In this issue of Blood, Yao and colleagues report that the morphogen sonic hedgehog (Shh) is driven by platelet-derived growth factor B (PDGF-BB) in vascular smooth muscle cells, contributing to vessel maturation in an autocrine manner.1
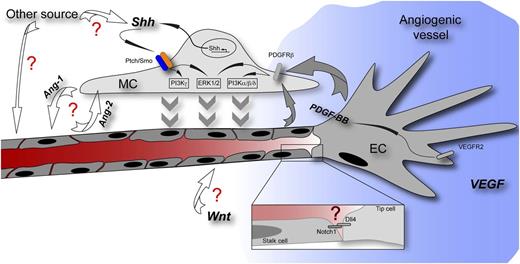
Scheme of an angiogenic vessel showing a tip cell responding to a VEGF gradient (blue) and followed by stalk cells of the growing vessel. Yao et al describe the function of Shh for MC recruitment to the stabilizing vessel. The question marks in red highlight pathways with which Shh might interact herein as well as other potential sources and functions of Shh. Ang-1/-2, angiopoietin-1/-2; Dll4, delta-like 4; PDGFRβ, platelet-derived growth factor receptor β; VEGFR2, vascular endothelial growth factor receptor 2; Wnt, Wnt growth factors.
Scheme of an angiogenic vessel showing a tip cell responding to a VEGF gradient (blue) and followed by stalk cells of the growing vessel. Yao et al describe the function of Shh for MC recruitment to the stabilizing vessel. The question marks in red highlight pathways with which Shh might interact herein as well as other potential sources and functions of Shh. Ang-1/-2, angiopoietin-1/-2; Dll4, delta-like 4; PDGFRβ, platelet-derived growth factor receptor β; VEGFR2, vascular endothelial growth factor receptor 2; Wnt, Wnt growth factors.
The hedgehog (Hh) pathway has originally been identified in Drosophila melanogaster as a gene affecting segment polarity during larval development.2 In the vascular system, initial evidence for a role of Shh was provided by the ectopic overexpression of Shh in the dorsal neural tube beyond the developmental phase of dorsoventral patterning, showing “prominent vasculature and hemorrhages” around the “hyperplastic spinal cord.”3 Since then, the Hh pathway, particularly via its ligand Shh, was shown to have mainly a proangiogenic and arterializing function during embryonic development as well as in diverse models of pathological and regenerative angiogenesis.4 The effect of Shh turned out to be mediated mostly by inducing various angiogenic factors in mesenchymal and vascular smooth muscle cells, among which vascular endothelial growth factor (VEGF), angiopoietin-1, and angiopoietin-2 are the most prominent ones.4 Nevertheless, there have been reports that the Shh ligand can elicit a signaling response also directly in endothelial cells, contributing to blood-brain barrier integrity in brain vessels and to angiogenesis in a Rho-kinase–dependent manner, respectively.5,6
In the absence of Hh ligands (indian hedgehog, desert hedgehog, or Shh), patched (Ptch) represses smoothened (Smo), thereby inhibiting Hh signaling transduction from the plasma membrane to the cytoplasm. Upon Hh binding to Ptch, its repressive function on Smo is released, thereby activating transcription via members of the glioma-associated homologs (Gli) of Kruppel family zinc-finger–containing transcription factors.
Particularly, Shh, which is named after Sega’s jump ‘n’ run character and is the best characterized of the 3 mammalian homologs, became the prototype of a morphogen, eliciting its function along gradients in a concentration-dependent manner.
In the vascular system, there is growing evidence that Shh can induce “canonical” (ie, transcriptional) signaling not only via Gli but also via transcription-independent pathways.7 Particularly, the chemotactic effect of Shh on mesenchymal cells was shown to be independent of Gli. In this regard, it is important to note that the signaling quality downstream of Shh and Ptch seems to be determined by the subcellular localization of Smo. Specifically, Smo recruitment to primary cilia fosters Gli transcription, whereas Smo outside of cilia confers signaling via G proteins, affecting cytoskeletal rearrangement and motility.7
For angiogenesis, vascular differentiation, and arterialization, the recruitment of mural cells (MCs) is crucial not only to gain vascular stability during development but also to normalize tumor vessels during pathological angiogenesis. In these conditions, Shh appears to act at different cellular sites and signaling platforms, affecting vessel formation and MC proliferation. However, the role of Shh in MCs during the establishment of an arterial vessel identity has been poorly characterized so far.
The paper by Yao et al now establishes Shh as an autocrine factor for MC motility, fostering chemotactic movement toward the PDGF-BB–releasing endothelium. PDGF-BB in turn acts upstream of Shh in MCs during this process, and both pathways feed into the phosphatidylinositol 3-kinase (PI3K) and extracellular signal-regulated kinase 1/2 (ERK1/2) cascades via specific kinase isoforms.1 Herein, Shh appears to play the role of a delayed reinforcement of the PI3K and ERK1/2 signal, possibly retaining pathway activity on an elevated level when the PDGF-BB boost has already vanished in the haze (see figure).
When putting these novel findings in the context of published data, they intriguingly fit to the described arterializing role of Shh that was suggested to act via upregulation of VEGF in somites that in turn leads to Notch pathway activity in the endothelium.4 Yao et al describe that VEGF-induced PDGF-BB release from the endothelium activates Shh expression in MCs so that one could envision a self-sustaining circle leading to vascular stability (see figure). Although this aspect was not touched on by Yao et al, a maintenance function in the vasculature might be suggestive, because Shh is important for blood-brain barrier integrity, which also relies on PDGF-BB–mediated pericyte recruitment.8,9
Besides the insufficiently understood cell-autonomous effects of Hh in the endothelium, these findings raise a number of important questions. For example, with which players other then PDGF-BB, such as angiopoietin/Tie, Dll4/Notch, and transforming growth factor β, does Shh interact during MC recruitment? Because the Wnt/β-catenin pathway has been suggested to regulate PDGF-BB in the endothelium, leading to MC recruitment to tumor vessels,10 it remains to be deciphered if this would contribute to the PDGF-BB–driven function of Shh in MCs observed by Yao et al. Furthermore, the contribution of other sources of Shh (ie, astrocytes in the brain) to MC recruitment needs to be clarified (see figure).
In conclusion, the study by Yao et al has successfully exposed that autocrine Shh signaling in MCs is crucial for their recruitment to endothelial cells and consequently for vessel stability. Future studies are required to determine whether targeting the Shh pathway in MCs might have beneficial effects in diseases involving pathologically increased or decreased MC recruitment, such as in regenerative and tumor angiogenesis.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal