Key Points
Detection of MRD by real-time quantitative PCR and flow cytometry is an important predictor of outcome in patients with Ph+ ALL.
Abstract
From 2001 to 2011, 122 patients with newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia were treated with chemotherapy + imatinib (n = 54) or + dasatinib (n = 68). One hundred fifteen (94%) achieved complete remission (CR) including 101 patients who achieved it with only 1 induction course and had at least 1 minimal residual disease (MRD) assessment; 25 patients underwent an allogeneic stem cell transplant in first CR and were excluded, leaving 76 patients as the subject of this report. MRD monitoring by multiparameter flow cytometry (MFC) and real-time quantitative polymerase chain reaction (PCR) was performed at the end of induction and at ∼3-month intervals thereafter. Median age was 54 years (range, 21-84 years). There was no difference in survival by achievement of at least a major molecular response (MMR; BCR-ABL/ABL < 0.1%) at CR (P = .22). Patients achieving MMR at 3, 6, 9, and 12 months had a better survival (P = .02, .04, .05, and .01, respectively). Negative MFC at CR did not predict for improved survival (P = .2). At 3 and 12 months, negative MRD by MFC was associated with improved survival (P = .04 and .001). MRD monitoring by PCR and MFC identifies patients who benefit from treatment intensification in first CR.
Introduction
The Philadelphia (Ph) chromosome resulting from a reciprocal translocation between chromosomes 9 and 22 and leading to the expression of the oncogenic protein, BCR-ABL, occurs in about a quarter to a third of adult patients with acute lymphoblastic leukemia (ALL).1 Prior to the introduction of the BCR-ABL–specific tyrosine kinase inhibitors (TKIs), the outcome of patients with this disease was poor, with long-term survival occurring almost exclusively in patients who underwent an allogeneic stem cell transplant (SCT) in first complete remission (CR).2
With the introduction of effective TKIs, the outcome of patients has significantly improved and whether allogeneic SCT is a necessary component of therapy for all patients with Ph+ ALL has been questioned. In early reports, many patients who received the TKI/cytotoxic chemotherapy combinations and who were unable to receive an allogeneic SCT in first CR, had durable remissions.3-5 Further follow-up reports of some of these studies have, however, suggested an advantage for undergoing an allogeneic SCT in first CR, at least in the younger age group. However, in a retrospective analysis from the Children’s Oncology Group, patients who received long-term continuous imatinib plus chemotherapy but no allogeneic SCT had a better, but statistically similar, outcome to those who underwent HLA-matched sibling or unrelated donor SCT.6
Clearly, these chemotherapy/TKI combination regimens are providing an alternate approach for patients who are unable to undergo allogeneic SCT in first CR. In large studies of patients with Ph+ ALL reported to date, this comprises a substantial proportion of patients.7 Whether these patients should be considered for alternative SCT options (such as reduced-intensity allogeneic SCT in the older patients or antigen mismatch unrelated donor, haploidentical, or cord blood SCT for patients without a sibling or matched unrelated donor) remains debatable.8 Clearly, a strategy identifying patients who are more likely to relapse may allow the selection of patients for the higher-risk SCT procedures while sparing those with minimal likelihood of relapse from the potential complications of such transplants.
In recent years, monitoring minimal residual disease (MRD) has been increasingly used in childhood and adult ALL with a number of studies demonstrating its importance as an independent prognostic factor.9 Bassan et al used real-time quantitative polymerase chain reaction (RQ-PCR) for leukemia-specific antigens using probes for the VDJ/VJ regions of immunoglobulin heavy chain (IGH) or the κ light chain (IGκ), and the T-cell receptor (TCR) γ, δ, and β genes as well as fusion genes including BCR-ABL, MLL-AF4, and others, to detect MRD in patients with ALL, and demonstrated that the MRD status was the most significant risk factor for relapse (hazard ratio [HR], 5.22).10 In a more recent report, Gokbuget et al demonstrated that using real-time PCR directed to TCR and IG gene rearrangements, molecular response was highly predictive for outcome in patients with Ph-negative ALL.11 Similar studies, both in the adult and pediatric population, have established the usefulness of MRD assessment leading to efforts to standardize its quantification.12
In this study, we evaluated the predictive value of MRD assessment by RQ-PCR (for BCR-ABL transcripts and for the IGH gene clonality) and by multiparameter flow cytometry (MFC) in patients with Ph+ ALL treated with combination chemotherapy and TKIs, but without an allogeneic SCT, on 2 frontline protocols conducted at our institution. Three different methods for MRD assessment were used to determine whether any 1 is superior to the others for predicting outcome.
Patients and methods
Patients
From April 2001 to March 2011, 122 patients with newly diagnosed Ph+ ALL were treated with 1 of the 2 frontline regimens combining hyperCVAD (hyperfractionated cyclophosphamide, doxorubicin, vincristine, and dexamethasone) with imatinib (n = 54) or dasatinib (n = 68).3,5 One hundred fifteen (94%) achieved CR, 2 (2%) CR without peripheral blood (PB) count recovery, and 1 (1%) partial response; 4 (3%) patients died at induction. Among the patients achieving CR, 14 achieved it with 2 cycles of therapy, and were excluded as they were already established as being at a higher risk of relapse. Therefore, 101 patients achieved CR with 1 induction course and had at least 1 MRD assessment; 25 patients underwent an allogeneic SCT in first CR and were also excluded from the analysis in order to arrive at a more uniform population with regards to relapse and survival, and the role of allogeneic SCT in first CR. The overall characteristics of the final 76 patients are shown in Table 1. All patients signed an approved informed consent form to participate in the trials in accordance with the Declaration of Helsinki. The 2 clinical trials that the study is based upon were approved by the institutional review board of The University of Texas MD Anderson Cancer Center.
Characteristics of previously untreated patients treated with hyperCVAD plus TKI and achieving CR after 1 course
| Characteristics . | HyperCVAD + dasatinib . | HyperCVAD + imatinib . | Overall . |
|---|---|---|---|
| N | 48 | 28 | 76 |
| Median age, y (range) | 55 (21-78) | 53 (28-84) | 54 (21-84) |
| Median WBC, ×109/L (range) | 11.4 (0.4-658.1) | 10.4 (3.7-211) | 10.8 (0.4-658.1) |
| Performance status, n (%) | |||
| 0-1 | 46 (96) | 24 (86) | 70 (92) |
| 2 | 2 (4) | 4 (14) | 6 (8) |
| CNS disease at Dx | 3 (6) | 0 | 3 (4) |
| Cytogenetics, n (%) | |||
| Ph+ | 9 (19) | 4 (14) | 13 (17) |
| Ph+ plus other | 34 (71) | 16 (57) | 50 (66) |
| IM/ND (BCR-ABL+) | 5 (10) | 8 (29) | 13 (17) |
| Molecular, n (%) | |||
| e1a2 or e1a3 | 38 (79) | 20 (71) | 58 (76) |
| e13a2 or e14a2 | 10 (21) | 8 (29) | 18 (24) |
| CD20 expression, ≥20% | 27 (56) | 19 (68) | 46 (61) |
| Characteristics . | HyperCVAD + dasatinib . | HyperCVAD + imatinib . | Overall . |
|---|---|---|---|
| N | 48 | 28 | 76 |
| Median age, y (range) | 55 (21-78) | 53 (28-84) | 54 (21-84) |
| Median WBC, ×109/L (range) | 11.4 (0.4-658.1) | 10.4 (3.7-211) | 10.8 (0.4-658.1) |
| Performance status, n (%) | |||
| 0-1 | 46 (96) | 24 (86) | 70 (92) |
| 2 | 2 (4) | 4 (14) | 6 (8) |
| CNS disease at Dx | 3 (6) | 0 | 3 (4) |
| Cytogenetics, n (%) | |||
| Ph+ | 9 (19) | 4 (14) | 13 (17) |
| Ph+ plus other | 34 (71) | 16 (57) | 50 (66) |
| IM/ND (BCR-ABL+) | 5 (10) | 8 (29) | 13 (17) |
| Molecular, n (%) | |||
| e1a2 or e1a3 | 38 (79) | 20 (71) | 58 (76) |
| e13a2 or e14a2 | 10 (21) | 8 (29) | 18 (24) |
| CD20 expression, ≥20% | 27 (56) | 19 (68) | 46 (61) |
Dx, diagnosis; IM/ND, insufficient metaphases/not done.
Treatment regimens
The details of the 2 regimens have been published previously.3,5,13 Odd courses (1, 3, 5, and 7) of hyperCVAD were given alternately with even courses (2, 4, 6, and 8) of high-dose cytarabine and methotrexate. Either imatinib 400 daily or dasatinib100 mg orally was administered in the first 14 days each of the above 8 courses. Later, in both studies, the dose of TKI was amended to imatinib 600 mg and dasatinib 70 mg daily continuously from the second course of chemotherapy onwards.3,5 Overall, 26 and 50 patients received continuous TKI vs the intermittent strategy, respectively. For central nervous system (CNS) prophylaxis, intrathecal therapy with methotrexate and cytarabine was given alternately on days 2 and 7 of each course for a total of 6 or 8 doses depending on the risk of CNS relapse.3,5 Maintenance therapy was given for 2 years with monthly courses of vincristine and prednisone and was initiated following the completion of the 8 courses of chemotherapy (or earlier due to poor tolerability and toxicity). Imatinib 800 mg daily or dasatinib100 mg daily was administered throughout the planned 2-year maintenance and was continued after that indefinitely. Patients had bone marrow (BM) evaluations for assessment of MRD at the time of achieving CR and approximately every 3 months thereafter until completion of maintenance or relapse or death.
Response and outcome definitions
CR was defined as the presence of <5% blasts in the BM with >1 × 109/L neutrophils and >100 × 109/L platelets in the PB with no extramedullary disease. Relapse was defined by recurrence of >5% blasts in a BM aspirate unrelated to recovery or by the presence of extramedullary disease. CR duration (CRD) was calculated from the time of CR until relapse. Disease-free survival (DFS) was calculated from the time of CR until relapse or death from any cause. Event-free survival (EFS) was calculated from the beginning of treatment until an event including relapse, death during induction, or death in CR. Overall survival (OS) was calculated from the time of initiation of treatment until death.
MRD by MFC
MRD assessment by MFC was performed on whole BM specimens using a standard stain-lyse-wash procedure. Cells (1 × 106) were stained per analysis tube, and data were acquired on at least 2 × 105 cells when specimen quality permitted. Specimens were excluded as inadequate if <5 × 104 cells were available for analysis. In patients treated earlier on in the course of the studies, data on 4-color staining combinations were acquired on FACSCalibur cytometers using CellQuest software (BD Biosciences) and analyzed using FlowJo (TreeStar). Starting in March 2009, data on 6-color stains were acquired on FACSCanto cytometers using FACSDiva software (BD Biosciences) and analyzed using FCS Express (De Novo Software). Four-color combinations contained CD34–fluorescein isothiocyanate (FITC) or CD34-PerCP-Cy5.5 as well as CD19–allophycocyanin (APC) in all tubes, with additional antigens conjugated to FITC and phycoerythrin (PE) including CD10, CD13, CD15, CD20, CD22, CD25, CD33, CD38, CD45, CD58, CD66c, and CD81 (all antibodies from BD except CD10 from Beckman Coulter and CD66c from Immunotech). Six-color combinations included CD34-PerCP-Cy5.5, CD10-PE-Cy7, and CD19-V450 or CD19-BV421 in each tube (with BV421 yielding a better signal-to-noise ratio than V450), with the additional antigens listed above conjugated to FITC, PE, and APC. MRD was identified in comparison with the known patterns of antigen expression by normal maturing CD19+ B cells, as described by Weir et al.14 A distinct cluster of at least 20 cells showing altered antigen expression was regarded as an aberrant population, yielding a sensitivity for both 4-color and 6-color assays of 1 in 104 cells, or 0.01% (for 80% of specimens where at least 2 × 105 cells could be collected). The sensitivity was at least 4 in 104 cells (0.04%) for all specimens. We required aberrant expression of at least 2 antigens for the description of positive MRD. We have found that specimens obtained from a regenerating BM may have lower cell numbers available for analysis (50 000-200 000 cells per tube). These specimens have a slightly lower sensitivity for MRD detection (between 0.01% and 0.04%), and therefore a negative result must be interpreted with caution. However, many of these lower-cellularity specimens nonetheless had sufficient material to generate a positive result for residual ALL, which could have a potential clinical impact. Therefore, we believe it is reasonable to include these specimens in our analysis.
MRD by PCR
BCR-ABL RQ-PCR was performed on total RNA extracted from leukocytes following red blood cell lysis. Reverse transcription was performed using random hexamers and PCR performed using TaqMan primer/probes for the e1a2, e13a2 (b2a2), and e14a2 (b3a2) BCR-ABL transcripts in a single tube with normalization to total ABL transcripts. Post-PCR capillary electrophoresis was used to type splice form, with the method having a sensitivity of ∼1 in 105BCR-ABL–expressing cells as established by periodic dilution studies.15 Specimens were considered as suboptimal when BCR-ABL fusion transcripts were not detected (negative) and ABL copies were below <104; these specimens were not included in the study. Major molecular response (MMR) was defined as a BCR-ABL/ABL ratio of <0.1%. Values were standardized to the international scale.
IGH-PCR was performed using variable region framework 1, 2, and 3 primers, each in combination with fluorescent dye, 6-carboxyfluorescein–labeled JH1245 consensus primer and JH3-specific primer as described previously.16,17 The fluorescent-labeled products were subjected to capillary electrophoresis and Genescan analysis. At least 1 clonal rearrangement was seen in all patients. Though allele-specific IGH-PCR is specific and more sensitive for MRD assessment, this requires sequencing the clone and designing clone-specific primers for each patient. Hence, though less sensitive, due to the tedious nature of allele-specific PCR, we limited our assessment to a simpler capillary electrophoresis and Genescan approach. Using this method, the sensitivity of detection of MRD in a sample with low numbers of polyclonal B cells (such as post-treatment BM) is ∼0.2% to 1%.
Statistical methods
Patient characteristics were summarized using the median (range) for numerical variables and frequencies (percentages) for categorical variables. Associations between categorical variables were assessed using Fisher exact tests. The Kaplan and Meier method was used to estimate the hazard functions of OS and DFS, and the 2-sided log-rank test was used to compare the hazard functions between groups. EFS was defined as time to disease relapse or death, whichever came first. Multivariate Cox proportional hazards models were used to assess the EFS by MMR, IGH, and MFC as time-dependent variables, respectively, adjusting for other baseline covariates. The cumulative incidence of relapse was estimated using competing risk analysis for death and relapse.
Results
Treatment outcomes and follow-up
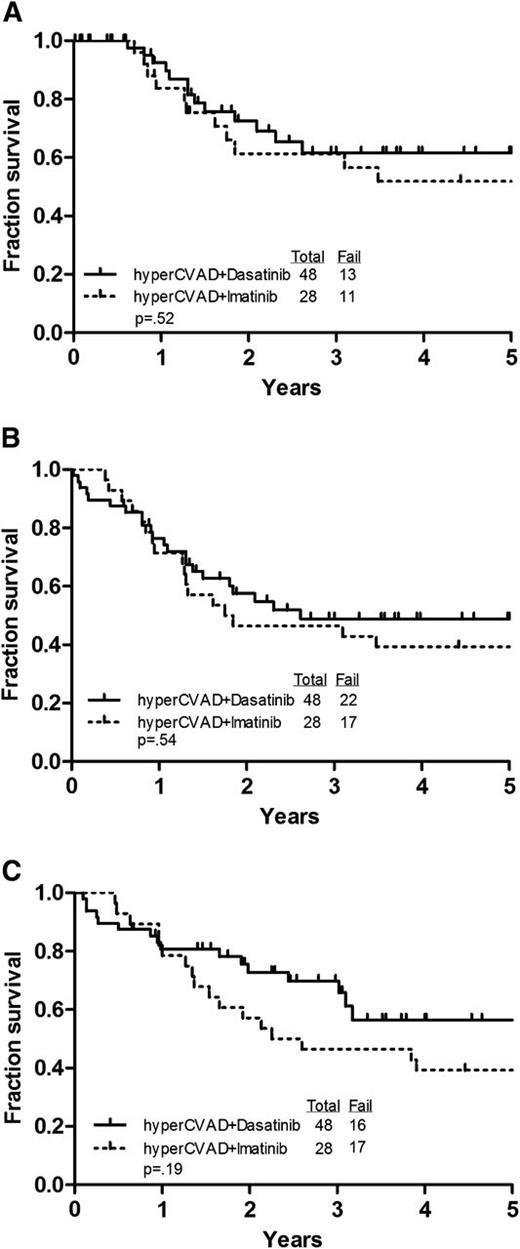
These studies were sequential frontline protocols for patients with Ph+ ALL treated from April 2001 to September 2006 (hyperCVAD + imatinib) and from September 2006 to March 2011 (hyperCVAD + dasatinib) with the median follow-up of surviving patients being 382 weeks (range, 232-501 weeks) and 123 weeks (range, 1-235 weeks), respectively. Figure 1 demonstrates CRD, DFS (from CR), and OS of the 76 patients treated on these protocols who did not receive an allogeneic SCT in first CR. Although there was a trend for better survival among the patients treated with dasatinib, this was not statistically significant.
Comparison of outcomes for the patients treated on the 2 regimens. (A) CRD. (B) DFS. (C) OS.
Comparison of outcomes for the patients treated on the 2 regimens. (A) CRD. (B) DFS. (C) OS.
Univariate analyses of EFS using Cox models with MMR, IGH, and MFC as time-dependent variables
As the status of MMR, IGH, or MFC changed over time after treatment, 3 univariate Cox regression models were used, respectively, to assess the EFS by achieving MMR, as well as IGH and flow negativity as time-dependent variables that can incorporate their status changes over time. Achieving MMR or better was associated with longer EFS (HR = 0.41; P = .002). Remaining positive for IGH by PCR was associated with a shorter EFS (HR = 1.96; P = .037), and remaining flow positive was associated with shorter EFS (HR = 3.27; P = .001).
Multivariate analysis on clinical predictors of outcome
We sought to identify characteristics at presentation that could predict relapse-free survival and OS. White blood count (WBC) at presentation, platelet count, percentage of BM blasts, presence of abnormalities in addition to Ph, type of fusion transcript (e1a2 or e1a3 vs e13a2 and e14a2), CD20 expression, and time to achieving hematologic and cytogenetic CR were evaluated. Only WBC at presentation was significantly associated with a better EFS (P = .036). Because the status of MMR, IGH, or MFC was changed over time after treatment, 3 multivariate Cox regression models were used to assess the EFS by MMR, IGH, and MFC as time-dependent variables, respectively, adjusting for other baseline covariates. Achievement of MMR (P = .002) and negative IGH (P = .018) and negative MFC (P = .028) was associated with a better EFS (results not shown). When MMR, IGH-negative status and MFC-negative status were all included in the Cox model, achievement of MMR remained significant but IGH and MFC status did not (Table 2). Due to the use of MMR, IGH, and MFC as internal time-dependent covariates in the multivariate model, the interpretation of the effects for baseline covariates in Table 2 needs to be cautious.
Multivariate analysis of predictors of long-term outcome
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| MMR | 0.248 | 0.110-0.559 | .001 |
| MRD positive by IGH | 1.651 | 0.714-3.819 | .242 |
| MRD positive by flow | 1.464 | 0.583-3.679 | .418 |
| Positive CD20 | 0.486 | 0.211-1.118 | .090 |
| Fusion protein = p210 | 0.532 | 0.179-1.582 | .256 |
| Cytogenetics status; Ph+ alone or + other | 0.665 | 0.220-2.010 | .470 |
| Platelet count | 0.996 | 0.989-1.002 | .212 |
| Blast % BM | 0.984 | 0.962-1.007 | .162 |
| Age | 1.014 | 0.985-1.045 | .347 |
| Time to CR | 0.971 | 0.897-1.051 | .460 |
| log. WBC | 2.083 | 1.050-4.133 | .036 |
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| MMR | 0.248 | 0.110-0.559 | .001 |
| MRD positive by IGH | 1.651 | 0.714-3.819 | .242 |
| MRD positive by flow | 1.464 | 0.583-3.679 | .418 |
| Positive CD20 | 0.486 | 0.211-1.118 | .090 |
| Fusion protein = p210 | 0.532 | 0.179-1.582 | .256 |
| Cytogenetics status; Ph+ alone or + other | 0.665 | 0.220-2.010 | .470 |
| Platelet count | 0.996 | 0.989-1.002 | .212 |
| Blast % BM | 0.984 | 0.962-1.007 | .162 |
| Age | 1.014 | 0.985-1.045 | .347 |
| Time to CR | 0.971 | 0.897-1.051 | .460 |
| log. WBC | 2.083 | 1.050-4.133 | .036 |
MRD by MFC as a predictor of CRD and OS
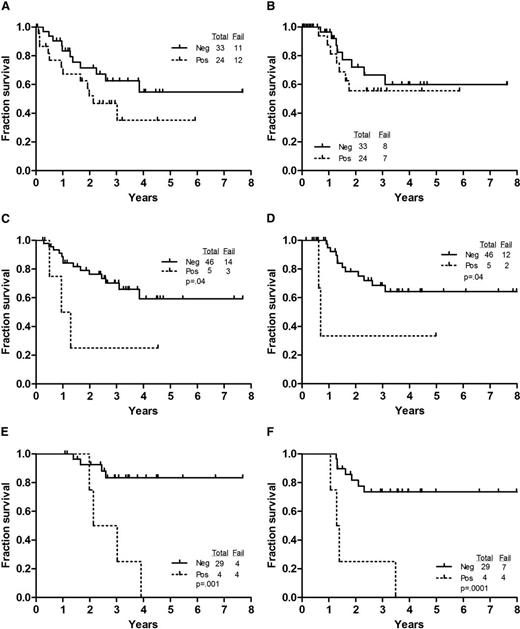
We evaluated the predictive value of detecting MRD by MFC at various time intervals after the initiation of treatment. Detection of MRD by MFC at the time of achieving CR did not affect OS or CRD (Figure 2A-B). However, patients who were positive for MRD by MFC at 3 months had a significantly shorter duration of OS and CR (P = .04 and .04, respectively) (Figure 2C-D). Furthermore, patients who were negative at 12 months had a significantly longer survival and CRD (P = .001 and .0001, respectively) (Figure 2E-F).
Outcome by achievement of negative flow at various time points. (A) OS by MFC status at CR. (B) CRD by MFC at CR. (C) OS by MFC at 3 months. (D) CRD by MFC at 3 months. (E) OS by MFC at 12 months. (F) CRD by MFC at 12 months.
Outcome by achievement of negative flow at various time points. (A) OS by MFC status at CR. (B) CRD by MFC at CR. (C) OS by MFC at 3 months. (D) CRD by MFC at 3 months. (E) OS by MFC at 12 months. (F) CRD by MFC at 12 months.
BCR-ABL ratio as a predictor of CRD and OS
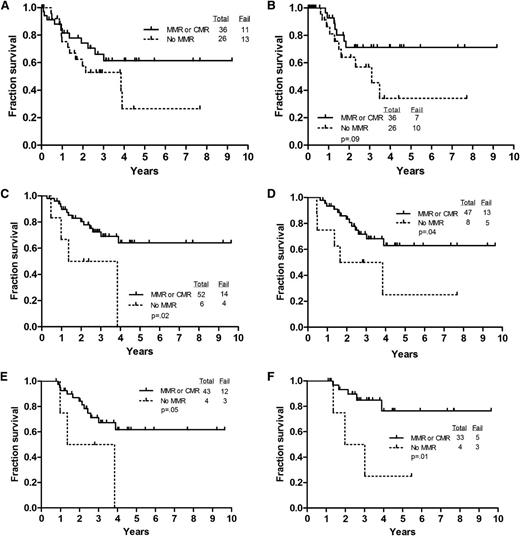
We examined the predictive value of achieving a MMR (BCR-ABL-ABL < 0.1%) at various time points, for CRD, EFS, and OS. We used MMR as a cutoff for several reasons. First, we have 7 patients with MMR who have remained in CR several years after completion of therapy. Overall, 47 (62%) patients achieved complete molecular remission sometime between achieving morphologic CR and 12 months. Twenty-four (32%) achieved MMR and 4 (5%) never achieved MMR or better. When we analyzed the data by MMR vs complete molecular remission, there was no difference in the outcome of these patients (supplemental Table 1; supplemental Figure 5, available on the Blood website). Although achieving MMR at CR was associated with longer CRD and OS, these did not reach statistical significance (Figure 3A-B). Achieving MMR at 3 and 6 months was associated with a significantly higher likelihood of longer survival (Figure 3C-D). Similarly, achieving MMR or better at 9 and 12 months was associated with longer survival (Figure 3E-F) and although CRD was longer for the patients achieving MMR at these time points, it did not reach statistical significance (figures not included).
Outcome by achievement of MMR or better at various time points. (A) OS by level of BCR-ABL to ABL transcripts at CR. (B) CRD by level of BCR-ABL to ABL transcripts at CR. (C) OS by level of BCR-ABL to ABL transcripts at 3 months. (D) OS by level of BCR-ABL to ABL transcripts at 6 months. (E) OS by level of BCR-ABL to ABL transcripts at 9 months. (F) OS by level of BCR-ABL to ABL transcripts at 12 months.
Outcome by achievement of MMR or better at various time points. (A) OS by level of BCR-ABL to ABL transcripts at CR. (B) CRD by level of BCR-ABL to ABL transcripts at CR. (C) OS by level of BCR-ABL to ABL transcripts at 3 months. (D) OS by level of BCR-ABL to ABL transcripts at 6 months. (E) OS by level of BCR-ABL to ABL transcripts at 9 months. (F) OS by level of BCR-ABL to ABL transcripts at 12 months.
MRD by IGH PCR as a predictor of CRD and OS
There was no association between achieving a negative IGH PCR at any time point and improved DFS and OS. This may be due to the use of consensus rather than leukemia-specific primers in this study.
Cumulative incidence of relapse using competing risk analysis (survival and relapse)
The cumulative incidence of relapse was estimated using competing risk analysis for death and relapse. There was no difference in the cumulative incidence of relapse by achieving MMR, or achieving MFC and IGH PCR negativity at any of the time points investigated with the exception of achieving a flow-negative status by 3 months that was associated with a lower incidence of relapse (Figure 4).
Cumulative incidence of relapse using competing risk analysis (death and relapse).
Cumulative incidence of relapse using competing risk analysis (death and relapse).
Concordance of MRD testing using different techniques
Fifteen patients had 1 or more specimens (total of 27 specimens) positive for BCR-ABL transcripts (>0.1%) but negative for MRD by MFC (supplemental Table 2). These discordances were not due to inadequate MFC specimen cell counts. Multilineage involvement with the presence of the Ph chromosome in B- and T-lymphoid, erythroid, and myeloid cells has been previously described18 and may well account for these discrepancies. This is an important consideration in the interpretation of MRD data in patients with Ph-positive ALL.
Discussion
The potential role of MRD detection after induction and consolidation in predicting the outcome has been increasingly evaluated in patients with ALL.9 A number of studies in both children and adults using either MFC or PCR-based methods have clearly demonstrated the ability to detect MRD and its potential role for stratifying the risk of relapse.17,19-27
Multiple clinical trials incorporating imatinib and dasatinib into the frontline regimens used for treating patients with Ph+ ALL have clearly established the superiority of this approach over comparable regimens without these TKIs.2,28 The majority of these trials have included imatinib, which in combination with traditional cytotoxic agents has produced higher CR rates and better long-term outcomes.28 Although dasatinib is a more potent inhibitor of the kinase activity of BCR-ABL, few studies have investigated its role in the frontline treatment of Ph+ ALL.3,29 Whether the addition of chemotherapy to dasatinib is necessary has been debated. In a mouse model of Ph+ ALL, intensive therapy with dasatinib led to the achievement of remission but invariably the disease relapsed with disseminated or CNS involvement. This was associated with the appearance of leukemic clones harboring BCR-ABL kinase domain mutations conferring resistance, in particular, the highly resistant T315I gatekeeper mutation.30 Furthermore, the addition of chemotherapy agents to dasatinib circumvented the emergence of these mutations.30 In the recently reported study by Foa et al, dasatinib was administered to patients with Ph+ ALL for 84 days combined with intrathecal chemotherapy and steroids for the first 32 days.29 Although all 53 patients enrolled in the study achieved CR, with a follow-up of 20 months, 23 patients had relapsed and 12 of 17 patients with mutational analysis had a T315I mutation.29 Of interest, on multivariate analysis, a BCR-ABL level of <10−3 at day 85 correlated with DFS.29 In our study of dasatinib with chemotherapy, with a median follow-up of 14 months, 5 of 35 patients had relapsed including 1 with the T315I mutation.3 Therefore, the available data suggests that a combination of known antileukemic agents with the available TKIs may be most effective in achieving a durable response. However, the best composition and intensity of such combination regimens is not yet fully clear.
The introduction of TKIs has revolutionized the management of patients with Ph+ ALL with several studies demonstrating improved outcome after the introduction of TKIs.31 Although allogeneic SCT remains the standard of care for these patients, a few studies have demonstrated the possibility of long-term survival without a transplant.3,6 In the pediatric study by Schultz et al, transplant plus imatinib offered no advantage over imatinib alone.6 However, few studies, have examined the potential role of monitoring for MRD to predict outcome and determine the potential role of intensification of therapy with an allogeneic SCT to eradicate it. Furthermore, it is not clear whether achieving a negative MRD status either by MFC or PCR can safely protect the patient against relapse and spare the patient the potential risks of transplant. In a study by Lee et al, the extent of reduction in the BCR-ABL transcripts after treatment with an imatinib-containing regimen was predictive of likelihood of relapse after allogeneic SCT.32
In this study, we attempted to establish whether achieving MMR or negative status for MRD by MFC and by IGH PCR could predict the outcome in a small cohort of patients with Ph+ ALL treated with combination of chemotherapy and TKIs without an allogeneic SCT. We were able to demonstrate that achieving MMR and negative MFC status at 3 months (and beyond) from starting treatment were associated with a decreased likelihood of relapse and with a longer OS. However, achieving a negative status for IGH by PCR did not predict for an improved outcome (data not shown). This may be attributable to the use of consensus rather than leukemia-specific primers in this study. We then estimated the cumulative incidence of relapse using competing risk analysis (death and relapse) and were able to demonstrate that achieving a negative MRD status by MFC at 3 months was associated with a statistically significant reduction in the risk of relapse (P = .039; Figure 4). This was not true for achieving MMR or better, or negative IGH PCR at 3 months (P = .93 and .92; data not shown). However, using achievement of MMR, negative MRD by IGH or by flow as time-dependent covariates, only achieving MMR and a lower WBC at presentation were associated with an improved EFS. Among the 44 patients who had a positive test for MRD in the first year of therapy (either by MFC or BCR-ABL transcripts), 13 have relapsed (supplemental Table 2). This includes 9 of 22 patients who were MFC positive or had less than MMR at 3 months or beyond; whereas only 13 of 54 patients who have remained negative by MFC and had BCR-ABL ≤0.1 at and beyond 3 months have relapsed. Among these 13 patients, 5 had molecular relapse preceding the morphologic relapse by 1 to 5 months which occurred after 12 months, further emphasizing the value of continued monitoring.
We note that 15 patients had 1 or more specimens (total of 27 specimens) positive for BCR-ABL transcripts (>0.1%) but negative for MRD by MFC (supplemental Table 2). These discordances were not due to inadequate MFC specimen cell counts. All subsequent relapse specimens contained CD19+ blast populations with antigenic aberrancies demonstrable using the same analysis strategies used on the specimens assessed for MRD. Several MRD specimens had high transcript levels (BCR-ABL/ABL transcript ratios of >20%), but <1% CD19+ cells by MFC, strongly suggesting that the residual Ph+ cells were present in a non-ALL compartment.18
These data clearly support the role of monitoring for MRD by MFC and by PCR for BCR-ABL in patients with Ph+ ALL who receive regimens containing TKIs. Whether these data can be used to determine whether an alternative donor transplant should be performed in first remission will require larger studies and collaborative international efforts to arrive at more concrete conclusions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ‘‘advertisement’’ in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by research funding from Bristol-Myers Squibb.
Authorship
Contribution: F.R. designed the trials and the study, treated patients, collected and analyzed data, and wrote the manuscript; D.A.T., S.O., and H.M.K. designed the trials, treated patients, and critically reviewed and approved the manuscript; R.G. collected and analyzed the data; X.H. and S.W. performed the statistical analyses; S.F., J.A.B., A.F., P.K., R.E.C., Z.E., and J.E.C. treated patients and reviewed and approved the manuscript; and S.A.W., J.L.J., R.L., and P.C. performed the MRD studies.
Conflict-of-interest disclosure: F.R. has received research funding and honoraria from Bristol-Myers Squibb, and honoraria from Novartis. J.E.C. and H.M.K. have received research funding from Bristol-Myers Squibb and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Farhad Ravandi, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: fravandi@mdanderson.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal