Key Points
Maturation of vascular endothelial growth factor–induced new vessels in cornea involves a PDGF-Shh axis.
Shh promotes PDGF-BB–mediated SMC migration by inducing ERK1/2 and phosphatidylinositol 3-kinase γ activation and increased motility.
Abstract
Recruitment of mural cells (MCs), namely pericytes and smooth muscle cells (SMCs), is essential to improve the maturation of newly formed vessels. Sonic hedgehog (Shh) has been suggested to promote the formation of larger and more muscularized vessels, but the underlying mechanisms of this process have not yet been elucidated. We first identified Shh as a target of platelet-derived growth factor BB (PDGF-BB) and found that SMCs respond to Shh by upregulating extracellular signal-regulated kinase 1/2 and Akt phosphorylation. We next showed that PDGF-BB–induced SMC migration was reduced after inhibition of Shh or its signaling pathway. Moreover, we found that PDGF-BB–induced SMC migration involves Shh-mediated motility. In vivo, in the mouse model of corneal angiogenesis, Shh is expressed by MCs of newly formed blood vessels. PDGF-BB inhibition reduced Shh expression, demonstrating that Shh is a target of PDGF-BB, confirming in vitro experiments. Finally, we found that in vivo inhibition of either PDGF-BB or Shh signaling reduces NG2+ MC recruitment into neovessels and subsequently reduces neovessel life span. Our findings demonstrate, for the first time, that Shh is involved in PDGF-BB–induced SMC migration and recruitment of MCs into neovessels and elucidate the molecular signaling pathway involved in this process.
Introduction
Formation of new vessels occurs during early development and adulthood and also in pathological conditions such as ischemia, tissue regeneration, inflammation, and cancer.1 Mural cell (MC) recruitment is essential for the stability and the functionality of newly formed vessels, particularly by controlling endothelial cell (EC) survival through the regulation of Ang-1/Ang-2 balance. MCs include pericytes and smooth muscle cells (SMCs), although a clear distinction between these 2 cell types remains controversial.2 Mechanisms involved in their recruitment on endothelial sprouts are complex. Pericytes are proposed to originate from surrounding mesenchymal precursors or to be recruited from the wall of adjacent vessels before differentiating into SMCs.3 It is also postulated that proliferation and migration of MCs toward ECs is dependent on a precisely orchestrated gradient of soluble chemotactic factors generated from ECs in their microenvironment.
Platelet-derived growth factor BB (PDGF-BB) is known to promote MC recruitment.4-7 It is secreted by ECs upon angiogenic stimulation8,9 or hypoxia.10 It is a potent chemotactic and growth factor for both vascular SMCs and pericytes expressing PDGF receptor β (PDGFR-β).5
Hedgehog (Hh) proteins, namely Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh), are involved in vessel formation during development11 and ischemia-induced postnatal neovascularization.12,13 Interestingly, in addition to increasing capillary density, Shh gene therapy in the ischemic myocardium was shown to promote the formation of larger and more muscularized blood vessels.14 Moreover, Shh was shown to upregulate MC-related markers during the formation of microvessel-like structures in vitro15 suggesting a potential action of Shh in vessel maturation. At the cellular level, recombinant Shh was shown to induce SMC and pericyte proliferation and survival16-19 and to contribute to PDGF-BB–induced myofibroblast proliferation.20 Even though Shh was shown to induce the migration of various cell types such as fibroblasts,21 ECs,22 endothelial progenitors,23 and monocytes,24 its role and mechanism in SMC migration in vitro and in MC recruitment in vivo have never been investigated.
The aim of this study was to identify the role of Hh proteins in SMC recruitment by neovessels and to characterize their mechanism of action. As such, we conducted a comprehensive series of experiments to demonstrate that Shh contributes to PDGF-BB–induced SMC migration in vitro and drives MC recruitment on growing vessels in the mouse corneal angiogenesis model.
Materials and methods
Products
Recombinant Shh, PDGF-BB, and vascular endothelial growth factor (VEGF) were purchased from R&D Systems, Smoothened (Smo) inhibitor cyclopamine from Sigma-Aldrich, and SANT2 from Enzo Life Sciences. The phosphatidylinositol 3-kinase (PI3K) inhibitors AS-252424 and Wortmannin and the PDGFR-β inhibitor imatinib were purchased from Santa Cruz Biotechnology, Inc.
Cell culture and migration assays
Rat aortic SMCs were obtained from Lonza. They were maintained in Dulbecco’s modified Eagle medium:F12 (1:1) supplemented with 20% fetal bovine serum, in a humidified 5% CO2 atmosphere at 37°C, and used between passages 2 to 12. Quiescent SMCs were obtained by a 24-hour culture in serum-free medium. Commercial (Lonza) rat aortic SMC phenotype was characterized by immunocytochemistry with anti–α-smooth muscle actin (α-SMA) (Sigma), anti–nerve/glial antigen-2 (NG2) (Millipore), and anti–PDGFR-β (R&D Systems) antibodies (supplemental Figure 1A; see the Blood Web site). They demonstrated the same phenotypic properties as aortic SMCs in situ (supplemental Figure 1B).
For the Transwell chemotactic assay, BD Falcon inserts were seeded with 65 000 cells per well. Compounds to be tested were added in the lower compartment. Migration was assessed as the mean of cells present below the porous membrane in 9 fields per well 6 hours after seeding.
The wound-healing assay was performed in a 12-well culture plate. A confluent layer of SMCs was scratched with a plastic tip, and pictures were shot at the beginning and 72 hours later.
The random motility test was performed on glass slides. SMCs were tracked using a time-lapse Zeiss microscope. More than 60 cells per condition were individually tracked by shooting pictures every 15 minutes for 15 hours (Axio Vision software). Each condition of the migration and motility assays was assayed in triplicate, and each experiment performed at least 3 times.
When indicated, the culture medium was supplemented with PDGF-BB (10 µg/mL), recombinant Shh (1 µg/mL), anti-Hh blocking antibody 5E1 (1.5 µg/mL; Developmental Studies Hybridoma Bank), cyclopamine (1 µmol/L), GANT58 (5 µmol/L; Tocris Biosciences), GANT61 (5 µmol/L; Alexis), or PI3Kγ selective inhibitor (AS252424; 100 nmol/L).
siRNA transfection and adenovirus transduction
Cells were transfected with short interfering RNA (siRNA) (Eurogentec) using Interferin (Polyplus transfection) according to the manufacturer’s instructions. Migration assays were performed 48 hours after transfection of quiescent cells. Adenovirus encoding for an inactive form of PI3Kγ (KRPI3Kγ) and control adenovirus were obtained from M. Laffargue’s laboratory.25 Cells were transduced at a multiplicity of infection of 50 overnight and then washed twice with serum-free medium. Fresh medium was added for 24 hours before using the cells.
RNA extraction, reverse transcription polymerase chain reaction (RT-PCR), and real-time quantitative PCR
Total RNA was extracted from cultured SMCs or from mouse corneas, according to the TriReagent manufacturer’s instructions (Molecular Research Center Inc.). Quantitative RT-PCR (RT-qPCR) was performed as detailed in supplemental Methods using the SYBR Green technique. Primer sequences are described in supplemental Methods.
Western blot and immunostaining techniques
Antibodies used in western blot analysis were as follows: anti–protein kinase B (Akt), anti–phospho-Akt (Ser473), anti–extracellular signal-regulated kinase (ERK) 1/2, and anti–phospho-ERK1/2 (Tyr202/Tyr204) (Cell Signaling Technology). Primary antibodies were then incubated with secondary Alexa 700-nm and Alexa 750-nm coupled antibodies (Invitrogen) and Alexa 800-nm coupled antibodies (LI-COR) and resolved with an infrared system (OdysseyR; LI-COR).
The concentration of Shh protein in cultured SMC media was measured using the Mouse Sonic Hedgehog N-Terminus Quantikine ELISA Kit (R&D Systems). Hh immunodetection on cells was performed with mouse anti-Hh antibody 5E1 (Developmental Studies Hybridoma Bank). Detection of PDGF-BB, Shh, ECs, and NG2+ MCs in the cornea model was performed with anti–PDGF-B subunit antibody (Santa Cruz Biotechnology, Inc.), anti–C-terminal Shh antibody (R&D Systems), anti-CD31 antibody (BMA Biomedicals), and anti-NG2 antibody (Millipore) and was revealed with fluorescent dye (Alexa 488, 564, and 647) coupled secondary antibodies (Invitrogen). Slides were mounted with Vectashield (Vector) imaging medium. Images were captured at 22°C with an Olympus FV1000 confocal microscope or a Zeiss Observer Z1 microscope equipped with a Zeiss digital camera. Three-dimensional image reconstruction was performed with Imaris Bitmap software.
Mouse cornea pocket angiogenesis assay
C3HeB/FeJ mice were obtained from Charles River Laboratories and bred in our animal facility. Mice were handled in accordance with the guidelines established by the National Institutes of Health and Medical Research (INSERM) and approved by the local Institutional Animal Care and Use Committee No. A501303-A. VEGF pellets were prepared and implanted in the corneas of 4- to 5-week-old C3HeB/FeJ mice as previously described.26 Pellets contain 5 µg VEGF and 20 nmol of cyclopamine or imatinib as indicated. Pellets without growth factor were implanted in control mice. Vessel formation and stability were examined by immunostaining 5 or 28 days after implantation.
Statistics
Results are reported as mean ± standard deviation (SD). Comparisons within groups were performed using analysis of variance. Comparisons between individual groups were performed with the Mann-Whitney U test.
Results
Hh proteins and Hh pathway activation are required for PDGF-BB–induced SMC migration
During angiogenesis, ECs have been shown to produce factors necessary for MC recruitment including PDGF-BB.27
Recent data demonstrated that Shh contributes to PDGF-BB–stimulated fibroblast migration.20 Because PDGF-BB is one of the main chemotactic factors for SMCs, we hypothesized that Hh proteins could work in combination with PDGF-BB to promote SMC migration. As shown in Figure 1A, Hh blocking antibody 5E1 decreased PDGF-BB–induced migration of commercial rat aortic primary-cultured SMCs. To confirm this data and further investigate whether activation of Hh signaling is necessary for PDGF-BB–induced SMC migration, we inhibited Smo activity with cyclopamine and SANT2. The 2 inhibitors decreased PDGF-BB chemotactic activity (Figure 1A and data not shown for SANT2). Taken together, these data demonstrate that Hh proteins and their subsequent activation of Hh signaling participate in PDGF-BB–induced SMC migration.
Shh mediates PDGF-BB chemotactic effect on SMCs. (A) PDGF-BB–stimulated (10 ng/mL) and nonstimulated (Ctrl) SMC migration was assessed in the presence of the Smo inhibitor cyclopamine (Cyclop; 1 µmol/L), the Hh blocking antibody 5E1 (1.5 µg/mL), an siRNA directed against Shh RNA (siShh) (30 nmol/L), or their respective controls (IgG1, ethanol 0.005% [EtOH], and siCtrl). (B) SMC migration in response to various concentrations of Shh (1 ng/mL to 1000 ng/mL), Ihh (1000 ng/mL), Dhh (1000 ng/mL), and PDGF-BB (10 ng/mL). (C) SMC motility was tested for 72 hours in wound-healing assays and 15 hours in random motility assays with or without stimulation by recombinant Shh (1 µg/mL). Data represent means ± SD of relative values vs Ctrl from 3 independent experiments performed in triplicate. P > .05 (NS); **P < .005; ***P < .0005. NS, nonsignificant.
Shh mediates PDGF-BB chemotactic effect on SMCs. (A) PDGF-BB–stimulated (10 ng/mL) and nonstimulated (Ctrl) SMC migration was assessed in the presence of the Smo inhibitor cyclopamine (Cyclop; 1 µmol/L), the Hh blocking antibody 5E1 (1.5 µg/mL), an siRNA directed against Shh RNA (siShh) (30 nmol/L), or their respective controls (IgG1, ethanol 0.005% [EtOH], and siCtrl). (B) SMC migration in response to various concentrations of Shh (1 ng/mL to 1000 ng/mL), Ihh (1000 ng/mL), Dhh (1000 ng/mL), and PDGF-BB (10 ng/mL). (C) SMC motility was tested for 72 hours in wound-healing assays and 15 hours in random motility assays with or without stimulation by recombinant Shh (1 µg/mL). Data represent means ± SD of relative values vs Ctrl from 3 independent experiments performed in triplicate. P > .05 (NS); **P < .005; ***P < .0005. NS, nonsignificant.
Because cultured vascular SMCs express the main Hh signaling pathway elements, including Hh receptor Patched-1, Smo, and Glioma-associated oncogene 1 (Gli1) transcription factor (supplemental Figure 2), we investigated the chemotactic effect of Hh proteins on SMCs. In contrast to PDGF-BB, Shh did not induce aortic SMC chemotaxis even at a wide range of concentrations (10 ng/mL to 1 µg/mL) (Figure 1B) or long-lasting migration time (24 hours) (supplemental Figure 3). Similarly, 1 µg/mL of Ihh or Dhh did not show any chemotactic effect on SMCs (Figure 1B). These data demonstrate that despite their involvement in PDGF-BB–driven SMC migration, Hh proteins alone are not chemotactic for SMCs.
We then tested whether Shh was able to promote SMC chemokinesis in the wound-healing assay and in the random motility test. We found that Shh was able to stimulate SMC motility in these 2 tests independently of the presence of a concentration gradient (Figure 1C). Altogether, these data suggest that Shh promotes PDGF-BB–induced SMC migration by stimulating SMC motility without inducing chemotaxis.
Shh expression is stimulated by PDGF-BB
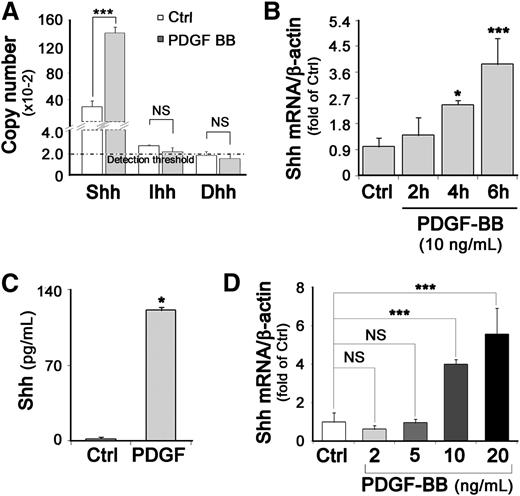
To determine whether PDGF-BB–treated SMCs were capable of producing Hh proteins, we measured the messenger RNA (mRNA) level of each Hh in SMCs by RT-qPCR. In the absence of PDGF-BB, Shh was the main Hh expressed (1.3 × 104 mRNA copies per µg RNA), Ihh was faintly expressed, and Dhh mRNA was in the range of the detection threshold (Figure 2A). Moreover, when SMCs were stimulated by PDGF-BB for 6 hours, the Shh mRNA level increased by 3.2-fold as compared with control unstimulated quiescent SMCs, whereas Ihh and Dhh expression remained unchanged (Figure 2A). The increase in Shh mRNA level became significant after a 4-hour PDGF-BB stimulation (Figure 2B). Shh protein accumulated in the cytoplasm and at the plasma membrane of PDGF-BB–stimulated SMCs (supplemental Figure 4) and was secreted by PDGF-BB–stimulated SMCs more abundantly than by control unstimulated SMCs (secreted Shh was 50 times more concentrated) (Figure 2C) in a dose-dependent manner (Figure 2D).
PDGF-BB stimulates Shh expression in SMCs. (A) SMCs were treated with 10 ng/mL PDGF-BB for 6 hours. The mRNA copy number of Shh, Dhh, and Ihh in stimulated (PDGF-BB) and unstimulated (Ctrl) SMCs was estimated by RT-qPCR. (B) Relative Shh mRNA expression in SMCs stimulated with PDGF-BB (10 ng/mL) for 2, 4, or 6 hours was determined by RT-qPCR; β-actin was used as housekeeping gene. (C) Concentrations of secreted Shh protein present in 24-hour–conditioned culture media from PDGF-BB–stimulated SMCs or unstimulated (Ctrl) SMCs were quantified by enzyme-linked immunosorbent assay. (D) Relative Shh mRNA expression in SMCs stimulated with different PDGF-BB concentrations was determined by RT-qPCR; data represent means ± SD from 3 independent experiments performed in triplicate. *P < .05; ***P < .0005.
PDGF-BB stimulates Shh expression in SMCs. (A) SMCs were treated with 10 ng/mL PDGF-BB for 6 hours. The mRNA copy number of Shh, Dhh, and Ihh in stimulated (PDGF-BB) and unstimulated (Ctrl) SMCs was estimated by RT-qPCR. (B) Relative Shh mRNA expression in SMCs stimulated with PDGF-BB (10 ng/mL) for 2, 4, or 6 hours was determined by RT-qPCR; β-actin was used as housekeeping gene. (C) Concentrations of secreted Shh protein present in 24-hour–conditioned culture media from PDGF-BB–stimulated SMCs or unstimulated (Ctrl) SMCs were quantified by enzyme-linked immunosorbent assay. (D) Relative Shh mRNA expression in SMCs stimulated with different PDGF-BB concentrations was determined by RT-qPCR; data represent means ± SD from 3 independent experiments performed in triplicate. *P < .05; ***P < .0005.
Additionally, when Shh expression was specifically inhibited by Shh siRNA, PDGF-BB–induced SMC migration was decreased by at least 50% (Figure 1A and supplemental Figure 5). Together these data demonstrate that Shh expression is induced by PDGF-BB and identify Shh as the Hh protein mediating PDGF-BB–induced SMC migration.
Because Gli1 overexpression is one of the main reporters of Hh signaling pathway activation, we first verified whether Gli1 expression increases upon PDGF-BB stimulation (supplemental Figure 6A). The role of Shh in PDGF-BB–induced Gli1 overexpression was controlled by Hh blocking antibodies (5E1). Moreover, we confirmed the involvement of Gli1 in PDGF-BB–induced SMC migration by using 3 Gli1 inhibitors including 2 small molecules, GANT58 and GANT61 (supplemental Figure 6B), and the physiological inhibitor of Gli1, the N-terminal part of Gli3 (supplemental Figure 6C-D). All 3 inhibitors significantly reduced PDGF-BB–mediated SMC migration. All together, these data confirm the activation of the Hh canonical signaling pathway by PDGF-BB.
Shh-dependent activation of PI3Kγ and ERK1/2 participates in PDGF-BB–induced SMC migration
Two of the main intracellular signaling pathways activated by PDGF-BB are mitogen-activated protein kinase and PI3K signaling, which lead to ERK1/2 and Akt phosphorylation, respectively.
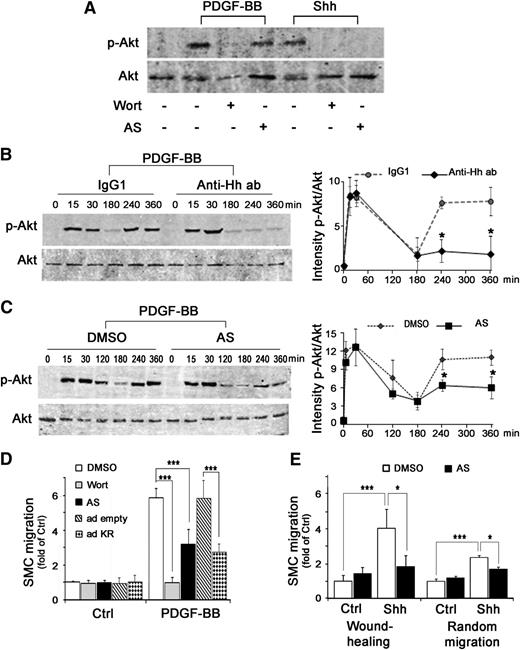
Previous studies have shown that Shh activates PI3K and subsequently leads to Akt phosphorylation in various cell types.21,23,28-30 We demonstrated that recombinant Shh is capable of inducing a fast (15-minute) Akt phosphorylation in SMCs. We showed that Shh-induced Akt phosphorylation is entirely inhibited by the pan PI3K inhibitor Wortmannin, the PI3Kγ selective inhibitor AS-252424 (Figure 3A), and competition with the inactive mutant of PI3Kγ25 (supplemental Figure 7A), demonstrating that Shh-induced Akt phosphorylation is mediated via PI3Kγ. In contrast, the fast Akt phosphorylation induced by PDGF-BB was only inhibited by Wortmannin (Figure 3A), but not by AS-252424 or the competitive inactive form of PI3Kγ (supplemental Figure 7A), confirming that PDGF-BB–induced Akt phosphorylation is instead mediated by PI3Kα/β/δ.
PDGF-BB–induced SMC migration implicates Shh-mediated PI3Kγ activation. (A) SMCs were pretreated for 30 minutes with Wortmannin, a pan PI3K inhibitor (Wort; 100 nmol/L), or AS-252424, a PI3Kγ selective inhibitor (AS; 100 nmol/L), then stimulated for 15 minutes with PDGF-BB (10 ng/mL) or Shh (1 µg/mL). Proteins were extracted and processed by western blot before Akt and phosphorylated Akt (p-Akt) detection. Kinetics of Akt phosphorylation in the presence or absence of the Hh blocking antibody 5E1 (Anti-Hh ab; 1.5 µg/mL) and control IgG1 (B) and in the presence or absence of the PI3Kγ selective inhibitor (AS) and dimethylsulfoxide (DMSO) (C) using western blot (left panels). Secondary Alexa 700-nm coupled antibodies (Invitrogen) and Alexa 800-nm coupled antibodies (LI-COR) were resolved with an infrared system (OdysseyR, LI-COR). Quantifications of band intensity performed with Odyssey software are shown (right panels). Immunoblots shown are representative of 3 independent experiments. (D) Basal and PDGF-BB–induced SMC migration in the presence or absence of PI3Kγ inhibitor (AS; 100 nmol/L) or Wortmannin (Wort; 100 nmol/L) or after transduction with empty (ad empty) or PI3Kγ inactive form (ad KR) adenoviruses. (E) SMC motility was measured after 72 hours in wound-healing assay and after 15 hours in random motility assay with or without stimulation by recombinant Shh (1 µg/mL) and in the presence or absence of PI3Kγ inhibitor (AS; 100 nmol/L). Data represent means ± SD from 3 independent experiments performed in triplicate. *P < .05; ***P < .0005.
PDGF-BB–induced SMC migration implicates Shh-mediated PI3Kγ activation. (A) SMCs were pretreated for 30 minutes with Wortmannin, a pan PI3K inhibitor (Wort; 100 nmol/L), or AS-252424, a PI3Kγ selective inhibitor (AS; 100 nmol/L), then stimulated for 15 minutes with PDGF-BB (10 ng/mL) or Shh (1 µg/mL). Proteins were extracted and processed by western blot before Akt and phosphorylated Akt (p-Akt) detection. Kinetics of Akt phosphorylation in the presence or absence of the Hh blocking antibody 5E1 (Anti-Hh ab; 1.5 µg/mL) and control IgG1 (B) and in the presence or absence of the PI3Kγ selective inhibitor (AS) and dimethylsulfoxide (DMSO) (C) using western blot (left panels). Secondary Alexa 700-nm coupled antibodies (Invitrogen) and Alexa 800-nm coupled antibodies (LI-COR) were resolved with an infrared system (OdysseyR, LI-COR). Quantifications of band intensity performed with Odyssey software are shown (right panels). Immunoblots shown are representative of 3 independent experiments. (D) Basal and PDGF-BB–induced SMC migration in the presence or absence of PI3Kγ inhibitor (AS; 100 nmol/L) or Wortmannin (Wort; 100 nmol/L) or after transduction with empty (ad empty) or PI3Kγ inactive form (ad KR) adenoviruses. (E) SMC motility was measured after 72 hours in wound-healing assay and after 15 hours in random motility assay with or without stimulation by recombinant Shh (1 µg/mL) and in the presence or absence of PI3Kγ inhibitor (AS; 100 nmol/L). Data represent means ± SD from 3 independent experiments performed in triplicate. *P < .05; ***P < .0005.
After PDGF-BB stimulation of SMCs, Akt phosphorylation demonstrated a rapid and transient increase (maximal at 15 minutes), then a rapid decrease, followed by a second wave of activation (Figure 3B). In the presence of the Hh blocking antibody 5E1, the delayed second wave of Akt phosphorylation was strongly decreased suggesting that the late activation of Akt was dependent on Shh autocrine action. Indeed, the early PDGF-BB–induced Akt phosphorylation was totally inhibited by Wortmannin (Figure 3A), but not by the PI3Kγ-specific inhibitor (Figure 3C). In contrast, the delayed Akt phosphorylation was strongly decreased by the PI3Kγ inhibitor (Figure 3C), suggesting that this delayed activation is attributable to Shh-induced PI3Kγ activation.
Finally, we demonstrated that PI3Kγ inhibition by a selective drug (AS-252424) or in the inactive PI3Kγ mutant-transduced cells reduced PDGF-BB–induced SMC migration by more than 50% (Figure 3D). We verified that PI3Kγ inhibitors block Shh-induced motility (Figure 3E and supplemental Figure 7B). Together these data suggest that Shh-activated PI3Kγ is one major determinant of PDGF-BB–induced SMC migration.
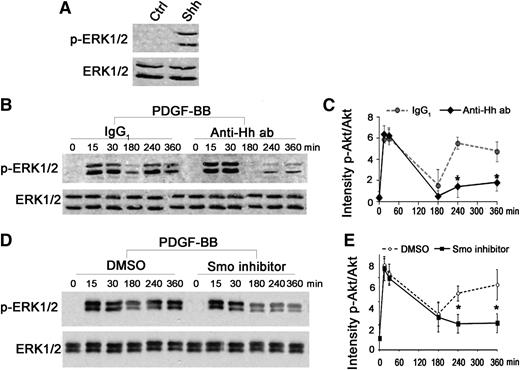
We also showed that recombinant Shh activates the mitogen-activated protein kinase pathway leading to ERK1/2 phosphorylation (Figure 4A). Kinetic analysis demonstrated a delayed reactivation of ERK1/2 phosphorylation after a 4-hour PDGF-BB stimulation (Figure 4B-C). In the presence of the Hh blocking antibody 5E1 or the Smo inhibitor cyclopamine, the early wave of ERK1/2 activation was not affected, whereas the delayed activation was strongly disrupted (Figure 4B-E), suggesting that PDGF-BB–induced Shh mediates the late ERK1/2 phosphorylation through Hh signaling pathway activation and may also participate, via this pathway, in SMC migration.
Shh is required to sustain ERK1/2 phosphorylation induced by PDGF-BB. (A) Quiescent SMCs were stimulated for 15 minutes with Shh (1 µg/mL). Proteins were extracted and processed for detection of ERK1/2 and phosphorylated ERK1/2 (p-ERK1/2) by western blot. (B) Kinetics of ERK1/2 phosphorylation in the presence or absence (Ctrl) of the Hh blocking antibody 5E1 (Anti-Hh ab; 1.5 µg/mL). Immunoblots shown are representative of 3 independent experiments. (C) Quantification of band intensity. (D) Kinetics of ERK1/2 phosphorylation in the presence or absence (Ctrl) of the Smo inhibitor cyclopamine (1 µmol/L). Immunoblots shown are representative of 3 independent experiments. (E) Quantifications of band intensity. *P < .05.
Shh is required to sustain ERK1/2 phosphorylation induced by PDGF-BB. (A) Quiescent SMCs were stimulated for 15 minutes with Shh (1 µg/mL). Proteins were extracted and processed for detection of ERK1/2 and phosphorylated ERK1/2 (p-ERK1/2) by western blot. (B) Kinetics of ERK1/2 phosphorylation in the presence or absence (Ctrl) of the Hh blocking antibody 5E1 (Anti-Hh ab; 1.5 µg/mL). Immunoblots shown are representative of 3 independent experiments. (C) Quantification of band intensity. (D) Kinetics of ERK1/2 phosphorylation in the presence or absence (Ctrl) of the Smo inhibitor cyclopamine (1 µmol/L). Immunoblots shown are representative of 3 independent experiments. (E) Quantifications of band intensity. *P < .05.
Shh is required for PDGF-BB–mediated MC recruitment on neovessels in the mouse cornea
We investigated Shh involvement in MC recruitment during neovessel formation in vivo using a mouse cornea micropocket angiogenesis assay. VEGF pellet implantation strongly increased Shh expression (Figure 5A) mainly at the leading edge of neovessels (Figure 5B-C). At the tip of the growing vessel, we found both CD31+ ECs and an SMC subpopulation characterized by the expression of α-SMA, NG2 proteoglycan marker, and PDGFR-β (supplemental Figure 7). Shh expression was mainly colocalized with the NG2+-expressing cells at the interface between ECs and SMCs (Figure 5D).
PDGF-BB–dependent Shh expression in VEGF-mediated corneal angiogenesis model. VEGF-containing (5 µg) pellets were implanted into the cornea of C3HeB/FeJ mice (n = 8 per group). (A) Five days later, the Shh mRNA level was evaluated by RT-qPCR in Ctrl and VEGF-treated corneas with or without the PDGFR-β inhibitor imatinib (20 nmol); β-actin was used as a housekeeping reference gene. Data of RT-qPCR represent means ± SD of relative expression vs control corneas. **P < .005; ***P < .0005. (B) Shh (green, Alexa 488) and NG2 (red, Alexa 568) expression was detected by immunostaining and imaged with a Zeiss Observer Z1 fluorescence microscope. (C-D) Shh (green, Alexa 488), EC (CD31, red, Alexa 568), and NG2 (pink, Alexa 647) expression was revealed by immunohistology. Nuclei were counterstained using 4,6 diamidino-2-phenylindole (DAPI; blue). Images were shot with an Olympus FV1000 confocal microscope at low (×20) (C) or high (×40) (D) magnification, respectively. In D, far-right panel shows isosurface analysis by Imaris Bitmap software.
PDGF-BB–dependent Shh expression in VEGF-mediated corneal angiogenesis model. VEGF-containing (5 µg) pellets were implanted into the cornea of C3HeB/FeJ mice (n = 8 per group). (A) Five days later, the Shh mRNA level was evaluated by RT-qPCR in Ctrl and VEGF-treated corneas with or without the PDGFR-β inhibitor imatinib (20 nmol); β-actin was used as a housekeeping reference gene. Data of RT-qPCR represent means ± SD of relative expression vs control corneas. **P < .005; ***P < .0005. (B) Shh (green, Alexa 488) and NG2 (red, Alexa 568) expression was detected by immunostaining and imaged with a Zeiss Observer Z1 fluorescence microscope. (C-D) Shh (green, Alexa 488), EC (CD31, red, Alexa 568), and NG2 (pink, Alexa 647) expression was revealed by immunohistology. Nuclei were counterstained using 4,6 diamidino-2-phenylindole (DAPI; blue). Images were shot with an Olympus FV1000 confocal microscope at low (×20) (C) or high (×40) (D) magnification, respectively. In D, far-right panel shows isosurface analysis by Imaris Bitmap software.
To test whether Shh expression was induced by PDGF-BB in vivo, we first verified that Pdgfb was increased after VEGF pellet implantation (supplemental Figure 8). Then imatinib, an inhibitor of PDGFR phosphorylation, was added together with VEGF in the pellet. In this condition, VEGF-induced Shh mRNA expression in neovessels was strongly reduced, demonstrating that PDGF-BB is required for induction of Shh expression by VEGF in vivo (Figure 5A).
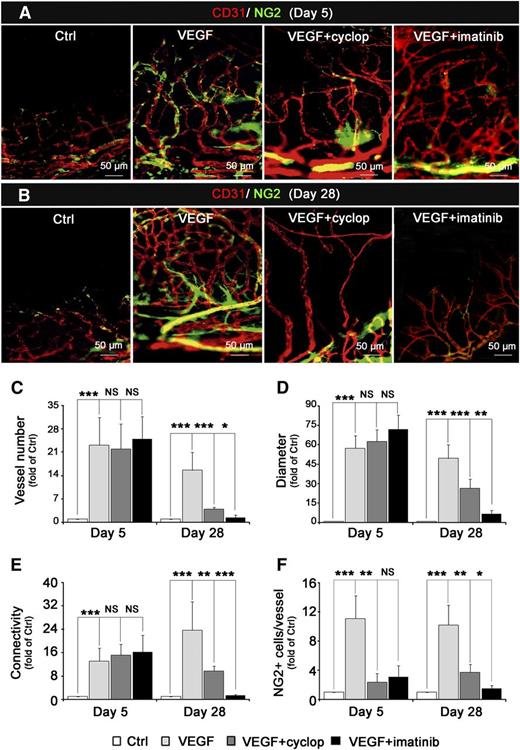
The role of Shh in MC recruitment was investigated using the Hh pathway inhibitor cyclopamine. Five days after corneal implantation of pellets containing both VEGF and cyclopamine, the growing new vessels showed a significant decrease in the number of recruited NG2+ MCs (Figure 6A,F). The number of vessels, their diameters, and vessel network connections remained unchanged (Figure 6C-E). An identical decrease in the number of recruited NG2+ MCs was observed when PDGFR was inhibited with imatinib (Figure 6A).
Inhibition of Hh signaling pathway stimulates neovessel maturation and stability. VEGF-containing (5 µg) pellets in the presence or absence of Smo inhibitor (20 nmol cyclopamine, cyclop) or PDGFR-β inhibitor (20 nmol imatinib) were implanted into the cornea of C3HeB/FeJ mice (n = 8 per group) for 5 days (A) or 28 days (B). ECs were immunostained using anti-CD31 antibody (red, Alexa 564) and MCs with anti-NG2+ antibody (green, Alexa 488). Image capture was performed with Zeiss Observer Z1 microscope (×20). Quantification of total vessel number (C), vessel diameter (D), vessel connections (E), and number of NG2+ cells per vessel (F), 5 days or 28 days after VEGF pellet implantation in the presence (gray histograms) or absence of cyclopamine (black). Data represent means ± SD of relative values vs control corneas. P > .05 (NS); **P < .005; ***P < .0005.
Inhibition of Hh signaling pathway stimulates neovessel maturation and stability. VEGF-containing (5 µg) pellets in the presence or absence of Smo inhibitor (20 nmol cyclopamine, cyclop) or PDGFR-β inhibitor (20 nmol imatinib) were implanted into the cornea of C3HeB/FeJ mice (n = 8 per group) for 5 days (A) or 28 days (B). ECs were immunostained using anti-CD31 antibody (red, Alexa 564) and MCs with anti-NG2+ antibody (green, Alexa 488). Image capture was performed with Zeiss Observer Z1 microscope (×20). Quantification of total vessel number (C), vessel diameter (D), vessel connections (E), and number of NG2+ cells per vessel (F), 5 days or 28 days after VEGF pellet implantation in the presence (gray histograms) or absence of cyclopamine (black). Data represent means ± SD of relative values vs control corneas. P > .05 (NS); **P < .005; ***P < .0005.
Twenty-eight days later, as expected, inhibition of the PDGF pathway by imatinib led to a vessel destabilization and a complete blood vessel regression. Cyclopamine-treated corneas were less drastically affected but also showed a marked reduction in blood vessel number (Figure 6B-C), diameter, and network connections (Figure 6D-E), suggesting that the Shh pathway is involved in maintaining/improving neovessel life span (Figure 6F). Altogether, those latter data demonstrate the role of Shh expression in neovessel maturation and stabilization in vivo. Moreover, it confirms that Shh acts downstream of PDGF-BB.
Early recruitment of NG2+ MCs in the leading edge of the growing vessel does not require their proliferation
Because Shh was previously shown to promote SMC proliferation in vitro16-18 and to drive the proliferative effect of PDGF-BB,20 we checked the role of the Hh pathway in NG2+ MC proliferation in vivo.
NG2+ MC proliferation was examined after mice were administered with 5-bromo-2′-deoxyuridine, 3 and 5 days after induction of angiogenesis by VEGF. Double BrdU+ NG2+ labeled cells were found on the preexisting parental/limbal vessels (supplemental Figure 9B), whereas we did not find any proliferating NG2+ cells in the growing new blood vessels either at day 3 or 5 (supplemental Figure 9A,C), demonstrating that NG2+ cell recruitment at the tip of the growing vessels does not require cell proliferation at least during the first 5 days.
Thus, these data suggest that new vessel formation in the mouse cornea mainly involves vessel remodeling with endothelial and MC migration at least during the first days after angiogenesis was induced, and that these processes are dependent of the Shh pathway.
Moreover, we found that addition of cyclopamine did not reduce the number of proliferating NG2+ cells on limbal vessels (supplemental Figure 9D), demonstrating that activation of the Hh signaling pathway is not necessary for MC proliferation in this model of angiogenesis.
Discussion
Previous investigations have shown that the morphogen Shh is upregulated in different pathological conditions, such as ischemia,12 and enhances revascularization of ischemic tissues. In the ischemic heart, exogenous Shh administration not only promotes neovascularization, but also increases vessel muscularization, indicating that Shh may play a role in vessel maturation.14 In the present study, we confirmed the essential role of Shh in blood vessel muscularization and revealed that Shh participates in PDGF-BB–induced SMC migration by increasing cell motility but not chemotaxis, while PDGF-BB drives the direction of the movement. Indeed, we next demonstrated that both Shh expression and secretion were increased after SMC stimulation by PDGF-BB and that autocrine production of Shh was involved in PDGF-BB–stimulated SMC migration. This finding reveals a new effect of Shh on SMCs, in addition to its previously demonstrated role in proliferation of these cells.16-18
Even if Hh proteins are suggested to act on angiogenesis only indirectly,12,31,32 ECs were shown to respond to Shh by increasing their migration and the impermeability of the brain endothelium.33 In the context of growing vessels, SMC-derived Shh may not only act on SMCs themselves, but also on the neighborhood ECs by stimulating their migration and consequently by promoting vessel growth. However, our data showed that inhibition of the Hh signaling pathway has no effect on vessel growth at least during the first days after induction, suggesting that Hh has only a minor effect on this process. Moreover, an effect of Shh on endothelial permeability of newly formed corneal vessel cannot be ruled out, but the fact that Shh is mainly produced at the edge of growing vessels suggests that this effect probably remains minor.
Moreover, this study characterizes Shh-dependent signaling in SMCs and demonstrates that PDGF-BB–stimulated Shh induces the canonical Hh signaling pathway and a delayed activation of ERK1/2 and PI3K/Akt phosphorylation, 2 key mediators of the cell migration process. Indeed, PI3K targets [PtdIns(4,5)P2 and PtdIns(3,4,5)P3] mediate cell migration via several downstream molecules, such as Akt, guanine nucleotide exchange factors, GTPase-activating proteins, and protein tyrosine kinases that control cell movement.34 To sustain this process, PI3K must be reactivated by a Rho GTPase–dependent activation loop.34 The potentiating effect of Shh appears to be mediated by Smo, a member of the G protein-coupled receptor (GPCR) superfamily.35 In contrast to PDGFR-β, a tyrosine kinase receptor that activates PI3Kα, β, and δ isoforms, GPCRs are known to activate the PI3Kγ subclass.36 Our data demonstrate, for the first time, that Shh activates PI3Kγ via Smo, and that PDGF-BB–mediated Shh/Smo signaling leads to a delayed PI3Kγ activation, allowing long-lasting cell activation, which finally increases PDGF-BB–induced migration.
Previous data demonstrated that PI3Kγ was required for reparative angiogenesis by contributing to angiogenic factor production by ECs.37 We present here strong evidence that Shh-activated PI3Kγ is one of the events triggering PDGF-BB–induced SMC chemotaxis and could potentially also be involved in MC recruitment. Cotreatment with both Hh and PI3Kγ inhibitors had a stronger inhibitory effect than Hh inhibitors alone suggesting that PI3Kγ activity is not exclusively regulated by Shh (supplemental Figure 10). Interestingly, autocrine production of monocyte chemotactic protein-1, another GPCR agonist, has also been shown to implicate PI3Kγ in PDGF-BB–induced aortic SMC migration.25 Our study brings to light a novel function of Shh, implicating this morphogenic protein in PDGF-BB–induced SMC chemotaxis in a similar fashion to osteopontin38 or monocyte chemotactic protein-1.25 Shh is expressed and secreted under PDGF-BB stimulation of SMCs and triggers an array of intracellular pathways (PI3Kγ and ERK1/2), which converge to allow long-term PDGF-BB–induced signaling mechanisms to initiate the cellular response (ie, SMC migration).
MC coverage of growing vessels is the result of different processes including MC proliferation and migration from preexisting vessels or MC differentiation from progenitor cells. MCs include smooth muscle lineage cells at different differentiation stages such as pericytes and SMC subtypes.39 In mouse cornea, we found that MCs of growing vessels coexpress α-SMA, NG2, and PDGFR-β that are commonly associated with phenotypes of human pericytes (supplemental Figures 11A-B and 12A) and rat aortic SMCs (supplemental Figure 1). Moreover, the migratory response to PDGF-BB of these MCs from human and rat depends identically on the Hh pathway (supplemental Figure 12B). These findings suggest that data from in vitro experiments of rat aortic SMCs are relevant to understanding mechanisms of PDGF-dependent MC recruitment in vivo at least in the mouse corneal angiogenesis model.
In the pathophysiological context, notably during angiogenesis, PDGF-BB is one of the major factors involved in MC recruitment.40 This growth factor is produced in neovessels and is highly responsive to angiogenic factors, including VEGFA.41,42 Expression of PDGF-BB by neovessels is essential for proper recruitment and organization of MCs, which express PDGFR-β and can proliferate and migrate along the PDGF-BB gradient generated by ECs.43 Our in vivo data show that Shh is required for the previously characterized effects of PDGF-BB on vessel maturation. Blocking either PDGF-BB or Shh action strongly reduced NG2+ MC recruitment and consequently reduced both vessel maturation and life span.
Because blocking the Hh signaling pathway was well shown to inhibit SMC proliferation in vitro,16-18 the effect of Shh on MC recruitment in vivo may be the result of its effect on MC proliferation instead of migration. However, our data show that in the cornea early steps of new vessel formation do not involve either EC or MC proliferation and moreover that blocking the Hh signaling pathway does not reduce cell proliferation on preexisting limbal vessels, thus suggesting that inhibition of MC proliferation is probably not the target of Hh inhibitors. Our in vitro data, demonstrating that Shh is involved in PDGF-BB–mediated SMC migration, suggest that one potential effect of Shh in vivo is to promote this effect.
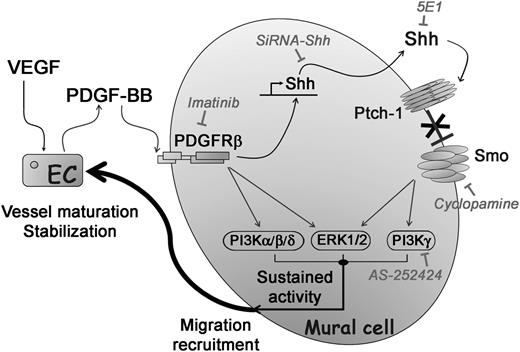
In summary, the present study demonstrates that (1) Shh expression is induced by PDGF-BB in MCs both in vivo in the mouse corneal angiogenesis model and in vitro in cultured SMCs, and (2) inhibition of Shh signaling leads to a decrease in the MC number on new vessels and a subsequent decrease in vessel life span. Taken together, these findings strongly suggest that autocrine production of Shh by MCs is a downstream effector of PDGF-BB for MC migration and recruitment in neovessels (Figure 7), documenting an enigmatic role of Shh in vessel maturation.14 Finally, our work underscores the potential therapeutic application of Shh to increase the endogenous effect of PDGF-BB in promoting vessel maturation by enhancing MC migration toward PDGF-BB–producing ECs.
Role of autocrine Shh signaling in the potentiation of PDGF-BB–induced SMC migration and recruitment. PDGF-BB secreted by VEGF-stimulated ECs induces Shh expression in MCs. Locally, autocrine Shh promotes a sequence of events mediated by downstream signaling pathways via PI3Kγ and ERK1/2, leading to costimulation of PDGF-BB pathway targets. By this novel mechanism, Shh potentiates PDGF-BB–mediated NG2+ MC migration and recruitment on growing vessels allowing their long-lasting stabilization.
Role of autocrine Shh signaling in the potentiation of PDGF-BB–induced SMC migration and recruitment. PDGF-BB secreted by VEGF-stimulated ECs induces Shh expression in MCs. Locally, autocrine Shh promotes a sequence of events mediated by downstream signaling pathways via PI3Kγ and ERK1/2, leading to costimulation of PDGF-BB pathway targets. By this novel mechanism, Shh potentiates PDGF-BB–mediated NG2+ MC migration and recruitment on growing vessels allowing their long-lasting stabilization.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
For their excellent technical assistance, the authors thank Jérôme Guignard (INSERM U1034, Pessac) at the animal facility, Annabel Reynaud for molecular biology experiments, and Myriam Petit for histology.
This work was supported by grants from the Conseil Régional d’Aquitaine (action inter-régionale Aquitaine-Midi Pyrénées); Communauté de Travail des Pyrénées; ANR, program (ANR-07-PHYSIO-010-02); Fondation Recherche Médicale, program on cardiovascular aging (DCV20070409258); and a postdoctoral fellowship from La Ligue Nationale Contre le Cancer (M.-A.R.).
Authorship
Contribution: Q.Y. performed research, analyzed data, and wrote the manuscript; M.-A.R. performed research, analyzed data, and edited the manuscript; C.C., S.V., I.B., and B.J.-V. performed research; J.-M.D.-L. analyzed data; M.L. contributed vital new reagents; A.M. designed research and edited the manuscript; C.D. designed research, performed research, analyzed data, and wrote the manuscript; and A.-P.G. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alain-Pierre Gadeau, INSERM U1034, Cardiovascular adaptation to ischemia, 1 Ave de Magellan, Pessac, 33600, France; e-mail: alain.gadeau@inserm.fr.

![Figure 1. Shh mediates PDGF-BB chemotactic effect on SMCs. (A) PDGF-BB–stimulated (10 ng/mL) and nonstimulated (Ctrl) SMC migration was assessed in the presence of the Smo inhibitor cyclopamine (Cyclop; 1 µmol/L), the Hh blocking antibody 5E1 (1.5 µg/mL), an siRNA directed against Shh RNA (siShh) (30 nmol/L), or their respective controls (IgG1, ethanol 0.005% [EtOH], and siCtrl). (B) SMC migration in response to various concentrations of Shh (1 ng/mL to 1000 ng/mL), Ihh (1000 ng/mL), Dhh (1000 ng/mL), and PDGF-BB (10 ng/mL). (C) SMC motility was tested for 72 hours in wound-healing assays and 15 hours in random motility assays with or without stimulation by recombinant Shh (1 µg/mL). Data represent means ± SD of relative values vs Ctrl from 3 independent experiments performed in triplicate. P > .05 (NS); **P < .005; ***P < .0005. NS, nonsignificant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/15/10.1182_blood-2013-06-508689/4/m_2429f1.jpeg?Expires=1765921284&Signature=2tqjq70vH1U6nBMbjnAPOrbjgwoQS8TkLNoFsraOW9mjVdFpd~xS43lfDc-QuQ8~IWKjDA9KoQcOZZCMLQUM4kDz7tHrwjmfilT9LuvKegJOYmaeSlMYMkUpDu8AVGZMslF2Qj51QkqKVuypdGBrc5uzvj715slGlnuHLq5GG1DPjzJIO5VA4~IuRY1mx43HFUyhXJnS8UafX6ughBewnwuynea2PfbzDcDZ-RO2yrnuur0gNg4KCktypUC88i3L-DCMQsw4KWOS6rIBxreTO3xSoo0ji8zq6QRo8EBZ7yNYZU0XIzdduBh0Z-ljDxPdyslHp8cdbNI20bZbHCKkig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal