Key Points
Deletion of Fanca or Fancd2 dysregulates the activity and function of regulatory T cells.
The loss of FA proteins alters the expression of Foxp3 target genes.
Abstract
Fanconi anemia (FA) is a genetic disorder associated with bone marrow (BM) failure and leukemia. Recent studies demonstrate variable immune defects in FA. However, the cause for FA immunodeficiency is unknown. Here we report that deletion of Fanca or Fancd2 dysregulates the suppressive activity of regulatory T cells (Tregs), shown functionally as exacerbation of graft-vs-host disease (GVHD) in mice. Recipient mice of Fanca−/− or Fancd2−/− BM chimeras exhibited severe acute GVHD after allogeneic BM transplantation (BMT). T cells from Fanca−/− or Fancd2−/− mice induced higher GVHD lethality than those from wild-type (WT) littermates. FA Tregs possessed lower proliferative suppression potential compared with WT Tregs, as demonstrated by in vitro proliferation assay and BMT. Analysis of CD25+Foxp3+ Tregs indicated that loss of Fanca or Fancd2 dysregulated Foxp3 target gene expression. Additionally, CD25+Foxp3+ Tregs of Fanca−/− or Fancd2−/− mice were less efficient in suppressing the production of GVHD-associated inflammatory cytokines. Consistently, aberrant NF-κB activity was observed in infiltrated T cells from FA GVHD mice. Conditional deletion of p65 in FA Tregs decreased GVHD mortality. Our study uncovers an essential role for FA proteins in maintaining Treg homeostasis, possibly explaining, at least in part, the immune deficiency reported in some FA patients.
Introduction
Fanconi anemia (FA) is a genetic disorder associated with the progressive development of BM failure (BMF) and high risk of cancer including leukemia.1-3 FA is genetically heterogeneous, with 16 causative genes identified.4-6 Studies have demonstrated that most, if not all, of the FA proteins function in a single pathway to regulate important cellular processes such as DNA replication, cell-cycle control, and DNA damage response and repair.5,7
It remains possible that some or all of the FA proteins might have additional functions outside of the principal pathway. For example, FANCC functions independently to modulate expression of a family of interferon γ (IFN-γ)-inducible genes and protects cells from apoptotic cues in ways that do not require an intact FA core complex.1 We previously reported that the FA pathway counteracts oxidative stress through selective protection of antioxidant defense gene promoters, suggesting a role for the FA pathway in cellular antioxidant defense.8 It has been shown that several components of the FA core complex, including FANCA, FANCF, and FANCG, functionally interact with HES1, a key player in the Notch signaling pathway.9 Consistently, a recent study using FA mouse models demonstrated a functional crosstalk between Notch signaling and the FA proteins in regulation of hematopoietic stem cell (HSC) differentiation.10 Furthermore, recent studies uncovering a novel role of FANCD2 in transcriptional regulation of Tap63 and ΔNp63 advanced our understanding of how defective FA proteins contribute to the pathogenesis of human cancer.11,12
Many studies indicate that FA proteins play specific roles in hematopoiesis by governing the responses of hematopoietic cells to both genotoxic and cytotoxic stresses. Loss of FA functions causes excessive apoptosis of hematopoietic stem and progenitor cells (HSC/P) leading to BMF in the early stage of FA.13-17 As the disease progresses, apoptosis as well as genomic instability impose a selective pressure on FA HSC/P cells. The loss of stem cell fitness in FA HSC/P cells permits the emergence of resistant clones, which could be transformed to leukemia.14,15 Complications of BMF are the major causes of the morbidity and mortality of FA. To date, HSC transplantation (HSCT) is considered the best treatment of BMF and leukemia in FA patients.1-3,16 Immune deficiency, in contrast to the myeloid insufficiency associated with BMF in FA, has been recently described. For example, in the first systematic and broad evaluation of immune function in FA patients, Myers and colleagues reported that absolute numbers of B cells and natural killer (NK) cells were reduced in FA patients compared with controls, whereas the absolute number of T cells was within normal range, although cytotoxic T-cell function was reduced.17 Perforin and granzyme content of NK-cells was reduced along with NK-cell cytotoxicity (P < .001). Antigen proliferation in response to tetanus was also decreased whereas responses to candida and phytohemagglutinin were preserved.17 Furthermore, impaired cytoskeletal rearrangements in Fancc−/− macrophages from mice deleted for the Fancc gene (Fancc−/−) have been linked to the cell-autonomous defects detected in vitro, as well as altered monocyte/macrophage trafficking in vivo.18 More recent studies, using primary samples, linked immunological alterations to cell apoptosis and tissue inflammation in FA patients.19,20 However, mechanisms responsible for these described immune deficiencies remain poorly understood. In the present study, we show for the first time that the function of Tregs that is deficient for Fanca or Fancd2 is decreased, a finding that may contribute mechanistically to clinical immune deficiency in FA patients.
Materials and methods
Mice and treatment
Fanca+/− and Fancd2+/− mice (C57BL/6: B6, H-2b+, CD45.2+) were provided by Dr Madeleine Carreau (Laval University) and Dr Markus Grompe (Oregon Health & Sciences University), respectively.21,22 Conditional p65f/fFanca−/− mice were generated by interbreeding the RelA/p65 mice23 with Fanca+/−. p65 gene deletion was accomplished by Cre-mediated deletion of floxed alleles by crossing the p65f/fFanca+/− mice with a Cre-ERT2 strain24 and intraperitoneally injecting 100 μL of Tamoxifen (20 mg/mL; Sigma-Aldrich, St. Louis, MO) daily for 3 days. All the animals including BoyJ (C57BL/6: B6, H-2b+, CD45.1+) and Balb/c (C57BL/6: B6, H-2d+, CD45.2+) mice were maintained in the animal barrier facility at Cincinnati Children’s Hospital Medical Center. Animals were kept in accordance with the Institutional Animal Care and Use Committees (2C06040.) All the animals used for the experiments were 8 to 12 weeks old.
BM transplantation
To establish bone marrow (BM) chimeras, 5 × 106 whole BM cells (WBMCs) plus 5 × 106 splenocytes from wild-type (WT) C57BL/6 mice (C57BL/6: B6, H-2b+, CD45.2+) or 10 × 106 WBMCs plus 5 × 106 splenocytes from Fanca−/− or Fancd2−/− mice (C57BL/6: B6, H-2b+, CD45.2+) were transplanted into lethally (11 Gy) irradiated BoyJ mice (B6, H-2b+, CD45.1+) to accomplish comparable donor cell reconstitution. Four months after primary BMT, donor-derived chimerism (CD45.2+) was measured by flow cytometry (FACSCanto I, BD Biosciences, San Jose, CA). After substantial donor-derived reconstitution (CD45.2+) was confirmed (>95%), recipient cells were used for 2nd BMT with 9 Gy irradiation and transplantation using 5 × 106 WBMCs plus 2 × 106 T cells from either syngeneic C57/BL6J mice (H-2b+, CD45.2+) or allogeneic Balb/c (H-2d+, CD45.2+) mice. To test FA T-cell–induced GVHD lethality, 5 × 106 of T cell deleted total BMCs (TCD) from WT mice plus or minus 3 × 106 T cells from either WT or FA mice were transplanted to lethally irradiated Balb/c recipients. For BMT with Tregs, 5 × 106 T cell depleted (TCD) BM cells (BMCs) plus 5 × 105 Teff cells with or without 5 × 105 Treg cells from either WT or Fanca−/− or Fancd2−/− mice (C57/BL6; H-2b+) were transplanted to lethally irradiated Balb/c recipients (C57/BL6; H-2d+). For transplant with cells expressing the conditional p65 allele, Treg cells isolated from 8- to 12-week-old p65f/fFanca+/+ or p65f/fFanca−/− mice plus TCD + Teff cells were transplanted to lethally irradiated Balb/c recipients (∼10 weeks old). Five days after transplantation, the recipients were injected intraperitoneally with DMSO or Tamoxifen (100 μL, 20 mg/mL) injection for 3 days to delete p65 gene. Survival data were plotted by the Kaplan-Meier curve method and analyzed by the log-rank test.
Flow cytometry analysis
Donor-derived chimerism was determined by staining peripheral blood from primary recipient mice with antibodies specific for CD45.1, CD45.2 (BD Biosciences, San Jose, CA). Cells isolated from the spleen of native or recipient mice were used for flow cytometry analysis by staining with antibodies specific for Foxp3, H-2b, H-2d, CD4, CD25 (BD Biosciences, San Jose, CA). Annexin V and 7AAD staining determined cell apoptosis (BD Biosciences Pharmingen, San Diego, CA). Data were analyzed using BD Diva software (BD Biosciences, San Jose, CA) for dot graph or FlowJo software for histogram (TreeStar, Ashland, OR).
For intracellular cytokine staining, lymphocytes from the small intestine of recipient mice were enriched using a pan-T cell isolation kit (Miltenyi Biotech, Auburn, CA) according to the manufacturer’s instructions. T cells were then stimulated with 50 ng/mL PMA (Calbiochem, Darmstadt, Germany) and 2 μg/mL Ionomycin (Invitrogen, Grand Island, NY) for 1 hour, followed by 3 hours incubation in the presence of 1 μg/mL Brefeldin A (Sigma-Aldrich, St. Louis, MO), as described in the suppliers’ manual of Intracellular Cytokine Staining Starter Kit (BD PharMingen, San Jose, CA). Treated cells were stained H-2b+ and CD4+ antibodies before the treatment with Cytofix/Cytoperm reagent (BD PharMingen, San Jose, CA). After fixation and permeabilization, cytokines were intracellularly stained with antibodies specific for TNF-α, IFN-γ, and IL-6 followed by flow cytometric analysis gated on the H-2b+CD4+ cell compartment.
For other experimental procedures, see supplemental Methods, available on the Blood Web site.
Results
FA Treg cells exhibit reduced suppression potential in vitro and in vivo
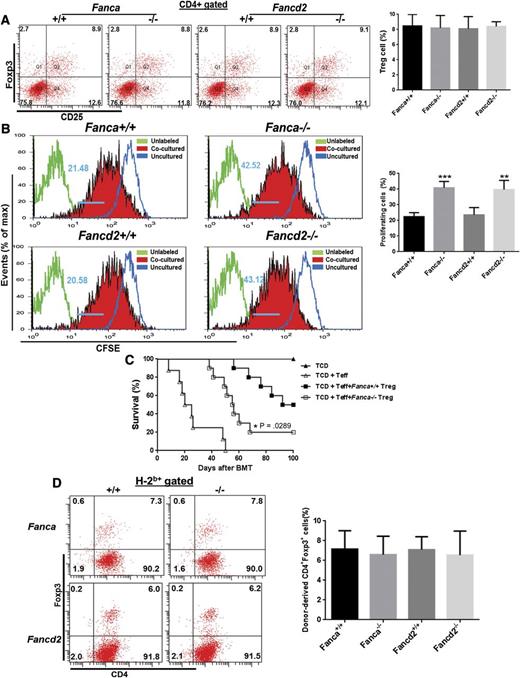
To explore immune function in FA-deficient cells, we examined numbers and function of Tregs in Fanca−/− or Fancd2−/− mice. We did not find a difference in Treg cell numbers between WT and FA mice (Figure 1A). However, Treg cells from Fanca−/− or Fancd2−/− mice (B6, H-2b+, CD45.2+) are less effective in suppressing the proliferation of CD4+CD25− Teff cells from allogeneic Balb/c mice (H-2d+, CD45.2+) in vitro compared with WT Treg cells, as determined by CFSE retention assay by coculturing CFSE labeled Teff cells with CD4+CD25+ Treg cells freshly isolated from either WT, Fanca−/−, or Fancd2−/− mice at a ratio of 2:1 for 5 days followed by flow cytometry analysis (Figure 1B). To substantiate this in vitro finding, we performed allogeneic BMT by transplanting Teff cells along with or without Treg cells isolated from WT or FA mice (B6, H-2b+, CD45.2+) into lethally irradiated Balb/c mice (H-2d+, CD45.2+). We found that recipients transplanted with Fanca−/− or Fancd2−/− Treg cells died of GVHD more rapidly than those receiving WT Treg cells (Figure 1C; supplemental Figure 1). The impaired function of the FA Treg cells might be a result of a loss of Treg cells after BMT. To test this possibility, we examined donor Treg cell frequency in the recipients. Again, we found no difference in the number of donor-derived H-2b+CD4+Foxp3+ Treg cells in recipients of the tested groups regardless of the genotype of Treg cells (Figure 1D). These data indicate a functional deficit of FA Treg cells.
FA Treg cells show lower suppression potential. (A) FA deficiency does not alter Treg cell number in native mice. Splenocytes from mice with the indicated genotypes were subjected to flow cytometry analysis for CD4, CD25, Foxp3 staining. CD4+ cells were gated for analysis of CD25+Foxp3+ cell portion. Representative flow graph (left) and quantification (right) are shown. Results are means plus or minus SD of 2 independent experiments (n = 6 per group). (B) FA Treg cells fail to suppress Teff cell proliferation in vitro. Carboxyfluorescein diacetate succinimidyl ester (CFSE) labeled WT CD4+CD25− Teff cells were cocultured with CD4+CD25+ cells freshly isolated from either WT or Fanca−/−, Fancd2−/− mice at a ratio of 2:1 in Dulbecco's modified Eagle's medium containing 10% fetal calf serum for 5 days followed by flow cytometry analysis for CFSE retention. Data were analyzed by FlowJo software. Representative flow graph (left) and quantification (right) are shown. Results are means plus or minus SD of 3 independent experiments (n = 9 per group). (C) FA Treg cells are less suppressive in preventing GVHD in vivo. Lethally irradiated Balb/c recipients were transplanted with 5 × 106 TCD BMCs from WT C57BL/6 animals alone, or with sorted WT Teff cells (CD4+CD25−, 5 × 105) plus or minus equal numbers of sorted CD4+CD25+ Treg cells from either WT C57BL/6, Fanca−/−. Survival of recipients was monitored by Kaplan-Meier curve method. Each group includes 7 to 10 mice. (D) No difference in cell number of donor-derived Treg cells. Splenocytes isolated from recipients described in (C) were subjected to Flow cytometric analysis for H-2b, CD4, Foxp3 staining. Donor H-2b+ cells were gated for CD4+Foxp3+ cell portion. Representative flow graph (left) and quantification (right) are shown.
FA Treg cells show lower suppression potential. (A) FA deficiency does not alter Treg cell number in native mice. Splenocytes from mice with the indicated genotypes were subjected to flow cytometry analysis for CD4, CD25, Foxp3 staining. CD4+ cells were gated for analysis of CD25+Foxp3+ cell portion. Representative flow graph (left) and quantification (right) are shown. Results are means plus or minus SD of 2 independent experiments (n = 6 per group). (B) FA Treg cells fail to suppress Teff cell proliferation in vitro. Carboxyfluorescein diacetate succinimidyl ester (CFSE) labeled WT CD4+CD25− Teff cells were cocultured with CD4+CD25+ cells freshly isolated from either WT or Fanca−/−, Fancd2−/− mice at a ratio of 2:1 in Dulbecco's modified Eagle's medium containing 10% fetal calf serum for 5 days followed by flow cytometry analysis for CFSE retention. Data were analyzed by FlowJo software. Representative flow graph (left) and quantification (right) are shown. Results are means plus or minus SD of 3 independent experiments (n = 9 per group). (C) FA Treg cells are less suppressive in preventing GVHD in vivo. Lethally irradiated Balb/c recipients were transplanted with 5 × 106 TCD BMCs from WT C57BL/6 animals alone, or with sorted WT Teff cells (CD4+CD25−, 5 × 105) plus or minus equal numbers of sorted CD4+CD25+ Treg cells from either WT C57BL/6, Fanca−/−. Survival of recipients was monitored by Kaplan-Meier curve method. Each group includes 7 to 10 mice. (D) No difference in cell number of donor-derived Treg cells. Splenocytes isolated from recipients described in (C) were subjected to Flow cytometric analysis for H-2b, CD4, Foxp3 staining. Donor H-2b+ cells were gated for CD4+Foxp3+ cell portion. Representative flow graph (left) and quantification (right) are shown.
FA deficiency affects Foxp3 target gene expression
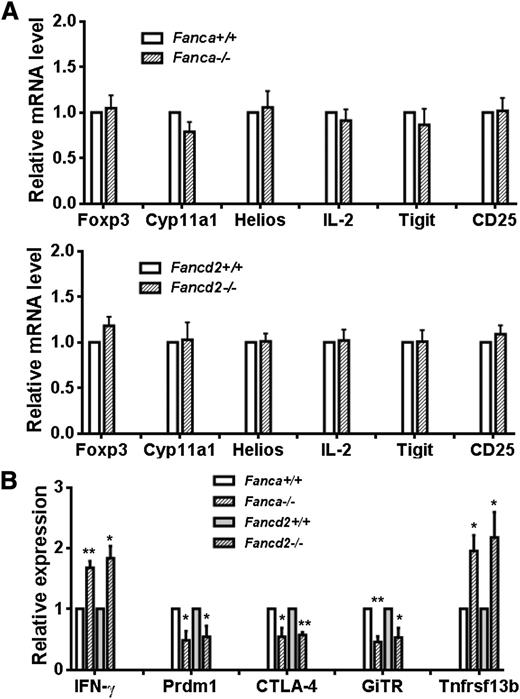
The forkhead transcription factor, Foxp3, is a key molecule required for the development and function of Tregs.25-28 Foxp3 is also involved in regulating B lymphopoiesis.29,30 To identify the molecular mechanism of functional defect in FA Tregs, we attempted to assess Foxp3 target gene expression in infiltrated donor T cells (H-2b+CD4+) from recipients transplanted with Teff cells plus WT or FA Treg cells. Interestingly, we found that the expression level of Foxp3 was comparable among the groups (Figure 2A). In addition, the expression of certain Foxp3 target genes was comparable between WT and FA T cells. Specifically, genes induced, such as CD25 and Helios,25,26 or suppressed, such as IL-2, Tigit and Cyp11a1,25,27 by Foxp3 were also induced or suppressed in the recipients of WT or FA Treg cells (Figure 2A). However, loss of Fanca or Fancd2 altered the expression levels of some other Foxp3 target genes that mediate either active (Prdm1, CTLA-4, GiTR) or repressive (IFN-γ, Tnfrsf13b) effects of Foxp3 (Figure 2B).25-27 These data indicate that loss of FA function deregulates Foxp3 transcriptional program and alters the expression of some Foxp3-target genes, which may contribute to the impaired function of FA Foxp3 Treg cells.
FA deficiency affects Foxp3 transcriptional activity. (A) Relative expression levels of Foxp3 signature genes in WT and Fanca−/− (upper) or Fancd2−/− (lower) donor T cells. RNA extracted from H-2b+CD4+ cells isolated from recipients transplanted with Treg cells from WT, Fanca−/−, or Fancd2−/− mice was used for real-time PCR analysis using primers specific for the indicated Foxp3 target genes. Samples were normalized to the level of GAPDH mRNA. (B) Change in expression levels of Foxp3 target genes in FA infiltrated donor T cells (H-2b+CD4+). RNA extracted from H-2b+CD4+ cells of the recipients described in (A) was used for real-time PCR analysis using primers specific for the indicated Foxp3 target genes. Each group includes 6 mice.
FA deficiency affects Foxp3 transcriptional activity. (A) Relative expression levels of Foxp3 signature genes in WT and Fanca−/− (upper) or Fancd2−/− (lower) donor T cells. RNA extracted from H-2b+CD4+ cells isolated from recipients transplanted with Treg cells from WT, Fanca−/−, or Fancd2−/− mice was used for real-time PCR analysis using primers specific for the indicated Foxp3 target genes. Samples were normalized to the level of GAPDH mRNA. (B) Change in expression levels of Foxp3 target genes in FA infiltrated donor T cells (H-2b+CD4+). RNA extracted from H-2b+CD4+ cells of the recipients described in (A) was used for real-time PCR analysis using primers specific for the indicated Foxp3 target genes. Each group includes 6 mice.
Fanca−/− and Fancd2−/− chimeras exhibit increased GVHD-inducing potential
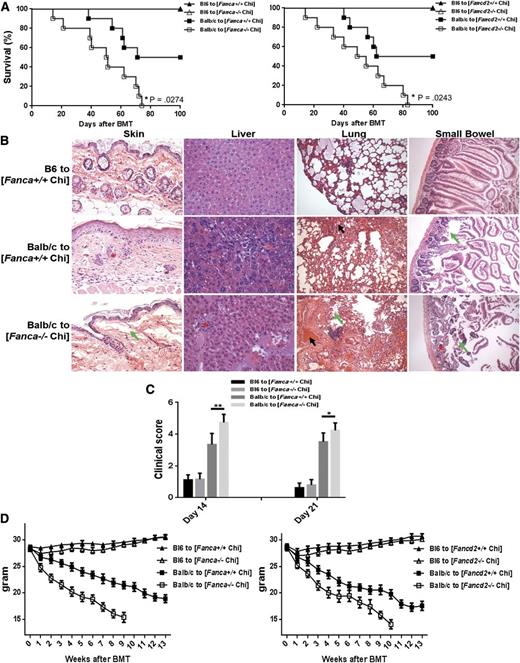
To explore further the function of FA-deficient Tregs in vivo, we used an allogeneic transplant model using FA-deficient and nondeficient BM as we hypothesized that GVHD would be increased in the presence of reduced Treg function. In the clinical transplant setting, FA patients receive normal donor cells. Therefore, the ideal allogeneic transplant model would be to use FA mice as recipients. However, FA mice are sensitive to radiation. To circumvent this, we generated FA BM chimeras to evaluate GVHD, by transplanting 10 × 106 of BM cells from Fanca−/− or Fancd2−/− mice (B6, H-2b+, CD45.2+) into congeneic BoyJ recipient mice (B6, H-2b+, CD45.1+). Flow cytometric analysis of donor hematopoietic cells (CD45.2+) demonstrated that these animals were complete donor chimeras (>95%; supplemental Figure 2) 4 months after BMT. The recipients were then used for secondary BMT by injecting 5 × 106 BM cells along with 2 × 106 T cells isolated from either B6 (syngeneic; H-2b+, CD45.2+) or Balb/c (allogeneic; H-2d+, CD45.2+) mice. We found that although all of the chimeras that received syngeneic cells survived with no signs of GVHD (Figure 3A), more than 60% of the allogeneic chimeras died with signs of GVHD by 100 days after BMT. More strikingly, all of the Fanca−/− and Fancd2−/− chimeras receiving allogeneic cells died within 85 days post-BMT with signs of severe clinical GVHD, whereas approximately 40% of the allogeneic WT chimeras were still alive at this time point. This indicates significantly greater mortality of FA chimeras compared with WT chimeras. Increased mortality in the allogeneic cell-transplanted Fanca−/− and Fancd2−/− chimera was associated with greater severity of GVHD as early as day 14 in the 4 GVHD-specific target organs, that is, liver, lung, small bowel, and skin, than that in the WT allogeneic recipients and syngeneic controls (Figure 3B). Histological findings included lymphocytic infiltration and vacuolar alteration at the dermoepidermal junction, and individual keratinocyte apoptosis in skin, liver cell necrosis and inflammatory infiltration, peribronchial infiltration and pneumonitis in the lung, and inflammation in the epithelium and lamina propria of the small intestine. The GVHD phenotype persisted in the FA chimeras on day 21 after BMT (Figure 3C, supplemental Figure 3). We also observed rapid weight loss, a key feature of clinical GVHD,31 in the allogeneic cell-transplanted Fanca−/− and Fancd2−/− chimera (Figure 3D). Together, these data indicate that FA deficiency exacerbates the severity of acute GVHD in recipient mice of Fanca−/− and Fancd2−/− chimeras, and suggest that the increased GVHD potential is likely a result of a functional alteration in the hematopoietic lineages but not the BM microenvironment of the FA mice.
FA chimeras exhibit increased GVHD-inducing potential. (A) Survival of secondary BM transplanted chimeras. 5 × 106 BMCs plus 5 × 106 splenocytes from WT C57BL/6 mice (B6, H-2b+, CD45.2+) or 10 × 106 WBMCs plus 5 × 106 splenocytes from Fanca−/− (left) or Fancd2−/− (right) mice (C57BL/6: B6, H-2b+, CD45.2+) were transplanted to lethally irradiated Boy J recipients (C57BL/6: B6, H-2b+, CD45.1+). Donor-derived chimera (CD45.2+) were assessed at 4 months after BMT. The recipients with greater than 95% donor-derived chimera were then subjected to 2nd BMT by 9 Gy irradiation and injecting 5 × 106 BM cells along with 2 × 106 T cells isolated from either B6 (syngeneic; H-2b+, CD45.2+) or Balb/c (allogeneic; H-2d+, CD45.2+) mice. Survival of the mice was monitored by Kaplan-Meier curve method. (B) Histopathologic examination of GVHD target organs. Tissue sections (skin, liver, lung, and small intestine) were stained with hematoxylin and eosin and examined by microscope. Asterisks show lymphocytic infiltrates; green arrows show tissue destruction; black arrows show focal alveolar hemorrhages in lung. The specimens shown are representative images of 5 mice in each group with similar histologic features. (C) GVHD clinical scores were determined as a measure of GVHD severity on days 14 and 21 after allogeneic BMT. Data are presented as means plus or minus SD of 2 independent experiments (n = 7 to 10 per group). (D) Weight loss of the recipients. Average weights are shown for mice described in (A). Data are presented as means plus or minus SD of 2 independent experiments (n = 7 to 10 per group).
FA chimeras exhibit increased GVHD-inducing potential. (A) Survival of secondary BM transplanted chimeras. 5 × 106 BMCs plus 5 × 106 splenocytes from WT C57BL/6 mice (B6, H-2b+, CD45.2+) or 10 × 106 WBMCs plus 5 × 106 splenocytes from Fanca−/− (left) or Fancd2−/− (right) mice (C57BL/6: B6, H-2b+, CD45.2+) were transplanted to lethally irradiated Boy J recipients (C57BL/6: B6, H-2b+, CD45.1+). Donor-derived chimera (CD45.2+) were assessed at 4 months after BMT. The recipients with greater than 95% donor-derived chimera were then subjected to 2nd BMT by 9 Gy irradiation and injecting 5 × 106 BM cells along with 2 × 106 T cells isolated from either B6 (syngeneic; H-2b+, CD45.2+) or Balb/c (allogeneic; H-2d+, CD45.2+) mice. Survival of the mice was monitored by Kaplan-Meier curve method. (B) Histopathologic examination of GVHD target organs. Tissue sections (skin, liver, lung, and small intestine) were stained with hematoxylin and eosin and examined by microscope. Asterisks show lymphocytic infiltrates; green arrows show tissue destruction; black arrows show focal alveolar hemorrhages in lung. The specimens shown are representative images of 5 mice in each group with similar histologic features. (C) GVHD clinical scores were determined as a measure of GVHD severity on days 14 and 21 after allogeneic BMT. Data are presented as means plus or minus SD of 2 independent experiments (n = 7 to 10 per group). (D) Weight loss of the recipients. Average weights are shown for mice described in (A). Data are presented as means plus or minus SD of 2 independent experiments (n = 7 to 10 per group).
Increased GVHD lethality by FA T cells
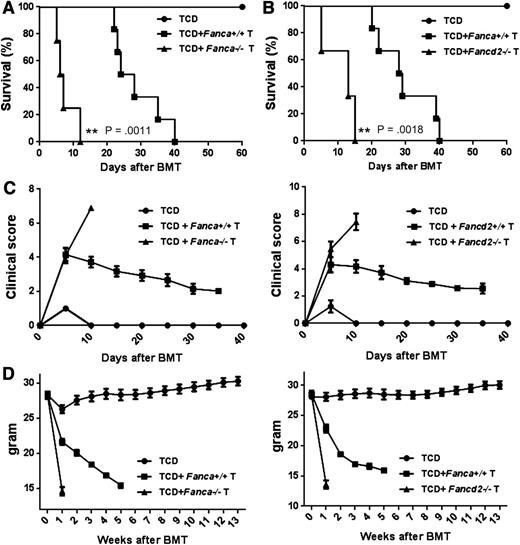
Donor T cells in the graft are the major cause of GVHD.32-34 We next attempted to determine whether FA T cells were responsible for the increased mortality observed in Fanca−/− and Fancd2−/− chimeras by transplanting TCD WT B6 BMCs with or without T cells isolated from WT, Fanca−/−, or Fancd2−/− mice into lethally irradiated allogeneic Balb/c recipients, and we monitored survival and GVHD development in recipient mice. We found that mice transplanted with Fanca−/− or Fancd2−/− T cells died much more rapidly than those receiving WT T cells (Figure 4A-B). Mice transplanted with FA-deficient T cells also showed more severe GVHD (Figure 4C) and faster weight loss (Figure 4D) than those with WT T cells. These results indicate that FA-deficient T cells contributed to increased GVHD lethality in allogeneic BMT.
FA T cell induces increased GVHD lethality. Fanca−/− (A) and Fancd2−/− (B) T cells induce higher GVHD lethality. 5 × 106 of T cell deleted total BMCs (TCD) from WT mice plus 3 × 106 T cells from either WT or FA mice were transplanted to lethally irradiated Balb/c recipients. Survival of recipients was monitored by Kaplan-Meier curve method. Each group includes 6 to 10 mice. (C) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals on the indicated time points after allogeneic BMT. Data are presented as means plus or minus SD of 2 independent experiments. (D) Weight loss of the recipients. Average weights are shown for mice described in (A) and (B). Data are presented as means plus or minus SD of 2 independent experiments.
FA T cell induces increased GVHD lethality. Fanca−/− (A) and Fancd2−/− (B) T cells induce higher GVHD lethality. 5 × 106 of T cell deleted total BMCs (TCD) from WT mice plus 3 × 106 T cells from either WT or FA mice were transplanted to lethally irradiated Balb/c recipients. Survival of recipients was monitored by Kaplan-Meier curve method. Each group includes 6 to 10 mice. (C) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals on the indicated time points after allogeneic BMT. Data are presented as means plus or minus SD of 2 independent experiments. (D) Weight loss of the recipients. Average weights are shown for mice described in (A) and (B). Data are presented as means plus or minus SD of 2 independent experiments.
Exacerbated inflammation contributes to FA GVHD
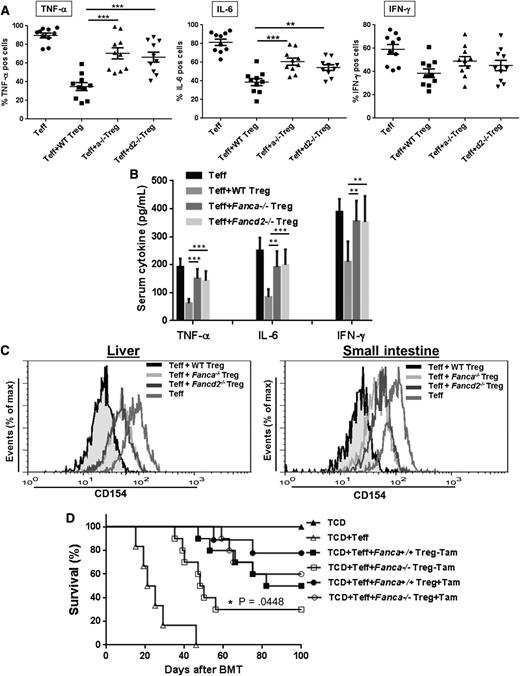
Perturbation of the cytokine network may function as a common signaling pathway in GVHD-induced organ damage, and the rapid onset of GVHD is a consequence of a “cytokine storm.”35-38 GVHD severity is positively correlated with production of proinflammatory cytokines such as IFN-γ, TNF-α, and IL-6 in the target organs.3,39 We thus determined the cytokine profile in donor-derived T cells infiltrating the small intestine of BMT recipients. Intracellular cytokine staining using donor H-2b+CD4+ T cells isolated from the small intestine of the Balb/c recipients showed that the number of TNF-α and IL-6 positive cells was significantly elevated in FA Treg-transplanted mice compared with WT Treg-transplanted recipients (Figure 5A). FA Treg-transplanted recipients also had higher donor-derived IFN-γ positive cells, albeit not statistically significant compared with WT Treg-transplanted mice. Consistent with these results, a significant increase in serum IFN-γ, TNF-α, and IL-6 was observed in FA Treg-transplanted mice (Figure 5B).
Exacerbated inflammation partially contributes to GVHD in FA. (A) Expression of GVHD effective cytokines in FA Treg-transplanted mice. Lethally irradiated Balb/c recipients were transplanted with 5 × 106 TCD cells from WT C57Bl/6 animals alone, or with sorted WT Teff cells (CD4+CD25−, 5 × 105) plus or minus equal numbers of sorted CD4+CD25+ Treg cells from either WT C57BL/6, Fanca−/− or Fancd2−/− mice. Donor T cells isolated from the small intestine of the Balb/c recipients transplanted with the indicated donor cells were stimulated with 50 ng/mL PMA and 2 μg/mL Ionomycin for 1 hour, followed by 3 hours incubation in the presence of 1 μg/mL Brefeldin A. Treated cells were stained H-2b+ and CD4+ antibodies before the treatment with Cytofix/Cytoperm reagent. Cytokines were intracellularly stained with antibodies specific for TNF-α, IFN-γ, and IL-6 followed by flow cytometric analysis gated on the H-2b+CD4+ cell compartment. Each group includes 6 to 10 mice. (B) Levels of TNF-α, IFN-γ, and IL-6 in sera of recipient mice described in (A). (C) Increased NF-κB transcription activity in infiltrated FA T cells. H-2b+CD4+ T cells isolated from liver (left) and small intestine (right) of the recipients described in (A) were used for flow cytometric analysis for CD154. Representative flow graphs are shown. (D) p65 deletion partially reduces FA GVHD mortality. Treg cells from p65f/fFanca+/+ or p65f/fFanca−/− mice plus TCD + Teff cells were transplanted to lethally irradiated Balb/c recipients followed by DMSO or Tamoxifen treatment of 3 days. Survival of recipients was monitored by Kaplan-Meier curve method. Each group includes 6 to 9 mice.
Exacerbated inflammation partially contributes to GVHD in FA. (A) Expression of GVHD effective cytokines in FA Treg-transplanted mice. Lethally irradiated Balb/c recipients were transplanted with 5 × 106 TCD cells from WT C57Bl/6 animals alone, or with sorted WT Teff cells (CD4+CD25−, 5 × 105) plus or minus equal numbers of sorted CD4+CD25+ Treg cells from either WT C57BL/6, Fanca−/− or Fancd2−/− mice. Donor T cells isolated from the small intestine of the Balb/c recipients transplanted with the indicated donor cells were stimulated with 50 ng/mL PMA and 2 μg/mL Ionomycin for 1 hour, followed by 3 hours incubation in the presence of 1 μg/mL Brefeldin A. Treated cells were stained H-2b+ and CD4+ antibodies before the treatment with Cytofix/Cytoperm reagent. Cytokines were intracellularly stained with antibodies specific for TNF-α, IFN-γ, and IL-6 followed by flow cytometric analysis gated on the H-2b+CD4+ cell compartment. Each group includes 6 to 10 mice. (B) Levels of TNF-α, IFN-γ, and IL-6 in sera of recipient mice described in (A). (C) Increased NF-κB transcription activity in infiltrated FA T cells. H-2b+CD4+ T cells isolated from liver (left) and small intestine (right) of the recipients described in (A) were used for flow cytometric analysis for CD154. Representative flow graphs are shown. (D) p65 deletion partially reduces FA GVHD mortality. Treg cells from p65f/fFanca+/+ or p65f/fFanca−/− mice plus TCD + Teff cells were transplanted to lethally irradiated Balb/c recipients followed by DMSO or Tamoxifen treatment of 3 days. Survival of recipients was monitored by Kaplan-Meier curve method. Each group includes 6 to 9 mice.
Because NF-κB controls the expression of many inflammatory genes,40 and the aberrant activation of NF-κB-dependent transcriptional activity has been observed in FA cells,41,42 we next examined NF-κB activity in FA Treg-transplanted mice. We focused on CD154 (also known as CD40L), because it is a direct NF-κB target gene,43,44 and because CD40-CD154 signaling is required for donor T cell expansion and the development of anti-host cytotoxic T lymphocyte activity.45 Flow cytometric analysis showed high levels of CD154 expression in donor H-2b+CD4+ T cells isolated from both liver (Figure 5C left) and small intestine (Figure 5C right) of recipients transplanted with TCD + Teff cells, indicating active NF-κB transcription during GVHD development. Recipient mice transplanted with WT Tregs suppressed NF-κB target expression; whereas this suppressive function was greatly diminished in mice receiving either Fanca−/− or Fancd2−/− Tregs (Figure 5C). Thus, loss of FA function enhanced expression of NF-κB target genes in donor-infiltrated T cells of GVHD mice.
To validate the role of NF-κB in FA GVHD development, we tested whether inhibition of NF-κB can restore FA Treg function and can reduce FA GVHD mortality. To this end, we inactivated NF-κB by conditionally deleting its subunit p65 in the allogeneic recipients transplanted with either p65f/fFanca+/+ or p65f/fFanca−/− Treg cells. Induced deletion of the p65 gene did not significantly affect cellular senescence or apoptosis in either WT or Fanca−/− Treg cells (supplemental Figure 4). However, we found that NF-κB inactivation partially prolonged the survival of p65f/fFanca−/− Treg cells-transplanted Balb/c recipients (Figure 5D). These data suggest that dysregulated NF-κB activity contributes at least in part to FA GVHD in these experimental murine models.
Discussion
In this study, we have investigated Treg cell function and have found that the loss of Fanca or Fancd2 dysregulates Treg activity with reduced suppressive activity. We demonstrated the in vivo significance of this dysfunction by showing increased incidence and severity of GVHD in mice in an allogeneic transplant model that used FA-deficient donor T cells. There are several findings that highlight the functional significance of FA deficiency in Tregs: first, recipient mice of Fanca−/− and Fancd2−/− BM chimeras exhibit greater severity of acute GVHD after secondary allogeneic BMT; second, FA-deficient T cells induce higher GVHD lethality than WT T cells; third, FA Tregs have lower Teff proliferation suppression potential than WT Tregs; fourth, FA deficiency deregulates the expression of some Foxp3 target genes in FA-deficient T cells infiltrating GVHD target organs; last, FA-deficient donor T cells in the GVHD recipient mice show exacerbated inflammation, which is correlated with dysregulated expression of NF-κB target genes, and gene deletion of p65 in FA Tregs ameliorates GVHD mortality. We recognize that our mouse model does not reflect what happens in clinical BMT in FA-deficient children, in whom the donor cells are FA sufficient, but we have used this GVHD model simply to indicate that the Treg deficiency we noted in the FA-deficient setting has functional significance.
Increasing evidence suggests dysfunction of the immune system in FA.17,46,47 Specifically, patients with FA exhibit significant deficits in the NK compartment, a quantitative B-cell defect, as well as abnormal cytotoxic T-cell function. Our observation of Treg defect in the FA mouse models may be relevant to these FA-immune deficiencies in the clinical setting. Studies have demonstrated that Tregs regulate the function and development of NK cells and B cells by diverse suppressor mechanisms.48 For example, Tregs have been described to suppress NK cells and in particular to impair NK-cell effector functions.49 Mechanistically, a reduced CD4+CD25+Foxp3+ Treg cell number or reduced Foxp3 expression and functionality result in NK-cell suppression via enhancing the proliferation of autoreactive CD4+ T cells, which produce IL-21, leading to NK-cell degeneration and reduction in NK-cell function and numbers.50,51 Moreover, Tregs control the number of NK cells and CD8a+ immature dendritic cells in the lymph node paracortex.52 In the context of B-cell regulation by Tregs, it is known that CD4+CD25+ Treg cells suppress B-cell proliferation in response to polyclonal B-cell activators by inducing death of the responding B cells through a granzyme-dependent, partially perforin-dependent pathway.53 It has also been shown that natural Tregs can directly suppress autoreactive B cells in systemic lupus erythematosus.54 These studies suggest an important role of Tregs in regulating the function and development of B cells and NK cells. In our study we show reduced proliferation and suppressive activity of Tregs in FA-deficient cells. Although further mechanistic studies are needed to elucidate whether the suppressive deficit of FA Tregs noted in our model links to the B- and NK-cell defects observed in FA patients, our finding implicates a novel role of FA proteins in immune regulation. It is important to note that in this study we studied the effect of deficiency in 2 of the 16 FA genes, selecting Fanca, the most frequently mutated human gene, and Fancd2, the gene in which mutation is associated with the most severe phenotype in humans. It is possible that deficiency in other FA genes might lead to similar or different immune defects, and this will need to be addressed in future studies.
An important finding of the current study is the observation that FA Tregs have a reduced suppressive capacity both in vitro and in vivo (Figure 1). Treg-mediated suppression serves as a vital mechanism of negative regulation of immune-mediated inflammation and features prominently in autoimmune and autoinflammatory disorders such as acquired aplastic anemia (AA), which is characterized by the destruction of HSCs by cytotoxic T lymphocytes.55 Indeed, decreased Treg frequency and suppressive capacity have been observed in AA patients.56,57 Although FA patients develop marrow aplasia, the mechanism of marrow failure may be different from typical autoimmune marrow, as there is generally a lesser response to immune suppression in FA than in aplastic anemia, although a small proportion of FA patients do show benefit.58 Treg deficiency has not been described in patients with FA, and a prospective evaluation at our center is underway.
The forkhead transcription factor, FOXP3, is selectively expressed and required for the development and function of Treg cells through the regulation of the expression of genes involved in cellular immune responses.28 Dysregulation of FOXP3 transcriptional activity has been linked to many human diseases. For example, children born with a mutation of FOXP3 develop IPEX syndrome (immune deficiency, polyendocrinopathy, enteropathy, X-linked), and typically have profound immune deficiency with severe autoimmunity,59 likely reflecting a more profound degree of defective development of CD4+CD25+ Tregs than the reduced function we have identified in the Tregs in FA-deficient mice. It is possible that Treg deficiency is not manifested as autoimmune disease in FA because patients are commonly transplanted in the first 2 decades of life, probably restoring normal Treg function. Interestingly, a 2-bp insertion in the Foxp3 gene leads to a mouse model of IPEX, the Scurfy mouse, which exhibits a lethal lymphoproliferative autoimmune syndrome resulting from a deficiency in CD4+CD25+ Tregs but not CD4+CD25− Teff cells.60,61 Analogously, reduced suppressive capacity of CD4+CD25+ Tregs, albeit less severe than the IPEX or Scurfy mice, was observed in our FA mouse models. Interestingly, the Scurfy mouse also shows a B-cell defect,62 as we have previously reported in children with FA.17 It is unclear at this time whether Treg deficiency contributes directly to B-cell deficiency in children with FA, or whether B-cell deficiency is simply a manifestation of marrow failure with reduced hematopoietic cell production.
Another interesting finding is that FA deficiency altered both active and repressive transcriptional activities of Foxp3 (Figure 2). Although the expression of certain Foxp3 target genes was comparable between WT and FA T cells, loss of Fanca or Fancd2 altered the expression levels of the Foxp3 target genes that mediate either active or repressive effects of Foxp3. Specifically, INF-γ, Prdm1, Tnfrsf13b, and GiTR are known to be involved in inflammation,25,26 whereas Prdm1 and CTLA-4 are important to T-cell or B-cell proliferation and activation.27 These alterations of Foxp3 target gene expression may contribute to the pathophysiology of the severe GVHD we observed in the FA mouse models, either exacerbating the inflammatory reaction or deregulating of T-cell and/or B-cell development and activation. We found that the GVHD cytokines TNF-α, IFN-γ, and IL-6 were overproduced in the donor-infiltrated T cells from target organs of FA Tregs-transplanted recipients (Figure 5A-B). Consistently, the activity of NF-κB, a major transcriptional activator of numerous proinflammatory genes, was higher in these infiltrated T cells than those of WT Tregs-transplanted recipients (Figure 5C). Moreover, conditional inactivation of p65 in FA Tregs ameliorated GVHD mortality (Figure 5D). Based on these results, we suspect that there is a link between the dysregulation of Foxp3 transcriptional program and aberrant NF-κB signaling in FA Treg cells. It has been shown that Foxp3 expression and thymic Treg development requires TCR signaling via the NF-κB subunit p65.59 More recent studies identified p65 binding sites in the promoter region of the Foxp3 gene.63 ,64 In addition, increased production of inflammatory cytokines could affect Foxp3 activity in Tregs. It has been shown that increased production of inflammatory cytokines by NF-κB, such as IL-6 known to inhibit Foxp3 expression, negatively regulates Foxp3 transcription in Tregs.65 In this context, overproduced IL-6 in the GVHD target organs of FA Treg-transplanted recipients may repress Foxp3 gene expression and lead to the downregulation of some Foxp3 target genes. Whether and how NF-κB influences Foxp3 transcription remains for further investigation; nevertheless, our results favor a model for NF-κB-Foxp3 crosstalk in FA GVHD: FA deficiency leads to upregulation of NF-κB, which in turn induces inflammatory cytokine gene transcription leading to repression of Foxp3 transcription.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Madeleine Carreau (Laval University) for Fanca+/− mice, Dr Markus Grompe (Oregon Health & Sciences University) for Fancd2+/− mice, and the Comprehensive Mouse and Cancer Core of the Cincinnati Children's Research Foundation (Cincinnati Children’s Hospital Medical Center) for BM transplantation service.
This work was supported by National Institutes of Health (Heart, Lung and Blood Institute) grant R01 HL076712 and (National Cancer Institute) grant R01 CA157537. Q.P. is supported by a Leukemia and Lymphoma Scholar award. W.D. is supported by a National Institutes of Health, Institutional Research Training T32 grant.
Authorship
Contribution: W.D. designed research, performed research, analyzed data, and wrote the paper; O.E., A.W., J.M.S., and J.S. performed research; P.M. and K.C.M. designed research; K.A.S. contributed vital new reagents; and S.M.D. and Q.P. designed research and wrote the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Qishen Pang, Division of Experimental Hematology and Cancer Biology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: qishen.pang@cchmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal