Key Points
Imatinib-resistant/-intolerant patients with chronic myeloid leukemia in chronic phase can experience long-term benefit with dasatinib.
Early (3- and 6-month) molecular and cytogenetic responses were associated with improved progression-free survival and overall survival.
Abstract
We present long-term follow-up of a dasatinib phase 3 study of patients with imatinib-resistant/-intolerant chronic myeloid leukemia (CML). In the CA180-034 study, 670 patients with imatinib-resistant/-intolerant CML in chronic phase (CML-CP) received dasatinib 100 mg once daily, 50 mg twice daily, 140 mg once daily, or 70 mg twice daily. At 6 years, 188 (28%) of 670 patients remained on study treatment. Estimated 6-year protocol-defined progression-free survival (PFS) rates were 49%, 51%, 40%, and 47%, respectively, and estimated 6-year overall survival (OS) rates were 71%, 74%, 77%, and 70%, respectively (intent-to-treat population, including protocol-defined progression or death after discontinuation). Estimated 6-year rates of survival without transformation on study treatment were 76%, 80%, 83%, and 74%, respectively. Major molecular response was achieved in 43% (100 mg once daily) and 40% (all other arms) of patients by 6 years. Molecular and cytogenetic responses at 3 and 6 months were highly predictive of PFS and OS. Notably, estimated 6-year PFS rates based on ≤1%, >1% to 10%, and >10% BCR-ABL transcripts at 3 months were 68%, 58%, and 26%, respectively. Most adverse events occurred by 2 years. Imatinib-resistant/-intolerant patients with CML-CP can experience long-term benefit with dasatinib therapy, particularly if achieving BCR-ABL ≤10% at 3 months. This study was registered at ClinicalTrials.gov: NCT00123474.
Introduction
Chronic myeloid leukemia (CML) is characterized by the presence of a constitutively active tyrosine kinase, BCR-ABL, which drives the malignant phenotype of leukemic stem cells. Treatment of CML generally relies on BCR-ABL tyrosine kinase inhibitors.1-7 Dasatinib, a potent oral BCR-ABL inhibitor, is approved as first-line therapy for newly diagnosed patients with CML in the chronic phase (CML-CP), and as second-line therapy for patients with any phase CML or Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia with resistance or intolerance to prior therapy, including imatinib.8 The approved dosing regimen for imatinib-resistant or -intolerant patients with CML-CP was changed from dasatinib 70 mg twice daily to 100 mg once daily based on the results of an ongoing phase 3 dose-optimization study (CA180-034).8-10 After 2 years, dasatinib 100 mg once daily maintained efficacy similar to other dasatinib dosing regimens while minimizing toxicity.10
Identifying early and meaningful predictors of long-term outcomes in patients with CML-CP receiving first-line or second-line BCR-ABL inhibitor therapy is critically important to inform changes in therapy and to minimize adverse outcomes.11-14 In this analysis of the CA180-034 study, which represents the longest follow-up of patients with CML-CP treated with a second-generation BCR-ABL inhibitor, we have evaluated early molecular and cytogenetic responses for predicting long-term progression-free survival (PFS) and overall survival (OS) with dasatinib in imatinib-resistant or -intolerant patients. We have also continued to analyze efficacy and safety of dasatinib 100 mg once daily in this heavily pretreated population.
Methods
Study design and patient eligibility
Study design and eligibility criteria have been described previously.9,10 CA180-034 is a randomized, phase 3 dose-optimization study in adults with CML-CP who were resistant to or intolerant of imatinib. Patients were randomly assigned to dasatinib 100 mg once daily, 50 mg twice daily, 140 mg once daily, or 70 mg twice daily. To manage inadequate response or adverse events (AEs), the protocol allowed dose escalation (up to a total daily dose of 180 mg) or dose interruption or reduction (down to a total daily dose of 20 mg). Additionally, the protocol allowed switching from a twice-daily to a once-daily regimen with the same total daily dose after at least 1 dose reduction for recurrent anemia, thrombocytopenia, neutropenia, pleural effusion, or any other fluid retention (any grade) at the investigator’s discretion. Treatment was administered until protocol-defined disease progression or death, unacceptable toxicity, or patient/investigator request to stop threatment.9 The protocol defined progression as increasing white blood cell count, loss of complete hematologic response (CHR) or major cytogenetic response (MCyR), ≥30% increase in Ph+ metaphases, or transformation to accelerated phase (AP) or blast phase (BP) disease. Thus, PFS in this protocol (which considered events of protocol-defined progression or death from any cause) is similar but not identical to what the European LeukemiaNet (ELN) and previous studies have reported as event-free survival.3,15 Patients who had MCyR and subsequently no longer met the criteria for MCyR after starting their maximum dose of dasatinib were considered to have lost MCyR. Progression (including loss of MCyR) was assessed by the investigator. This study was approved by local ethics committees and conducted in accordance with the Declaration of Helsinki, its amendments, and Good Clinical Practice. All patients provided written, informed consent.
Evaluations and analyses
Efficacy and safety assessments have been described previously.9 Major molecular response (MMR) was reported as a percentage (number of responders divided by the intent-to-treat population). PFS and OS were estimated by using Kaplan-Meier product-limit methodology. Efficacy analyses, with the exception of landmark analyses, included randomly assigned patients. For landmark analyses, patients not assessed at 3 or 6 months were not included in the corresponding analyses. Safety analyses included treated patients, and AEs were reported as cumulative incidence.
BCR-ABL transcripts were measured by using blood samples collected at baseline, once per month (first 3 months), every 3 months (from 3 months to 2 years), at 2.5 years, yearly thereafter, and at the end of assigned treatment. Molecular data were collected in 1 central laboratory (Clinical Biomarker Development lab at Bristol-Myers Squibb) through the 2-year measurement, after which they were collected by MolecularMD (Portland, OR). Standard conversion factors for each laboratory (4.72 and 0.81, respectively) were used to express BCR-ABL transcripts on the International Scale. MMR was defined as BCR-ABL ≤0.1%.3
PFS was defined as time from random assignment until progression (described in the “Study design and patient eligibility” section). For PFS analyses, patients who had not died or otherwise progressed were censored at their last cytogenetic or hematologic assessment to ensure that laboratory data supported lack of progression. OS was defined as time from random assignment until death. For OS analyses, patients who had not died and patients lost to follow-up were censored on the last date they were known to be alive. Patients were considered to be off study treatment if they discontinued study treatment and off study if they died, were lost to follow-up, or withdrew informed consent. Patients who were off study treatment but on study were observed for PFS and OS. Protocol-defined progression (assessed by the investigator) and death after discontinuation of study treatment were included in the analyses to the extent these were reported. The number of patients with known progression status at 5 years, known progression status at 6 years, known survival status at 5 years, and known survival status at 6 years is presented in the “Results” section. Reason for progression, such as transformation to AP or BP, was recorded during study treatment only; therefore, analyses involving transformation were restricted accordingly. The cumulative incidence of transformation to AP or BP on study treatment and death as a result of disease was analyzed with the following competing risks: discontinuation, death for any reason other than disease, and progression (as defined by the protocol) for a reason other than transformation to AP or BP.
Landmark analyses estimated 6-year rates of PFS, OS, and survival without transformation on study according to molecular and cytogenetic responses at 3 and 6 months by using the Kaplan-Meier method. The effects of imatinib resistance, baseline mutation status, and baseline response on the PFS landmark analyses were investigated. PFS and OS were also analyzed on the basis of CHR and BCR-ABL at 3 months, after excluding patients with CHR or BCR-ABL ≤10% at baseline. Time to progression was estimated in patient subgroups that achieved BCR-ABL ≤10% at 3, 6, 12, or >12 months. Discontinuation of study treatment according to 3-month molecular response or baseline mutation status was also investigated. Mutational analyses were conducted as described in earlier reports.9,10
Significance thresholds for efficacy analyses were not prespecified. Comparisons were for exploratory purposes; therefore, P values were not adjusted for multiple comparisons. AEs during the 6 years of follow-up were graded on the basis of the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 and recorded as worst-grade occurrences. Hematologic abnormalities were assessed throughout the duration of treatment.
Results
Patient disposition
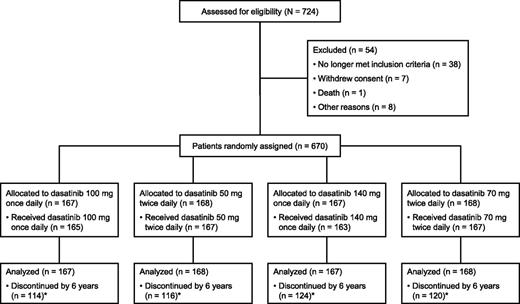
Of 724 patients enrolled from July 2005 to March 2006, 670 were randomly assigned and 662 were treated, as previously described (Figure 1).9 Most patients (74%) were imatinib-resistant; the remaining patients were imatinib-intolerant. Baseline characteristics were similar among treatment arms.9,10 All data are reported according to a database lock of approximately 72 months. Median duration of therapy was 29.3 months overall (range, 0.1 to 78.0 months; n = 662): 28.6 months in imatinib-resistant patients (range, 0.1 to 77.7 months; n = 490), and 31.2 months in imatinib-intolerant patients (range, 0.2 to 78.0 months; n = 172). At the time of analysis, 188 (28%) of 670 patients remained on study treatment (Table 1). Assessment of dosing by last dose available showed that 144 (77%) of 188 patients received once-per-day dosing; of these 144 patients, 60 (42%) received 100 mg, 22 (15%) received >100 mg, and 62 (43%) received <100 mg. Of the 167 patients randomly assigned to receive 100 mg once daily, 51 (31%) were still receiving treatment at the time of analysis. Of these 51 patients, 28 (55%) received 100 mg, 5 (10%) received >100 mg, and 17 (33%) received <100 mg.
CONSORT diagram for the CA180-034 study. One of the 167 patients treated with dasatinib 50 mg twice daily had been randomly assigned to receive 100 mg once daily. Adapted from Shah et al9 and reproduced with permission from the American Society of Clinical Oncology. (*) Reasons for discontinuation are presented in Table 1.
CONSORT diagram for the CA180-034 study. One of the 167 patients treated with dasatinib 50 mg twice daily had been randomly assigned to receive 100 mg once daily. Adapted from Shah et al9 and reproduced with permission from the American Society of Clinical Oncology. (*) Reasons for discontinuation are presented in Table 1.
Patient disposition
| . | Treated patients . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 mg once daily (n = 165) . | 50 mg twice daily (n = 167) . | 140 mg once daily (n = 163) . | 70 mg twice daily (n = 167) . | All arms (n = 662) . | ||||||
| . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . |
| On treatment | 51 | 31 | 51 | 31 | 39 | 24 | 47 | 28 | 188 | 28 |
| Reason for discontinuation | ||||||||||
| Disease progression* | 34 | 21 | 26 | 16 | 42 | 26 | 26 | 16 | 128 | 19 |
| Study drug toxicity | 34 | 21 | 40 | 24 | 42 | 26 | 48 | 29 | 164 | 25 |
| Patient or investigator request | 24 | 15 | 21 | 13 | 23 | 14 | 19 | 11 | 87 | 13 |
| Adverse event unrelated to drug | 7 | 4 | 8 | 5 | 4 | 2 | 6 | 4 | 25 | 4 |
| Other† | 15 | 9 | 21 | 13 | 13 | 8 | 21 | 13 | 70 | 11 |
| . | Treated patients . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 mg once daily (n = 165) . | 50 mg twice daily (n = 167) . | 140 mg once daily (n = 163) . | 70 mg twice daily (n = 167) . | All arms (n = 662) . | ||||||
| . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . |
| On treatment | 51 | 31 | 51 | 31 | 39 | 24 | 47 | 28 | 188 | 28 |
| Reason for discontinuation | ||||||||||
| Disease progression* | 34 | 21 | 26 | 16 | 42 | 26 | 26 | 16 | 128 | 19 |
| Study drug toxicity | 34 | 21 | 40 | 24 | 42 | 26 | 48 | 29 | 164 | 25 |
| Patient or investigator request | 24 | 15 | 21 | 13 | 23 | 14 | 19 | 11 | 87 | 13 |
| Adverse event unrelated to drug | 7 | 4 | 8 | 5 | 4 | 2 | 6 | 4 | 25 | 4 |
| Other† | 15 | 9 | 21 | 13 | 13 | 8 | 21 | 13 | 70 | 11 |
Discontinuations at up to 73.9 months are reflected. Reasons for discontinuation were reported by the investigator on the case report form.
Progression was defined as increasing white blood cell count, loss of complete hematologic response, loss of major cytogenetic response, ≥30% increase in Ph+ metaphases, or transformation to AP or BP disease.
Includes commercial supply, travel requirements, transplant, noncompliance, avoidance of toxicity, development of mutation, pregnancy, no response (as reported by the investigator), death unrelated to drug, other malignancy, and other reasons.
Efficacy rates
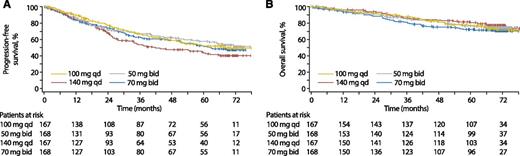
Efficacy is summarized in Table 2, with PFS and OS rates over time in Figure 2. Protocol-defined PFS and OS rates were calculated on an intent-to-treat basis (for all randomly assigned patients) and included events after study treatment discontinuation, as assessed by the investigator and to the extent reported. At 5 years, PFS status was available for 477 patients, and 113 additional patients were being observed but were censored per protocol (because they were off study treatment and had not progressed). Only 80 patients were no longer being observed at 5 years. Therefore, 5-year PFS rates could be estimated reliably based on the 6-year database lock. Six-year PFS rates were also estimated on the basis of the more limited number of patients with PFS data at 6 years. PFS and OS rates were comparable among all arms at 5 and 6 years, with no statistically significant differences.
Efficacy and outcome rates
| . | Treated patients . | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 mg once daily . | 50 mg twice daily . | 140 mg once daily . | 70 mg twice daily . | |||||||||||||||||||||
| All (n = 167) . | Res (n = 124) . | Int (n = 43) . | All (n = 168) . | Res (n = 124) . | Int (n = 44) . | All (n = 167) . | Res (n = 123) . | Int (n = 44) . | All (n = 168) . | Res (n = 126) . | Int (n = 42) . | |||||||||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| MMR by 6 y | 71 | 43 | 50 | 40 | 21 | 49 | 67 | 40 | 43 | 35 | 24 | 55 | 67 | 40 | 37 | 30 | 30 | 68 | 68 | 40 | 47 | 37 | 21 | 50 |
| Patients with protocol-defined PFS status at 5 y (ie, not censored at ≤5 y) | 123 | 74 | — | — | 114 | 68 | — | — | 118 | 71 | — | — | 122 | 73 | — | — | ||||||||
| Protocol-defined PFS at 5 y | 52 | 50 | 58 | 58 | 56 | 61 | 44 | 35 | 71 | 52 | 50 | 59 | ||||||||||||
| 95% CI | 43-60 | 40-59 | 41-75 | 49-66 | 46-66 | 45-77 | 35-52 | 26-45 | 55-87 | 44-61 | 40-60 | 41-77 | ||||||||||||
| Patients with protocol-defined PFS status at 6 y (ie, not censored at ≤6 y) | 80 | 48 | — | — | 80 | 48 | — | — | 93 | 56 | — | — | 83 | 49 | — | — | ||||||||
| Protocol-defined PFS at 6 y | 49 | 46 | 58 | 51 | 50 | 53 | 40 | 33 | 67 | 47 | 45 | 55 | ||||||||||||
| 95% CI | 41-58 | 36-56 | 41-75 | 42-61 | 40-61 | 33-73 | 31-49 | 23-42 | 49-84 | 38-56 | 35-55 | 37-73 | ||||||||||||
| Patients with OS status at 5 y (ie, not censored at ≤5 y) | 143 | 86 | — | — | 135 | 80 | — | — | 132 | 79 | — | — | 139 | 83 | — | — | ||||||||
| OS at 5 y | 77 | 75 | 82 | 76 | 74 | 82 | 80 | 78 | 87 | 72 | 69 | 81 | ||||||||||||
| 95% CI | 70-84 | 67-83 | 70-94 | 69-83 | 65-82 | 71-94 | 74-87 | 70-86 | 77-98 | 65-79 | 61-78 | 68-94 | ||||||||||||
| Patients with OS status at 6 y (ie, not censored at ≤6 y) | 77 | 46 | — | — | 75 | 45 | — | — | 67 | 40 | — | — | 72 | 43 | — | — | ||||||||
| OS at 6 y | 71 | 69 | 78 | 74 | 73 | 79 | 77 | 73 | 87 | 70 | 67 | 81 | ||||||||||||
| 95% CI | 64-79 | 60-77 | 64-92 | 67-81 | 64-81 | 66-92 | 70-84 | 65-82 | 77-98 | 63-78 | 58-76 | 68-94 | ||||||||||||
| . | Treated patients . | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 mg once daily . | 50 mg twice daily . | 140 mg once daily . | 70 mg twice daily . | |||||||||||||||||||||
| All (n = 167) . | Res (n = 124) . | Int (n = 43) . | All (n = 168) . | Res (n = 124) . | Int (n = 44) . | All (n = 167) . | Res (n = 123) . | Int (n = 44) . | All (n = 168) . | Res (n = 126) . | Int (n = 42) . | |||||||||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| MMR by 6 y | 71 | 43 | 50 | 40 | 21 | 49 | 67 | 40 | 43 | 35 | 24 | 55 | 67 | 40 | 37 | 30 | 30 | 68 | 68 | 40 | 47 | 37 | 21 | 50 |
| Patients with protocol-defined PFS status at 5 y (ie, not censored at ≤5 y) | 123 | 74 | — | — | 114 | 68 | — | — | 118 | 71 | — | — | 122 | 73 | — | — | ||||||||
| Protocol-defined PFS at 5 y | 52 | 50 | 58 | 58 | 56 | 61 | 44 | 35 | 71 | 52 | 50 | 59 | ||||||||||||
| 95% CI | 43-60 | 40-59 | 41-75 | 49-66 | 46-66 | 45-77 | 35-52 | 26-45 | 55-87 | 44-61 | 40-60 | 41-77 | ||||||||||||
| Patients with protocol-defined PFS status at 6 y (ie, not censored at ≤6 y) | 80 | 48 | — | — | 80 | 48 | — | — | 93 | 56 | — | — | 83 | 49 | — | — | ||||||||
| Protocol-defined PFS at 6 y | 49 | 46 | 58 | 51 | 50 | 53 | 40 | 33 | 67 | 47 | 45 | 55 | ||||||||||||
| 95% CI | 41-58 | 36-56 | 41-75 | 42-61 | 40-61 | 33-73 | 31-49 | 23-42 | 49-84 | 38-56 | 35-55 | 37-73 | ||||||||||||
| Patients with OS status at 5 y (ie, not censored at ≤5 y) | 143 | 86 | — | — | 135 | 80 | — | — | 132 | 79 | — | — | 139 | 83 | — | — | ||||||||
| OS at 5 y | 77 | 75 | 82 | 76 | 74 | 82 | 80 | 78 | 87 | 72 | 69 | 81 | ||||||||||||
| 95% CI | 70-84 | 67-83 | 70-94 | 69-83 | 65-82 | 71-94 | 74-87 | 70-86 | 77-98 | 65-79 | 61-78 | 68-94 | ||||||||||||
| Patients with OS status at 6 y (ie, not censored at ≤6 y) | 77 | 46 | — | — | 75 | 45 | — | — | 67 | 40 | — | — | 72 | 43 | — | — | ||||||||
| OS at 6 y | 71 | 69 | 78 | 74 | 73 | 79 | 77 | 73 | 87 | 70 | 67 | 81 | ||||||||||||
| 95% CI | 64-79 | 60-77 | 64-92 | 67-81 | 64-81 | 66-92 | 70-84 | 65-82 | 77-98 | 63-78 | 58-76 | 68-94 | ||||||||||||
MMR was recorded on study treatment and is presented as a percentage (number of responders divided by the intent-to-treat population). Protocol-defined PFS and OS rates at 5 and 6 years were estimated by using Kaplan-Meier product-limit methodology. After discontinuation of study treatment, events of protocol-defined progression (assessed by the investigator) and death were included in the analysis to the extent these were reported.
Int, imatinib-intolerant patients; Res, imatinib-resistant patients.
Kaplan-Meier analyses (all patients). (A) PFS. (B) OS. bid, twice daily; qd, once daily.
Kaplan-Meier analyses (all patients). (A) PFS. (B) OS. bid, twice daily; qd, once daily.
While protocol-defined progression (including events other than transformation) was monitored after discontinuation, the specific reason for progression was not collected after patients discontinued study treatment. Therefore, the actual rates of transformation would likely be higher than those reported here. By 6 years, 10 treated patients (9 imatinib-resistant and 1 imatinib-intolerant) in the 100 mg once-daily arm transformed to AP or BP during study treatment (2 in year 1, 2 in year 2, 4 in year 3, 1 in year 4, 0 in year 5, and 1 in year 6). Across all arms, the cumulative incidence of death by 6 years (during and after study treatment) was 24%. Disease was the most common reason for death, as reported by the investigator (supplemental Table 1). The estimated 6-year rate of survival without transformation to AP or BP on study treatment was 78% overall (76%, 100 mg once daily; 80%, 50 mg twice daily; 83%, 140 mg once daily; 74%, 70 mg twice daily). The cumulative incidence of transformation to AP or BP on study treatment and death resulting from disease by 6 years was 24% (sum of 8.0% [100 mg once daily], 6.0% [50 mg twice daily], 5.5% [140 mg once daily], and 4.8% [70 mg twice daily]) when analyzed with the following competing risks: discontinuation, death for any reason other than disease, and progression (as defined by the protocol) for a reason other than transformation to AP or BP (supplemental Figure 1). These rates should be considered minimal estimates because information regarding transformation off study treatment was not collected.
Landmark analyses
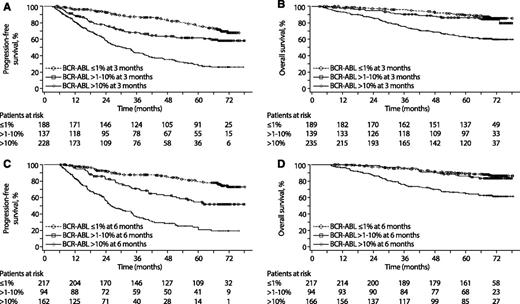
To evaluate whether early molecular and cytogenetic responses predicted long-term PFS and OS with dasatinib in this population, 4 sets of landmarks were considered: molecular response (BCR-ABL ≤1%, >1% to 10%, and >10%) and cytogenetic response (complete cytogenetic response [CCyR], partial cytogenetic response [PCyR], and neither) at 3 and 6 months (Table 3). Considering patients with molecular or cytogenetic data at the relevant time points and events of protocol-defined progression and death after the landmark time (including events off study treatment), PFS and OS rates were higher (P < .0001 comparing entire Kaplan-Meier curves) in patients with BCR-ABL ≤10% vs >10% at 3 months. PFS and OS rates over time for the various landmark groupings are provided in Figure 3 and supplemental Figure 2. The PFS landmark analyses showed a trend similar to that of the overall population in imatinib-resistant and -intolerant subgroups (supplemental Table 2). Patients who achieved optimal levels of response at 3 and 6 months, as defined by the ELN, were more likely to remain on study and less likely to discontinue because of progression (supplemental Table 3). As of the database lock, 39% of patients with BCR-ABL ≤10% at 3 months and 15% of patients with BCR-ABL >10% at 3 months remained on study treatment.
6-Year PFS and OS rates according to molecular and cytogenetic responses at 3 and 6 months
| . | At 3 months . | At 6 months . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | 6-y PFS (%) . | 95% CI . | P* . | 6-y OS (%) . | 95% CI . | P* . | No. . | 6-y PFS (%) . | 95% CI . | P* . | 6-y OS (%) . | 95% CI . | P* . | |
| Molecular response (BCR-ABL) | 565 | 477 | ||||||||||||
| ≤1% | 189 | 68 | 59-76 | .003 | 86 | 80-91 | .285 | 217 | 73 | 65-80 | .001 | 86 | 81-91 | .554 |
| >1%-10% | 139 | 58 | 49-67 | <.0001 | 84 | 78-91 | <.0001 | 94 | 52 | 41-63 | .017 | 84 | 76-91 | .001 |
| >10% | 237 | 26 | 19-33 | 60 | 53-66 | 166 | 19 | 11-27 | 61 | 53-69 | ||||
| Cytogenetic response (Ph+ metaphases) | 450 | 437 | ||||||||||||
| 0% (CCyR) | 164 | 68 | 59-77 | .172 | 87 | 81-93 | .244 | 208 | 72 | 64-80 | .063 | 86 | 81-91 | .974 |
| 1%-35% (PCyR) | 83 | 62 | 51-74 | .006 | 81 | 73-90 | .033 | 62 | 61 | 48-74 | .015 | 88 | 79-96 | .005 |
| >35% (no CCyR or PCyR) | 203 | 30 | 23-37 | 65 | 58-72 | 167 | 24 | 16-31 | 60 | 52-68 | ||||
| . | At 3 months . | At 6 months . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | 6-y PFS (%) . | 95% CI . | P* . | 6-y OS (%) . | 95% CI . | P* . | No. . | 6-y PFS (%) . | 95% CI . | P* . | 6-y OS (%) . | 95% CI . | P* . | |
| Molecular response (BCR-ABL) | 565 | 477 | ||||||||||||
| ≤1% | 189 | 68 | 59-76 | .003 | 86 | 80-91 | .285 | 217 | 73 | 65-80 | .001 | 86 | 81-91 | .554 |
| >1%-10% | 139 | 58 | 49-67 | <.0001 | 84 | 78-91 | <.0001 | 94 | 52 | 41-63 | .017 | 84 | 76-91 | .001 |
| >10% | 237 | 26 | 19-33 | 60 | 53-66 | 166 | 19 | 11-27 | 61 | 53-69 | ||||
| Cytogenetic response (Ph+ metaphases) | 450 | 437 | ||||||||||||
| 0% (CCyR) | 164 | 68 | 59-77 | .172 | 87 | 81-93 | .244 | 208 | 72 | 64-80 | .063 | 86 | 81-91 | .974 |
| 1%-35% (PCyR) | 83 | 62 | 51-74 | .006 | 81 | 73-90 | .033 | 62 | 61 | 48-74 | .015 | 88 | 79-96 | .005 |
| >35% (no CCyR or PCyR) | 203 | 30 | 23-37 | 65 | 58-72 | 167 | 24 | 16-31 | 60 | 52-68 | ||||
Patients without a molecular or cytogenetic assessment at 3 or 6 months were not included in the corresponding analyses. Also, patients who progressed (for PFS) or died (for OS) before the landmark time point were excluded from those analyses. Two of the 237 patients shown as having BCR-ABL >10% at 3 months, 1 of the 164 patients shown as having CCyR at 3 months, and 1 of the 203 patients shown as having no CCyR or PCyR at 3 months had assays between 2 and 3 months and died before 3 months.
P values compare BCR-ABL ≤1% vs >1% to 10%, BCR-ABL >1% to 10% vs >10%, CCyR vs PCyR, and PCyR vs no CCyR or PCyR by the log-rank test based on full Kaplan-Meier curves, rather than 6-year rates. P values for BCR-ABL ≤1% vs >10% at both 3 and 6 months were <.0001 for both PFS and OS. P values for CCyR vs no CCyR or PCyR at both 3 and 6 months were <.0001 for both PFS and OS.
Kaplan-Meier landmark analyses (landmark populations). (A) PFS according to molecular response at 3 months. For BCR-ABL ≤1% vs >1% to 10%, P = .003; for >1% to 10% vs >10%, P < .0001. Of note, 212 (90%) of the 235 patients with BCR-ABL >10% at 3 months in this analysis had CHR. (B) OS according to molecular response at 3 months. For BCR-ABL ≤1% vs >1% to 10%, P = .285; for >1% to 10% vs >10%, P < .0001. (C) PFS according to molecular response at 6 months. For BCR-ABL ≤1% vs >1% to 10%, P = .001; for >1% to 10% vs >10%, P = .017. (D) OS according to molecular response at 6 months. For BCR-ABL ≤1% vs >1% to 10%, P = .554; for >1% to 10% vs >10%, P = .001. Patients without a molecular assessment at 3 or 6 months were not included in the corresponding analyses. In addition, patients who progressed (for PFS) or died (for OS) before the landmark time point were excluded from those analyses.
Kaplan-Meier landmark analyses (landmark populations). (A) PFS according to molecular response at 3 months. For BCR-ABL ≤1% vs >1% to 10%, P = .003; for >1% to 10% vs >10%, P < .0001. Of note, 212 (90%) of the 235 patients with BCR-ABL >10% at 3 months in this analysis had CHR. (B) OS according to molecular response at 3 months. For BCR-ABL ≤1% vs >1% to 10%, P = .285; for >1% to 10% vs >10%, P < .0001. (C) PFS according to molecular response at 6 months. For BCR-ABL ≤1% vs >1% to 10%, P = .001; for >1% to 10% vs >10%, P = .017. (D) OS according to molecular response at 6 months. For BCR-ABL ≤1% vs >1% to 10%, P = .554; for >1% to 10% vs >10%, P = .001. Patients without a molecular assessment at 3 or 6 months were not included in the corresponding analyses. In addition, patients who progressed (for PFS) or died (for OS) before the landmark time point were excluded from those analyses.
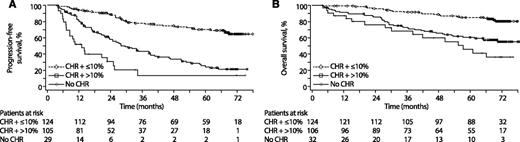
Furthermore, regardless of imatinib resistance (resistance vs resistance or intolerance), baseline mutation status (0 vs ≥1 mutations), or baseline response (CHR vs no CHR; PCyR vs no PCyR), patients had better PFS with BCR-ABL ≤10% vs >10% at 3 months (P < .001 for all cases; data not shown). In an analysis that excluded patients with CHR or BCR-ABL ≤10% at baseline, PFS and OS rates were higher in patients with CHR plus BCR-ABL ≤10% at 3 months vs those with CHR plus BCR-ABL >10%. Patients without CHR at 3 months had the worst outcome (Figure 4).
PFS and OS by CHR and molecular response at 3 months (all treatment arms combined). Analysis excludes patients with CHR or BCR-ABL ≤10% at baseline; only those patients who had not progressed (n = 258) or had not died (n = 262) by 3 months were included in (A) and (B), respectively. Four patients who had progressed but had not died by 3 months were included in (B). P < .0001 (log-rank) for CHR plus ≤10% vs CHR plus >10% at 3 months for PFS and OS. For CHR plus >10% vs no CHR at 3 months, P < .0001 (log-rank) for PFS and P = .0001 (log-rank) for OS.
PFS and OS by CHR and molecular response at 3 months (all treatment arms combined). Analysis excludes patients with CHR or BCR-ABL ≤10% at baseline; only those patients who had not progressed (n = 258) or had not died (n = 262) by 3 months were included in (A) and (B), respectively. Four patients who had progressed but had not died by 3 months were included in (B). P < .0001 (log-rank) for CHR plus ≤10% vs CHR plus >10% at 3 months for PFS and OS. For CHR plus >10% vs no CHR at 3 months, P < .0001 (log-rank) for PFS and P = .0001 (log-rank) for OS.
Estimated 6-year rates of survival without transformation to AP or BP on study treatment were 85% (95% confidence interval [CI], 78% to 92%), 88% (95% CI, 81% to 94%), and 63% (95% CI, 53% to 73%) for BCR-ABL ≤1%, >1% to 10%, and >10% at 3 months, respectively. For the same BCR-ABL levels at 6 months, rates were 87% (95% CI, 82% to 93%), 84% (95% CI, 74% to 93%), and 59% (95% CI, 45% to 72%), respectively. Regarding cytogenetic landmarks, estimated 6-year rates of survival without transformation to AP or BP on study treatment were 87% (95% CI, 80% to 94%), 89% (95% CI, 82% to 97%), and 65% (95% CI, 55% to 74%) for patients who achieved CCyR, PCyR, or neither, respectively, at 3 months; rates were 88% (95% CI, 82% to 93%), 90% (95% CI, 79% to 100%), and 61% (95% CI, 49% to 72%) based on the same cytogenetic landmarks at 6 months.
Multistate analysis
A total of 460 patients had BCR-ABL >10% at baseline, and a large subset of these patients (n = 317) had on-study molecular assessments to allow for investigating the importance of achieving BCR-ABL ≤10% by an earlier time point. A multistate analysis showed that achievement of BCR-ABL ≤10% at ≤3 months was a better predictor of longer PFS (P = .002) than achievement of BCR-ABL ≤10% at 6, 12, or >12 months.
Mutations
Response according to the presence of baseline mutations has been reported.9,10 Of the 581 patients with a mutational assessment at baseline, 369 patients had no baseline mutations, 22 patients had ≥1 dasatinib-resistant mutation (a mutation in V299, T315, or F317 of BCR-ABL), and 190 patients had dasatinib-sensitive mutations only (mutations in any residues other than V299, T315, and F317). At 6 years, none of the patients with a dasatinib-resistant mutation remained on treatment. More patients without a baseline mutation (31%) remained on treatment compared with patients who had ≥1 dasatinib-sensitive mutation (26%).
Safety
Safety data for the currently approved dose (100 mg once daily) are summarized here and in supplemental Table 4. AEs for all arms combined are presented in supplemental Table 5. Nonhematologic AEs (all grades) generally first occurred within the first 2 years of treatment when the most patients remained on study and were typically mild/moderate (grade 1/2). The most common nonhematologic AEs (all grades) occurring in ≥40% of patients were musculoskeletal pain (49%), headache (47%), infection (47%), and diarrhea (41%). The most common grade 3/4 AEs occurring in ≥5% of patients were infection (6%) and pleural effusion (5%). By 6 years, 7% of patients (100 mg once daily) and 9% of patients (all arms) discontinued treatment because of pleural effusion (supplemental Table 6). Few patients (≤2.4%, 100 mg once daily; ≤2.9%, all arms) discontinued treatment because of any other individual AE. Two cases with the term pulmonary arterial hypertension were reported, although diagnostic right-heart catheterization was not performed in either case. Grade 3/4 hematologic AEs generally first occurred within the first year of treatment.
Discussion
This analysis represents the longest reported follow-up of patients with CML-CP treated with a second-generation BCR-ABL inhibitor. Findings indicate that a consistent subgroup of CML-CP patients resistant to or intolerant of imatinib can have a long-term benefit from dasatinib therapy. In particular, those with faster and deeper responses to dasatinib (BCR-ABL ≤10% at 3 months) are more likely to have better long-term outcomes.
The range of 6-year PFS rates across treatments was 40% to 51%, with 49% in the 100 mg once-daily arm. Six-year OS rates were similar among arms (70% to 77%). The rather good survival of patients after they had a progression event indicates that alternative salvage therapies can rescue a considerable fraction of patients even in the most unfavorable (BCR-ABL >10%) group. At 6 years, 31% of patients remained on study treatment in the 100 mg once-daily arm; however, this population had a long history of CML and exposure to 1 or more prior therapies.9 Some patients had been treated with imatinib for a prolonged period despite resistance because there were no other options at the time, and such pretreatment could have possibly rendered the disease more resistant. After discontinuing study treatment, patients may have resumed treatment with commercially available dasatinib or another BCR-ABL inhibitor, although this information was not available for this analysis.
Landmark analyses that assessed the value of early responses for predicting long-term outcomes in patients receiving dasatinib as second-line BCR-ABL inhibitor therapy were performed. An ongoing concern with the use of BCR-ABL measurements is the accessibility of these tests and standardization of results in the general community. For this reason, we considered both molecular and cytogenetic landmarks. A reduction in BCR-ABL to ≤10% or PCyR/CCyR at 3 months was highly predictive of superior PFS and OS. Because CHR at 3 months was a key criterion for optimal response to first-line imatinib according to the 2009 ELN guidelines16 and because BCR-ABL ≤10% is currently considered optimal,3 we examined PFS and OS rates in patients with CHR plus BCR-ABL ≤10% vs CHR plus BCR-ABL >10% at 3 months. Among patients with CHR at 3 months, BCR-ABL transcript level was an important predictor of PFS and OS.
In the 100 mg once-daily arm, only 10 treated patients transformed to AP or BP on study treatment. Estimated 6-year rates of survival without transformation to AP or BP on study treatment were high (76%, 100 mg once daily). The main limitation of this study was the lack of data regarding transformation to AP or BP off study treatment; however, date of progression (as defined by the protocol and assessed by the investigator) was captured for many patients off study treatment. The protocol definition of PFS was determined before academic consensus on the definition and is similar but not identical to event-free survival.3,15 Because follow-up is ongoing, we cannot exclude the possibility that we may be overestimating PFS. On the basis of the 6-year database lock for this study, the data set is more complete at 5 years compared with 6 years, although the 5- and 6-year PFS estimates are generally similar.
Early response as well as baseline mutations affected rate of study continuation. More patients without baseline mutations remained on study at 6 years compared with patients with dasatinib-sensitive baseline mutations, and no patients with dasatinib-resistant mutations (defined as mutations in V299, T315, or F317) remained on study at 6 years. The association of baseline BCR-ABL mutations with early response is being explored in detail and will be reported separately.
Multiple studies have described the importance of an early response with imatinib. A long-term follow-up of patients treated with imatinib after interferon failure showed that achieving MCyR or better at 12 months had prognostic value in terms of outcomes at 10 years.17 With first-line imatinib, Hanfstein et al11 reported significantly better 5-year OS in patients who achieved early molecular or cytogenetic landmarks (BCR-ABL ≤1% or ≤10%, or Ph+ ≤35% or ≤65%, at 3 or 6 months). Similarly, Marin et al12 reported that patients with CML-CP who received first-line treatment with imatinib, followed by dasatinib or nilotinib if they failed imatinib, had improved rates of PFS, OS, CCyR, and complete molecular response (CMR) at 8 years, if they achieved BCR-ABL ≤9.84% at 3 months.
In a recent single-institution study of 119 imatinib-resistant patients receiving dasatinib, nilotinib, or bosutinib as second-line therapy for CML-CP, Milojkovic et al18 found that patients achieving BCR-ABL ≤10% at 3 months had significantly improved rates of PFS, OS, CCyR, MMR, and CMR. This analysis supports the value of early molecular and cytogenetic responses in predicting the outcome of patients treated with second-line dasatinib therapy after imatinib failure. Patients not achieving BCR-ABL ≤10% at 3 months with second-line dasatinib may face a worse outcome compared with those who achieve this milestone. However, prospective clinical studies are needed to explore the benefit of switching to an alternative BCR-ABL inhibitor at 3 months, 6 months, or a later time point. Allogeneic hematopoietic stem cell transplantation may be another reasonable alternative in eligible patients who have a suitable donor, particularly in patients receiving second or subsequent lines of therapy.
In this analysis, dasatinib was generally well tolerated, with no new safety signals identified. Two cases with the term pulmonary arterial hypertension were reported, although diagnostic right-heart catheterization was not performed in either case. Pulmonary hypertension was not specifically investigated and may be underrepresented. Both nonhematologic and hematologic AEs (all grades) typically occurred by 2 years, and dasatinib 100 mg once daily had the lowest rate of discontinuation as a result of study drug toxicity. Collectively, the data continue to support dasatinib use in patients with resistance or intolerance to imatinib and demonstrate the prognostic value of achieving BCR-ABL ≤10% and/or MCyR at 3 months.
Dasatinib 100 mg once daily offers a favorable long-term risk-benefit profile in patients with imatinib-resistant or -intolerant CML-CP. Molecular and cytogenetic responses at 3 and 6 months were highly predictive of long-term outcomes, and achievement of BCR-ABL ≤10% at 3 months was a particularly strong predictor. For the substantial proportion of patients who respond well to dasatinib, risk of progression to AP or BP is low.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank all participating study sites for this Bristol-Myers Squibb–sponsored study. They wish to thank David Dejardin from Bristol-Myers Squibb for data analyses and statistical input.
This work was supported by Bristol-Myers Squibb (with additional funding to Pamela Barendt, at StemScientific for writing and editorial support) and by a Leukemia and Lymphoma Society Scholar in Clinical Research grant (N.P.S.). The authors did not receive financial compensation from Bristol-Myers Squibb for authoring the manuscript.
Authorship
Contribution: N.P.S. and P.l.C. designed the research; N.P.S., F.G., J.E.C., C.A.S., P.l.C., T.H.B., H.M.K., A.H., P.R., D.H., M.C., and G.S. enrolled patients and/or collected data; N.P.S., F.G., J.E.C., C.A.S., P.l.C., H.M.K., A.H., H.M., and D.H. analyzed and interpreted data; and all authors wrote and/or critically reviewed the manuscript and agreed upon the final version.
Conflict-of-interest disclosure: N.P.S. has consulted for and received research funding from Bristol-Myers Squibb (BMS) and Ariad Pharmaceuticals. F.G. has consulted for Pfizer and BMS and has received honoraria from Pfizer, BMS, and Novartis. J.E.C. has consulted for Ariad Pharmaceuticals, BMS, Pfizer, and Teva and has received research funding from Novartis, Ariad Pharmaceuticals, BMS, Pfizer, and Teva. C.A.S. has consulted for Novartis, BMS, Celgene, Teva, and Eisai, received honoraria from BMS, and received research funding from Celgene, Novartis, Ariad Pharmaceuticals, BMS, and Pfizer. P.l.C. has received honoraria from Novartis, BMS, and Pfizer and has received research funding from Novartis. T.H.B. has consulted for BMS, Novartis, Pfizer, and Ariad Pharmaceuticals, has received honoraria from BMS, Novartis, and Pfizer, and has received research funding from Novartis. H.M.K. has received research funding from BMS, Novartis, Ariad Pharmaceuticals, and Pfizer. A.H. has received research funding from BMS, Novartis, Pfizer, Ariad Pharmaceuticals, and MSD. P.R. has received research funding from BMS, Novartis, and Ariad Pharmaceuticals. H.M., D.H., and M.C. are employees of BMS and declare competing financial interest. G.S. has acted as a consultant for and received honoraria from BMS, Novartis, Ariad Pharmaceuticals, and Celgene.
Correspondence: Neil P. Shah, The Helen Diller Family Comprehensive Cancer Center at University of California at San Francisco, 505 Parnassus Ave, Suite M1286, Box 1270, San Francisco, CA 94143; e-mail: nshah@medicine.ucsf.edu.