In this issue of Blood, McDermott et al present the intriguing, clinically relevant, and perhaps unexpected findings for the efficacy and safety of long-term administration of low-dose plerixafor treatment of patients with warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome.1
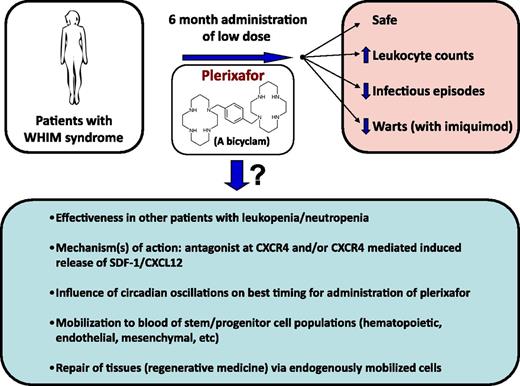
Schematic of results from the McDermott et al study,1 and questions emanating from the results.
Schematic of results from the McDermott et al study,1 and questions emanating from the results.
WHIM is a rare disorder of primary immunodeficiency associated with warts, hypogammaglobulinemia, retention of neutrophils in the bone marrow, and recurring bacterial infections. Until this study (Clinical Trials.gov identifier: NCT00967785),1 there was no treatment that allowed for long-term control of infections and warts in this syndrome. The rationale for using plerixafor (also known as AMD3100 and Mozobil) was to take advantage of the peripheral blood neutrophil and other leukocyte-mobilizing capacity of AMD3100/plerixafor to increase numbers of leukocytes in the circulation2 to alleviate some symptoms of this disease. This was a reasonable approach as a majority of patients with WHIM have an autosomal-dominant mutation of CXCR4, a chemokine receptor, believed to increase intracellular signaling that allows retention of neutrophils and other leukocytes at their tissue sites. The leukocyte-mobilizing effects of CXCR4 antagonism are relatively rapid, usually within minutes to hours, and cessation of administration of the antagonist is quickly (usually within hours) followed by decreased numbers of blood cells.2,3 Hence, maintaining increased levels of circulating leukocytes would require frequent dosing of patients with the antagonist. Frequent dosing, however, has implications for possible problems involving desensitization of the receptor to the antagonizing effector, and induction of serious side effects and safety concerns. In fact, CXCR4 is a coreceptor for HIV, and attempts at continuing antagonism of CXCR4 by AMD3100 to prevent or decrease HIV infection were associated with severe side effects.4 AMD3100 has been used successfully to mobilize hematopoietic stem (HSC) and progenitor (HPC) cells to the blood for collection for use in hematopoietic cell transplantation (HCT),3,5,6 but due to the rapid and effective mobilization of HSCs/HPCs, and its synergy in this effect with granulocyte colony-stimulating factor, the AMD3100 needed only to be administered short-term to donors for efficient mobilization of enough cells for effective HCT.6
The strength of the article by McDermott et al1 is that the 3 patients with WHIM assessed in this study were given plerixafor at low dose (twice a day by self-administration) for 6 months without side-effects (and with greatly decreased infectious episodes) and, in combination with imiquimod, improved control of warts (see figure). In retrospect, the design of this long-term study is quite impressive; the effectiveness of long-term low-dose administration of plerixafor was not necessarily predictable, even though it followed 2 phase 1 clinical trials with plerixafor administered for 1 to 2 weeks to patients with WHIM.7,8
There are interesting consequences of low-dose administration of plerixafor noted in this article,1 including the slow reversion to baseline levels of leukocytes after chronic plerixafor low-dosing was discontinued, and a thorough read of this clinical study is warranted with the understanding that this work is only a beginning to proving the clinical efficacy of plerixafor for treatment of some of the health issues associated with WHIM. It may not be a cure, but it clearly demonstrates health benefits for patients with WHIM syndrome, and follow-up confirmatory studies are warranted.
This study1 brings up a number of questions and possibilities (see figure) that could be experimentally evaluated in context of further clinical trials in this and other disorders associated with decreased circulating leukocytes, and in animal models of disease, as AMD3100 works in mice, and higher animals, as well as in humans. An intriguing finding was the apparent lack of desensitization of CXCR4 to the leukocyte-mobilizing effects of plerixafor, which is interesting because CXCR4 is a G-protein–linked 7-transmembrane spanning receptor that manifests desensitization to a natural ligand, stromal-derived factor-1 (SDF-1)/CXCL12. It has been reported that rapid mobilization of HPCs by AMD3100 in mice may be mediated at least in part by CXCR4-dependent release of SDF-1/CXCL12 from stromal cells in the bone marrow.9 In this context, it would be of interest to assess serum levels of SDF-1/CXCL12 in patients being treated with long-term low-dose plerixafor. Perhaps it is CXCR4-dependent release of SDF-1/CXCL12 that is in part responsible for the increase in circulating leukocytes and their sustained levels for a while after cessation of plerixafor. It would also be of interest to see whether, along with plerixafor-induced increases in circulating leukocytes, there is also an increase in phenotypically defined and functionally active subsets of long- and short-term repopulating human HSCs and HPCs, as well as other cells which may play a role in regenerative medicine, such as endothelial colony-forming cells (ECFCs) and mesenchymal stem/stromal cells (MSCs). Although mobilization of HSCs, HPCs, ECFCs, and/or MSCs may not play a role in helping to ameliorate some of the problems inherent in patients with WHIM syndrome, such information could be of importance for other disease states, as endogenous tissue repair from certain stress conditions may reflect the enhanced circulation of these stem/progenitor cell populations.
It is becoming clear that levels of circulating blood cells may be under control of circadian oscillations, as has recently been reported for HSCs,10 and correct timing of mobilization of such cells may greatly enhance the numbers of cells mobilized. Hence, studies of modified timing of low-dose plerixafor may increase efficacy. Whether clinical use of long-term administration of low-dose plerixafor can be adequately translated for treatment of other disorders associated with low blood leukocyte counts is of interest; this will require that there are enough leukocytes available in bone marrow and other tissue sites for potential mobilization, and the leukocytes in these patients would need to function normally, as in the evaluated 3 patients with WHIM syndrome. What the study by McDermott et al1 (and those publications preceding this work that established AMD3100/plerixafor as a mobilizing agent) demonstrates is that ingenuity, creativity, and flexibility in experimental design, starting at a basic science level and preceding through preclinical investigation, can lead to efficacious and safe manipulations that improve health care. This is what the scientific process is about.
Conflict-of-interest disclosure: The author declares no competing financial interests.