6 upregulates tissue factor in endothelial cells upon vessel injury (not depicted), leading to activation of the extrinsic coagulation pathway. ADP, adenosine diphosphate; PI3K, phosphatidylinositol 3-kinases; PSGL-1, P-selectin glycoprotein ligand-1.
Activation of the TAM receptors by Gas6 amplifies pro-inflammatory endothelial cell (EC) activation, leading to expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). In platelets and ECs, TAM receptor phosphorylation leads to increased expression of P-selectin. P-selectin glycoprotein ligand-1 on leukocytes binds to P-selectin. The enhanced expression of adhesion molecules induces sequestration of platelets and leukocytes to ECs and each other. Hereby, the TAM receptors support leukocyte extravasation and inflammation and adhesion of platelets to endothelial cells.57 Protein S levels are increased in atherosclerotic vessels. By activation of Mer, protein S inhibits macrophage scavenger receptor A–mediated acetylated low-density lipoprotein uptake in macrophages, thereby reducing the formation of foam cells.58 These mechanisms can possibly explain part of the recent findings that SNP mutations in TAM receptor genes are correlated with the formation of atherosclerotic plaques.59,60
Protein S is upregulated by interleukin-4 (IL-4) in primary T cells.61 Natural killer T cells require Mer to induce the transcription of IL-4 and interferon-γ (IFN-γ).62 Whether this results in a feedback loop in vivo is unknown. A cell-proliferative function of TAM signaling aids inflammation by stimulating maturation of natural killer cells.63 Similarly, in the kidney, activation of Axl promotes inflammation through increased proliferation of mesangial cells.64
In contrast to supporting the inflammatory response described previously, TAM receptor signaling inhibits inflammation by multiple mechanisms. Activation of Mer, in contrast to Axl, inhibits glomerular inflammation during glomerulonephritis.65 In antigen-presenting cells, TAM receptor signaling inhibits lipopolysaccharide (LPS)-induced cytokine production (eg, tumor necrosis factor-α [TNF-α]).11 Activation of cytokine receptors leads to an IFN-α/β receptor (IFNAR)/signal transducer and activator of transcription 1 (STAT1) upregulation of Axl (Figure 3). Together with the IFNAR/STAT1 signaling cassette, the TAM receptors induce the transcription of the anti-inflammatory suppressor of cytokine signaling protein 1 (SOCS1) and SOCS3 and inhibit both cytokine receptors and TLR signaling pathways.66,67 The decreased expression of these anti-inflammatory mediators in TAM−/− cells seems to point to a crucial role of SOCS proteins in the anti-inflammatory action of TAM receptors. However, a direct dependence of TAM anti-inflammatory function on SOCS has not yet been proven. TAM activation also induces Twist transcriptional repressors that suppress nuclear factor-κB (NF-κB)-dependent transcription.68 TLR signaling in its turn suppresses Gas6 and protein S expression via NF-κB in macrophages.69 Administration of recombinant Gas6 in a murine sepsis model results in less mortality because of reduced neutrophil migration.70
Effects of TAM receptors on inflammation. IFN-α induces TAM receptor expression. TAM signaling usurps the IFNAR/STAT1 cassette to inhibit TLR and JAK signaling via SOCS1 and SOCS3. TAM activation induces Twist, which suppresses NF-κB–dependent transcription reducing pro-inflammatory cytokine production. NF-κB inhibits GAS6 and protein S expression. ASK, apoptosis signal-regulating kinase; IFN-α, interferon-α; IRAK, interleukin-1 receptor–associated kinase; IRF, interferon regulatory factor; JAK, Janus kinase; MyD88, myeloid differentiation primary response gene 88; TRAF, TNF receptor–associated factor; TRIF, TIR domain–containing adapter-inducing IFN-β.
Effects of TAM receptors on inflammation. IFN-α induces TAM receptor expression. TAM signaling usurps the IFNAR/STAT1 cassette to inhibit TLR and JAK signaling via SOCS1 and SOCS3. TAM activation induces Twist, which suppresses NF-κB–dependent transcription reducing pro-inflammatory cytokine production. NF-κB inhibits GAS6 and protein S expression. ASK, apoptosis signal-regulating kinase; IFN-α, interferon-α; IRAK, interleukin-1 receptor–associated kinase; IRF, interferon regulatory factor; JAK, Janus kinase; MyD88, myeloid differentiation primary response gene 88; TRAF, TNF receptor–associated factor; TRIF, TIR domain–containing adapter-inducing IFN-β.
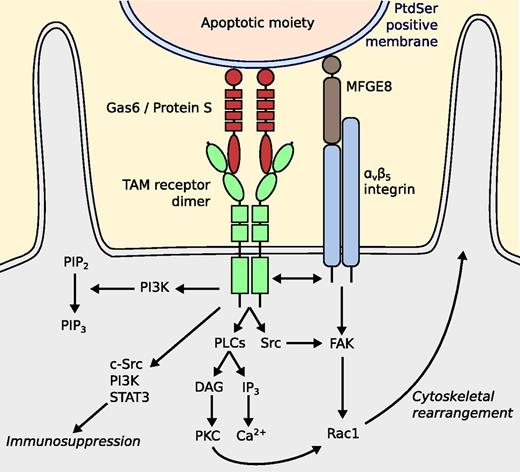
Another important anti-inflammatory mechanism is that TAM receptors enhance phagocytosis of apoptotic cells, also known as efferocytosis (Figure 4).71 Gas6 and protein S bind to phosphatidylserine-positive moieties with their N-terminal GLA domain (γ-carboxyglutamic domain).9,72,73 The C-terminal binds to Mer on macrophages and Axl and Tyro3 on dendritic cells,46 causing the intracellular kinase to phosphorylate.74-77 Although the exact signaling cascades remain unknown, a variety of signaling molecules has been shown to be relevant, such as PI3K, phospholipase Cγ2, Src family kinases, and interactions with the αvβ5 integrin. Rac1 is responsible for cytoskeletal rearrangement.77,78 Protection of the blood–brain barrier integrity also occurs through cytoskeletal rearrangement of brain endothelium by Rac1. It has been shown that this can be mediated by ligation of protein S to Tyro3, after which the protective sphingosine 1–phosphate receptor is activated.79
Putative model for TAM-mediated efferocytosis. The GLA domains of protein S and Gas6 bind to the phosphatidylserine-positive cell membrane of an apoptotic moiety. The SHBG domains bind to TAM receptors, which causes phosphorylation of the intracellular protein tyrosine kinase. Phosphorylated by the kinase, PI3K induces phosphorylation of PIP2 to PIP3, which facilitates phagocytosis. TAM receptor activation stimulates phospholipase Cγ2, leading to enhanced PKC activity. It has also been suggested that a Src family kinase is activated, resulting in recruitment of FAK, functionally cross-talking with αvβ5 integrin. It has also been suggested that a complex consisting of c-Src, PI3K, and STAT3 is established by Mer phosphorylation. This complex then inhibits inflammation in DCs. DAG, diacylglycerol; DC, dendritic cell; FAK, focal adhesion kinase; IP3, inositol trisphosphate; MFGE8, milk fat globule-EGF factor 8; PIP2, phosphatidylinositol (4,5)-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-triphosphate; PKC, protein kinase C; PLC, phospholipase C.
Putative model for TAM-mediated efferocytosis. The GLA domains of protein S and Gas6 bind to the phosphatidylserine-positive cell membrane of an apoptotic moiety. The SHBG domains bind to TAM receptors, which causes phosphorylation of the intracellular protein tyrosine kinase. Phosphorylated by the kinase, PI3K induces phosphorylation of PIP2 to PIP3, which facilitates phagocytosis. TAM receptor activation stimulates phospholipase Cγ2, leading to enhanced PKC activity. It has also been suggested that a Src family kinase is activated, resulting in recruitment of FAK, functionally cross-talking with αvβ5 integrin. It has also been suggested that a complex consisting of c-Src, PI3K, and STAT3 is established by Mer phosphorylation. This complex then inhibits inflammation in DCs. DAG, diacylglycerol; DC, dendritic cell; FAK, focal adhesion kinase; IP3, inositol trisphosphate; MFGE8, milk fat globule-EGF factor 8; PIP2, phosphatidylinositol (4,5)-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-triphosphate; PKC, protein kinase C; PLC, phospholipase C.
Accumulated apoptotic cell debris that exists when efferocytosis is impaired contains a variety of autoantigens that may cause lupus-like autoimmunity. Whether TAM receptors are involved in the etiology of lupus erythematosus (SLE) remains unknown. An association between decreased protein S levels and SLE has been described,80 whereas elevated Gas6 levels are associated with disease activity in SLE.81 Mutations in the murine and human genes coding for Mer lead to impaired phagocytosis by retinal pigment epithelial cells. This leads to cumulation of photoreceptor outer segments and retinal degeneration, causing retinitis pigmentosa in humans.82 This Mer-dependent signaling is mediated by both Gas6 and protein S as equally important ligands.30
TAM receptors do not mediate phagocytosis of bacteria, yeast, or latex particles.74,83 However, viral entry into target cells is facilitated by Axl by apoptotic mimicry of the viral envelope.84
Recently, new ligands for TAM receptor–mediated efferocytosis have been described: Tubby, tubby-like protein 1 (Tulp1),85 and galectin-3.86 Tubby and galectin-3 specifically bind to Mer, whereas Tulp1 can activate all 3 of the TAM receptors. Whether these new ligands can affect other mechanisms besides phagocytosis through TAM activation remains to be elucidated.
Knockout mice
Single, dual, and triple knockout mice for the Tyro3/Axl/Mer receptors have been described extensively, as have Gas6-deficient mice. Protein S knockout mice, however, had not been described until recently. The various phenotypes exhibited by the different knockout mice, with effects on hemostasis, inflammation, and other systems, are summarized in Table 1. As can be seen in this table, knockout mice for protein S, Gas6, and TAM receptors show a large variety of phenotypes. Protein S−/− mice have a lethal coagulopathy and vascular malformation. The latter is most probably the result of impaired blood flow caused by thrombi and—because the malformation is more severe than one would expect caused just by impaired blood flow—it could speculated to be due to insufficient Axl signaling. However, protein S/Axl binding has never been determined. Meanwhile, protein S has been shown to induce proliferation of vascular smooth muscle cells by receptor activation, a function known to be attributed to Axl when activated by Gas6.87
Summary of phenotypical effects on hemostasis, inflammation, and other systems as seen in various knockout mice
| Knockout type . | Effects on . | ||
|---|---|---|---|
| Hemostasis . | Inflammation . | Other . | |
| Protein S−/− | • Death by coagulopathy with macroscopic blood clots and extensive hemorrhages between E15.5 and E17.5. | Not described | • Vascular development is defective (caused by thrombosis and reduced protein S–dependent Axl signaling).88 |
| • Intravascular and interstitial fibrin depositions. | |||
| • Increased amounts of megakaryocytes in the liver, suggesting peripheral thrombocytopenia.88,93 | |||
| Protein S+/− | • 44% decrease in protein S levels. | Not described | • Defects in vascular development.88 |
| • 53% decrease in APC cofactor activity. | |||
| • FVa-based clotting time is shortened, thrombin generation is elevated, the lag time for thrombin generation is shortened.88,93 | |||
| Protein S−/− in hepatocytes (Alb-Cre/protein Sfl/fl) | • 15% show fibrin depositions in blood vessels. | Not described | Not described |
| • 55% decrease in protein S levels. | |||
| • 47% decrease in APC cofactor activity.88 | |||
| Protein S−/− in endothelial and hematopoietic cells (tie2-Cre/protein Sfl/fl) | • Fibrin depositions in blood vessels (but less severe than in Alb-Cre/protein S1fl/fl). | Not described | Not described |
| • 43% decrease in protein S levels. | |||
| • 49% decrease in APC cofactor activity.88 | |||
| Protein S−/− in vascular smooth muscle cells (Sm22-Cre/protein Sfl/fl/Gas6−/−) | Not described | Not described | • Vascular defects leading to permeation into liver parenchyma.88 |
| Gas6−/− | • The mice are protected against venous and arterial thrombosis but do not display spontaneous bleeding (caused by platelet dysfunction).38 | • Reduced inflammation and reduced myofibroblast activation in the steatotic liver, reducing liver fibrosis.94 | • Elevated vascular permeability.38 |
| • Reduced sequestration of platelets onto endothelium. | • Endothelial cells express less VCAM-1 and ICAM-1 when stimulated with TNF-α than WT. | • Less oligodendrocytes and microglial activation after demyelination.98 | |
| • Reduced thrombosis in models of endotoxinemia and vasculitis.57 | • Reduced sequestration of platelets onto endothelium, of leukocytes onto endothelium, and of platelets to leukocytes. | ||
| • Reduced expression and activity of tissue factor in vascular cells.53 | • Reduced leukocyte extravasation and inflammation in endotoxinemia, vasculitis, and heart transplantation.57 | ||
| • More hypoxia-induced cell death and higher IL-1β and TNF-α expression in murine macrophages.95 | |||
| • More graft-versus-host disease when receiving liver transplantation.96 | |||
| • Less mortality and proteinuria in accelerated nephrotoxic nephritis than in WT mice.64 | |||
| • More stable atherosclerotic plaques by increased fibrosis and fewer macrophages.97 | |||
| Gas6−/−, protein S−/− in retinal cells Prosfl/-/Nes-Cre/Gas6−/−) | Not described | Not described | • Blindness from impaired phagocytosis of photoreceptor outer segments by retinal pigment epithelial cells.31 |
| Tyro3/Axl/Mer−/− | • Recurrent thrombosis and hemorrhages in several tissues (including the brain), associated with the presence of antibodies to phospholipids as seen in autoimmune syndromes.50 | • After ∼4 weeks, spleens and lymph nodes enlarge. | • Spermatogenesis in males defected. |
| • Impaired hemostasis, thrombocytopenia resulting from platelet dysfunction and megakaryocytopoiesis.99 | • After 1 year, the spleens are about 10 times the normal size. | • Testes one third of WT size. | |
| • Hyperproliferation of constitutively activated B and T cells (the latter slightly more). | • Blindness from impaired phagocytosis of photoreceptor outer segments by retinal pigment epithelial cells. | ||
| • Ectopic lymphocytes in every researched organ. | • Young adults: diminished hippocampal long-term potential. | ||
| • Clinical manifestations mimic autoimmune diseases similar to rheumatoid arthritis, pemphigus vulgaris, and SLE. | • Aged: neural degeneration with seizures and paralysis.47 | ||
| • T cells express elevated amounts of IL-2 receptor and lectin CD69. | |||
| • B cells express Fas, CD44, and IFN-γ. | |||
| • Vascular endothelia express ICAM-I. Increased antibody titers can be found on double-stranded DNA, collagen, cardiolipin, phosphatidytidylserine, phosphatidylethanolamine, and phosphatidylinositol. | |||
| • Macrophages produce high levels of IL-12 and MHCII is strongly increased. | |||
| • When given LPS intraperitoneally, LPS-induced TNF-α response doubles in comparison with WT. | |||
| • Inactivation of Mer contributes the most to the previously mentioned scenario.50 | |||
| • Immature NK cell development.63 | |||
| Tyro3−/− | • Reduced thrombus formation. | Not described | • Young adults: diminished hippocampal long-term potential. |
| • Initial platelet aggregation is not reduced, but stabilization of the aggregates is, because of a decrease in outside-in signaling and platelet granule secretion.10 | • Aged: neural degeneration with seizures and paralysis.47 | ||
| Axl−/− | • Reduced thrombus formation. | • Increase in apoptosis in response to flow reduction in carotid artery. | • Elevated vascular permeability. |
| • Initial platelet aggregation is not reduced, but stabilization of the aggregates is, because of a decrease in outside-in signaling and platelet granule secretion.10 | • Impaired vascular remodeling: Increase in CD45+ cells and decrease in VSMC, macrophages, and neutrophils.100 | • Impaired vascular remodeling.100 | |
| • Enhanced inflammation in the CNS because of delayed removal of myelin debris during experimental autoimmune encephalomyelitis.101 | |||
| Merkd or Mer−/− | • Reduced thrombus formation. | • Delayed cell clearance of infused apoptotic cells. | • Blindness from impaired phagocytosis of photoreceptor outer segments by retinal pigment epithelial cells.30,49,82 |
| • Initial platelet aggregation is not reduced, but stabilization of the aggregates is, because of a decrease in outside-in signaling and platelet granule secretion.10,54 | • Animals develop a SLE-like autoimmunity with antibodies to chromatin, DNA, and IgG.74-102 | ||
| • Mice show increased susceptibility and death in response to endotoxic shock. | |||
| • Monocytes stimulated with LPS express more NF-κB and produce more TNF-α.11 | |||
| • NK T cells have a defect in in vivo GC-α–stimulated production of IL-4 and IFN-γ.62 | |||
| • Enhanced B-cell responses in splenic marginal zone.103 | |||
| • Increased migration of macrophages, DCs, plasmacytoid DCs, T cells, and B cells into the peritoneal cavity.104 | |||
| • Increased renal inflammation in nephrotoxic serum–induced nephritis.65 | |||
| • Decreased induction of c-Src and STAT3.105 | |||
| Knockout type . | Effects on . | ||
|---|---|---|---|
| Hemostasis . | Inflammation . | Other . | |
| Protein S−/− | • Death by coagulopathy with macroscopic blood clots and extensive hemorrhages between E15.5 and E17.5. | Not described | • Vascular development is defective (caused by thrombosis and reduced protein S–dependent Axl signaling).88 |
| • Intravascular and interstitial fibrin depositions. | |||
| • Increased amounts of megakaryocytes in the liver, suggesting peripheral thrombocytopenia.88,93 | |||
| Protein S+/− | • 44% decrease in protein S levels. | Not described | • Defects in vascular development.88 |
| • 53% decrease in APC cofactor activity. | |||
| • FVa-based clotting time is shortened, thrombin generation is elevated, the lag time for thrombin generation is shortened.88,93 | |||
| Protein S−/− in hepatocytes (Alb-Cre/protein Sfl/fl) | • 15% show fibrin depositions in blood vessels. | Not described | Not described |
| • 55% decrease in protein S levels. | |||
| • 47% decrease in APC cofactor activity.88 | |||
| Protein S−/− in endothelial and hematopoietic cells (tie2-Cre/protein Sfl/fl) | • Fibrin depositions in blood vessels (but less severe than in Alb-Cre/protein S1fl/fl). | Not described | Not described |
| • 43% decrease in protein S levels. | |||
| • 49% decrease in APC cofactor activity.88 | |||
| Protein S−/− in vascular smooth muscle cells (Sm22-Cre/protein Sfl/fl/Gas6−/−) | Not described | Not described | • Vascular defects leading to permeation into liver parenchyma.88 |
| Gas6−/− | • The mice are protected against venous and arterial thrombosis but do not display spontaneous bleeding (caused by platelet dysfunction).38 | • Reduced inflammation and reduced myofibroblast activation in the steatotic liver, reducing liver fibrosis.94 | • Elevated vascular permeability.38 |
| • Reduced sequestration of platelets onto endothelium. | • Endothelial cells express less VCAM-1 and ICAM-1 when stimulated with TNF-α than WT. | • Less oligodendrocytes and microglial activation after demyelination.98 | |
| • Reduced thrombosis in models of endotoxinemia and vasculitis.57 | • Reduced sequestration of platelets onto endothelium, of leukocytes onto endothelium, and of platelets to leukocytes. | ||
| • Reduced expression and activity of tissue factor in vascular cells.53 | • Reduced leukocyte extravasation and inflammation in endotoxinemia, vasculitis, and heart transplantation.57 | ||
| • More hypoxia-induced cell death and higher IL-1β and TNF-α expression in murine macrophages.95 | |||
| • More graft-versus-host disease when receiving liver transplantation.96 | |||
| • Less mortality and proteinuria in accelerated nephrotoxic nephritis than in WT mice.64 | |||
| • More stable atherosclerotic plaques by increased fibrosis and fewer macrophages.97 | |||
| Gas6−/−, protein S−/− in retinal cells Prosfl/-/Nes-Cre/Gas6−/−) | Not described | Not described | • Blindness from impaired phagocytosis of photoreceptor outer segments by retinal pigment epithelial cells.31 |
| Tyro3/Axl/Mer−/− | • Recurrent thrombosis and hemorrhages in several tissues (including the brain), associated with the presence of antibodies to phospholipids as seen in autoimmune syndromes.50 | • After ∼4 weeks, spleens and lymph nodes enlarge. | • Spermatogenesis in males defected. |
| • Impaired hemostasis, thrombocytopenia resulting from platelet dysfunction and megakaryocytopoiesis.99 | • After 1 year, the spleens are about 10 times the normal size. | • Testes one third of WT size. | |
| • Hyperproliferation of constitutively activated B and T cells (the latter slightly more). | • Blindness from impaired phagocytosis of photoreceptor outer segments by retinal pigment epithelial cells. | ||
| • Ectopic lymphocytes in every researched organ. | • Young adults: diminished hippocampal long-term potential. | ||
| • Clinical manifestations mimic autoimmune diseases similar to rheumatoid arthritis, pemphigus vulgaris, and SLE. | • Aged: neural degeneration with seizures and paralysis.47 | ||
| • T cells express elevated amounts of IL-2 receptor and lectin CD69. | |||
| • B cells express Fas, CD44, and IFN-γ. | |||
| • Vascular endothelia express ICAM-I. Increased antibody titers can be found on double-stranded DNA, collagen, cardiolipin, phosphatidytidylserine, phosphatidylethanolamine, and phosphatidylinositol. | |||
| • Macrophages produce high levels of IL-12 and MHCII is strongly increased. | |||
| • When given LPS intraperitoneally, LPS-induced TNF-α response doubles in comparison with WT. | |||
| • Inactivation of Mer contributes the most to the previously mentioned scenario.50 | |||
| • Immature NK cell development.63 | |||
| Tyro3−/− | • Reduced thrombus formation. | Not described | • Young adults: diminished hippocampal long-term potential. |
| • Initial platelet aggregation is not reduced, but stabilization of the aggregates is, because of a decrease in outside-in signaling and platelet granule secretion.10 | • Aged: neural degeneration with seizures and paralysis.47 | ||
| Axl−/− | • Reduced thrombus formation. | • Increase in apoptosis in response to flow reduction in carotid artery. | • Elevated vascular permeability. |
| • Initial platelet aggregation is not reduced, but stabilization of the aggregates is, because of a decrease in outside-in signaling and platelet granule secretion.10 | • Impaired vascular remodeling: Increase in CD45+ cells and decrease in VSMC, macrophages, and neutrophils.100 | • Impaired vascular remodeling.100 | |
| • Enhanced inflammation in the CNS because of delayed removal of myelin debris during experimental autoimmune encephalomyelitis.101 | |||
| Merkd or Mer−/− | • Reduced thrombus formation. | • Delayed cell clearance of infused apoptotic cells. | • Blindness from impaired phagocytosis of photoreceptor outer segments by retinal pigment epithelial cells.30,49,82 |
| • Initial platelet aggregation is not reduced, but stabilization of the aggregates is, because of a decrease in outside-in signaling and platelet granule secretion.10,54 | • Animals develop a SLE-like autoimmunity with antibodies to chromatin, DNA, and IgG.74-102 | ||
| • Mice show increased susceptibility and death in response to endotoxic shock. | |||
| • Monocytes stimulated with LPS express more NF-κB and produce more TNF-α.11 | |||
| • NK T cells have a defect in in vivo GC-α–stimulated production of IL-4 and IFN-γ.62 | |||
| • Enhanced B-cell responses in splenic marginal zone.103 | |||
| • Increased migration of macrophages, DCs, plasmacytoid DCs, T cells, and B cells into the peritoneal cavity.104 | |||
| • Increased renal inflammation in nephrotoxic serum–induced nephritis.65 | |||
| • Decreased induction of c-Src and STAT3.105 | |||
APC, antigen-presenting cell; CNS, central nervous system; MHC, major histocompatibility complex; NK, natural killer; VSMC, vascular smooth muscle cell; WT, wild-type.
Protein S knockouts with cell-specific Cre drivers give new insights into protein S production: endothelial and hematopoietic cells produce 43% of the circulating protein S, and local production of protein S in vascular smooth muscle cells is important for the vascular formation because Sm22-Cre/protein Sfl/fl knockout mice show increased vascular permeability.
Gas6 knockout mice exhibit a reduction of thrombus formation, improved survival when challenged with a thrombotic stimulus, vascular defects, and reduced liver inflammation. Knocking out any of the TAM receptors separately also protects mice against thrombosis.
When only Tyro3 is knocked out, the mice develop neurological disorders. Axl−/− mice have vascular defects (such as protein S and Gas6 knockouts), impaired vascular remodeling after hemodynamic stress, and an increased inflammatory response in the central nervous system caused by reduced debris removal. Mer knockout mice show many autoimmune-like features. This is probably from the loss of the inhibitory effect of Mer on the NF-κB pathway, leading to excessive amounts of pro-inflammatory cytokines, and because of the loss of efferocytosis. Triple TAM knockout mice seem to combine all individual phenotypes (Tyro3−/−, Axl−/−, and Mer−/−) and show a more severe phenotype (especially regarding inflammation).
Discussion
In this review of the literature, we have systematically explored the phenotypes of TAM/protein S/Gas6-deficient mice. Gas6, protein S, and the TAM receptors have effects on primary hemostasis and coagulation and display an anti-inflammatory or a pro-inflammatory effect, depending on cell type and even on the receptor. Besides describing these effects and their underlying mechanisms, the other goal of this review is to hypothesize about which functions of TAM receptors are attributable to protein S. Because of the severe impairment of the hemostatic function in protein S knockouts, it is difficult to assess the TAM-mediated effects in these mice. The disorders in vascular development seen are too severe to only be caused by coagulopathy. Although functional phenotypes in protein S–deficient mice may be hidden by redundant actions of Gas6,30 the local synthesis of protein S seems to have a role in the vascular development.88 Because mice lacking Axl suffer from similar underdeveloped blood vessels, this could imply locally produced protein S is required for activating Axl.
Because affinity between protein S and Axl has never been shown, it might be caused by protein S via a different mechanism than Axl activation. A recent study describes how protein S can inhibit vascular endothelial growth factor receptor-A–dependent EC migration, mitosis, and signaling via activation of Mer.89 Although by this mechanism, protein S–deficient mice would have increased vascular proliferation, one could hypothesize that an imbalance of proliferative and antiproliferative mechanisms could result in vascular malformation—especially because the Gas6/Axl stimulatory effect and the protein S/Mer inhibitory effect on vascular proliferation are both mediated by phosphorylation of SHP2. Apart from the vascular malformation, no other potential TAM receptor–mediated effects could be identified based on the experiments with protein S knockout mice.
Comparing Gas6 knockout mice with TAM receptor knockouts is another way to analyze the effects of Gas6-independent TAM activation. Specifically, the lupus-like autoimmune syndrome present in triple TAM knockout mice is less pronounced in Gas6−/− mice. This indicates that, in Gas6 knockout mice, another ligand fulfills the inhibitory task within the immune system, possibly protein S. This concurs with the findings that protein S plays an equivalent role to Gas6 in the efferocytosis stimulated by the TAM receptors74 and that SLE patients may show lower protein S levels and/or anti-protein S antibodies.87,90 However, the newly described ligands Tubby, Tulp1, and Galectin-3 are also likely candidates that could explain this difference between the phenotypes.
The physiological role of Gas6 has been deducted mainly in GAS6−/− mice, which are viable and able to reproduce. Until now, no humans have been identified with a total Gas6 deficiency, so either Gas6 deficiency has no major clinical consequences in man, or—although less probable—total Gas6 deficiency is not compatible with life in humans. Unlike human protein S, human Gas6 is a high-affinity ligand for all human TAM receptors with Kd in the nanomolar range (reviewed by Hafizi31 ). Redundancy with protein S in its function to activate Mer may actually mask important functions of Gas6, as has been demonstrated in the retina, in which locally produced protein S is as potent as Gas6 in activating Mer.30 Therefore, much remains to be elucidated on protein S–mediated TAM receptor activation.
Furthermore, certain molecular properties of the ligands require attention. The effect of multimerization of Gas6 and protein S on TAM receptor binding and activation is not completely clear. Upon purification, a major part of protein S is in a multimerized form with increased phospholipid-binding properties.91 Similar high-molecular-weight multimeres of protein S have been observed in plasma and reported to possess equal anticoagulant properties as the monomers.92 Membranes that contain phosphatidylserine serve as a scaffold for the auto-oxidation of Cys residues in protein S, which promotes the oligomerization of protein S required for Mer-dependent apoptotic cell clearance.73 This oligomerization may also provide a mechanism that allows the abundant protein S to activate Mer when necessary and not constitutively. Whether oligomerization of protein S increases the affinity (single bond interaction) or the avidity (combined strength of multiple bond interactions) with TAM receptors is still to be determined as well as whether murine protein S oligomerizes as human protein S does. Further research should be performed to elucidate when and how oligomerization is required for TAM-mediated functions of protein S and Gas6.
The importance of systemic circulating protein S to TAM receptor activation is unclear. Complete deletion of protein S results in embryonic malformation and thrombosis in protein S knockout mice. Furthermore, deletion of either hepatic or endothelial/hematopoietic production of protein S leads to ∼50% reduction in systemic levels and fibrin formation in blood vessels.89 Thus, it seems that to clarify the role of circulatory protein S as a TAM receptor ligand, it will be necessary to generate an animal with mutant protein S, which only displays anticoagulant activity and no TAM receptor–activating activity. This could potentially be obtained by the introduction of sequences in the PROS1 gene that would delete or mutate the SHBG-like region of protein S. Another way to generate an animal devoid of systemic TAM-activating protein S would be to silence the residual hepatic protein S production by small interfering RNA in tie2-Cre/protein Sfl/fl animals, which would then have to be supplemented pharmacologically with protein S that lacks the SHBG domain or protein S with an inactive SHBG domain to adequate anticoagulant levels. To generate such mice would be a major task and the relevance with regard to human disease could be questioned. However, such an approach may provide proof-of-principle data regarding the overall importance of nonredundant TAM-related protein S functions. A similar approach would be needed with a Gas6−/− background to investigate the TAM-related functions of protein S that are redundant with Gas6.
From a clinical perspective, further research in this field could provide therapeutic options for important diseases. A TAM receptor antagonist or a Gas6 antagonist could provide protection against thrombosis without a bleeding diathesis. Because vitamin K antagonists also inhibit Gas6 (and protein S), beside FII, FVII, FIX, and FX, anticoagulant effects of these drugs might partially also be caused by reduced stimulation of TAM receptors. The recent findings of a reduction of mortality in septic mice given recombinant Gas6, even after onset of the disease, brings a new potential supportive therapy for the critically ill. This also raises the question whether usage of vitamin K antagonists in septic patients might increase mortality. Further research could also provide more insight and possible therapeutic options for autoimmune diseases such as SLE, and, although not discussed in this review, cancer growth. That the described receptors and their ligands’ functions lie within 3 general groups of disease (hemostasis, inflammation, and cancer growth) should alert researchers to creating possible adverse effects when developing drugs. However, considering this information, it will be of great interest to see what the coming 2 decades of TAM receptor research will bring to medicine.
Authorship
Contribution: J.H.M.v.d.M., T.v.d.P., and C.v.V. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan H.M. van der Meer, Academic Medical Center, University of Amsterdam, Center for Experimental and Molecular Medicine, Meibergdreef 9, Room G2-132, 1105 AZ Amsterdam, The Netherlands; e-mail: j.h.vandermeer@amc.uva.nl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal