Key Points
AML blasts have an arginase-dependent ability to inhibit T-cell proliferation and hematopoietic stem cells.
AML blasts have an arginase-dependent ability to modulate the polarization of monocytes.
Abstract
Acute myeloid leukemia (AML) is the most common acute leukemia in adults and the second most common frequent leukemia of childhood. Patients may present with lymphopenia or pancytopenia at diagnosis. We investigated the mechanisms by which AML causes pancytopenia and suppresses patients’ immune response. This study identified for the first time that AML blasts alter the immune microenvironment through enhanced arginine metabolism. Arginase II is expressed and released from AML blasts and is present at high concentrations in the plasma of patients with AML, resulting in suppression of T-cell proliferation. We extended these results by demonstrating an arginase-dependent ability of AML blasts to polarize surrounding monocytes into a suppressive M2-like phenotype in vitro and in engrafted nonobese diabetic–severe combined immunodeficiency mice. In addition, AML blasts can suppress the proliferation and differentiation of murine granulocyte-monocyte progenitors and human CD34+ progenitors. Finally, the study showed that the immunosuppressive activity of AML blasts can be modulated through small-molecule inhibitors of arginase and inducible nitric oxide synthase, suggesting a novel therapeutic target in AML. The results strongly support the hypothesis that AML creates an immunosuppressive microenvironment that contributes to the pancytopenia observed at diagnosis.
Introduction
Studies during the last 20 years have led to an improved understanding of the genetic basis for acute myeloid leukemia (AML).1 However, the mechanisms by which AML blasts evade a patient’s immune system are poorly understood. Furthermore, the mechanisms by which AML blasts create an immune-privileged niche and suppress immune response are unknown.
Despite intensification of chemotherapy treatment, it is likely that a significant number of patients will require a hematopoietic stem cell (HSC) transplant.2 However, a significant number of patients will still relapse posttransplant and succumb to their disease.3,4 The ability of AML blasts to remain protected from the graft-versus-leukemia effect has been incompletely studied to date.5-7
In this study, we elucidate the mechanisms by which AML blasts hamper immune responses by demonstrating that secretion of arginase II from AML blasts significantly affects T-cell proliferation and polarizes monocytes toward an immunosuppressive M2-like phenotype. In addition, the increased arginine metabolism inhibits the proliferation of hematopoietic progenitors, contributing to a wider suppressive microenvironment in AML.
Methods
AML patient samples
Blood samples were obtained from 15 patients with AML who were treated at the John Radcliffe Hospital, Oxford; Hammersmith Hospital, London; or Hillingdon Hospital, London, United Kingdom. The samples were obtained from patients with newly diagnosed AML before the start of treatment. AML cells were separated from fresh samples using a Lymphoprep (Alere, Stockport, United Kingdom) gradient, followed by purification using anti-CD33 and anti-CD34 coated magnetic beads and magnetic-activated cell sorting (MACS) separation columns (Miltenyi Biotec, Bisley, United Kingdom). Samples from healthy donors were obtained from the John Radcliffe Hospital with informed consent.
Generation of human dendritic cells for mixed lymphocyte reaction
Dendritic cells were generated as previously described.8 Briefly, peripheral blood mononuclear cells were isolated by Lymphoprep gradient centrifugation. Monocytes were positively selected, using anti-CD14 magnetic beads (MACS; Miltenyi Biotec). The recovered cells were more than 99% CD14+, as determined by flow cytometry. Dendritic cells were generated by culturing monocytes in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 50 ng/mL granulocyte macrophage–CSF (GM-CSF; Peprotech, London, United Kingdom), and 1000 U/mL interleukin 14 (IL-14) for 5 days in a 6-well plate.
Mixed lymphocyte reaction
Peripheral blood lymphocytes (PBLs; 2 × 105) were cultured with allogeneic irradiated (5000 rad) dendritic cells in 200 μL RPMI 5% human AB serum (Sigma, Dorset, United Kingdom) in 96-well flat-bottom plates. Cells were incubated at 37°C, 5% CO2 for 4 days, and then 1 μCi/well 3H-thymidine (Perkin Elmer Life Sciences, Beaconsfield, United Kingdom) was added for 12 to 16 hours. 3H-thymidine incorporation was measured using a Wallac Microbeta Jet 1450 reader (Perkin Elmer). The suppressive ability of AML blasts from patients was assessed by coculturing purified blasts from patients with AML together with PBLs and irradiated dendritic cells. NG-hydroxy-l-arginine (NOHA; 0.5 mM) and L-NG-monomethyl arginine (L-NMMA; 0.5 mM) were added to mixed leukocyte reaction (MLR) cultures to inhibit arginase and inducible nitric-oxide synthase (iNOS) activity, respectively. Data are expressed as a percentage of PBL proliferation driven by allogeneic irradiated dendritic cells in the presence of AML blasts compared with alloreactive PBL proliferation in the absence of AML blasts (100%).
Immunophenotyping
PBLs from patients with AML were stained with anti-CD15 (eBioscience, Hatfield, United Kingdom), anti-CD14 (BD Bioscience, Oxford, United Kingdom), anti-CD33 (eBioscience), anti-CD34 (eBioscience), and anti-CD11b (eBioscience) antibody on ice. The cells were washed, and propidium iodide (1:200 final concentration) was added to allow viable cells to be gated. The immunophenotype was assessed by flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) in conjunction with CellQuest software. Data were analyzed using FlowJo 8.8 software (Tree Star Inc, Ashland, OR).
Reverse-transcription polymerase chain reaction analysis
Reverse-transcription polymerase chain reaction was used to detect arginase I, arginase II, and iNOS in patient-derived AML blasts. RNA was extracted using an RNeasy Mini kit (Qiagen, Manchester, United Kingdom). cDNA was prepared using SuperScript III Reverse Transcriptase (Invitrogen), following the manufacturer’s instructions. The polymerase chain reaction products were analyzed by gel electrophoresis on a 2% agarose gel and were visualized by staining with ethidium bromide (Table 1).
Clinical characteristics of AML patients and samples
| Target . | Forward primer . | Reverse primer . |
|---|---|---|
| Arginase I | 5′CTCTAAGGGACAGCCTCGAGGA3′ | 5′TGGGTTCACTTCCATGATATCTA3′ |
| Arginase II | 5′ATGTCCCTAAGGGGCAGCCTCTCGCGT3′ | 5′CACAGCTGTAGCCATCTGACACAGCTC3′ |
| iNOS | 5′CGGTGCTGTATTTCCTTACGAGGCGAAGAAGG3′ | 5′GGTGCTGCTTGTTAGGAGGTCAAGTAAAGGGC3′ |
| GAPDH | 5′CCAGCCGAGCCACATCGCTC3′ | 5′ATGAGCCCCAGCCTTCTC3′ |
| Target . | Forward primer . | Reverse primer . |
|---|---|---|
| Arginase I | 5′CTCTAAGGGACAGCCTCGAGGA3′ | 5′TGGGTTCACTTCCATGATATCTA3′ |
| Arginase II | 5′ATGTCCCTAAGGGGCAGCCTCTCGCGT3′ | 5′CACAGCTGTAGCCATCTGACACAGCTC3′ |
| iNOS | 5′CGGTGCTGTATTTCCTTACGAGGCGAAGAAGG3′ | 5′GGTGCTGCTTGTTAGGAGGTCAAGTAAAGGGC3′ |
| GAPDH | 5′CCAGCCGAGCCACATCGCTC3′ | 5′ATGAGCCCCAGCCTTCTC3′ |
Arginase II enzyme activity
The activity of arginase II present within AML blasts, culture supernatants, or patient plasma was determined by measuring the conversion of arginine into urea. Patient AML blasts or healthy donor–derived neutrophils and monocytes (1 × 106) were cultured in RPMI+10% FCS at 37°C, 5% CO2. After 24 hours, the supernatants were collected and the cells pelleted and lysed with 50 μL buffer containing 0.1% Triton X-100, 5 μg pepstatin, 5 μg aprotinin, and 5 μg antipain. The same buffer was added to 50-μL cell supernatants or patient plasma. The samples were placed on a 37°C heat block for 30 minutes before centrifugation at 14 000 rpm and collection of the supernatants. To activate the arginase enzyme, buffer containing Tris-HCl (25 mM) and MnCl2 (10 mM) was added and heated to 56°C for 10 minutes. l-arginine (0.5 M; Sigma) was added, and the samples were heated for 1 hour at 37°C. The hydrolysis of arginine was stopped with 800 μL of an acid solution mixture (H2SO4:H3PO4:H2O, 1:3:7). The amount of urea produced was determined using 9% α-isonitrosopropriophenone and compared with a standard curve with absorbance measured at 540 nm.
Monocyte polarization assay
Peripheral blood was collected from healthy donors, and monocytes were separated using a Lymphoprep gradient, anti-CD14 magnetic-beads, and MACS separation columns. Carboxyfluorescein succinimidyl ester (CFSE)-labeled monocytes were cultured in the presence or absence of AML blasts, AML culture supernatants (50% of final volume), or patient plasma (50% of final volume) for 36 hours. Cells were harvested and stained with anti-CD206 (BD Pharmingen, Oxford, United Kingdom) on ice for 30 minutes. Propidium iodide was added to allow viable cells to be gated. The expression of CD206 on monocytes was assessed by flow cytometry.
Bone marrow progenitor proliferation assay
Bone marrow was harvested from C57BL/6 mice. Murine granulocytic-monocytic precursors (GMPs) were isolated by flow cytometer sorting, following the methods described in Akashi et al.9 Purified human CD34+ HSCs were obtained from Lonza, Slough, United Kingdom. GMPs (30 × 103) were incubated with human IL-3 (2 ng/mL; Peprotech), murine GM-CSF (5ng/mL; Peprotech), human granulocyte colony-stimulating factor (10 ng/mL; Peprotech), and human FMS-related tyrosine kinase 3 ligand (10 ng/mL; Peprotech). CD34+ HSCs (30 × 103) were incubated with human stem cell factor (20 ng/mL; Peprotech), human FL3T-L (10 ng/mL), human granulocyte colony-stimulating factor (10 ng/mL), human IL-2 (10 ng/mL; Peprotech), human IL-15 (10 ng/mL; Peprotech), and Dup 697 (10−7 M; Cayman, Ann Arbor, MI). Murine GMPs and human CD34+ cells were incubated in the presence or absence of plasma (50% of final volume) from patients with AML at diagnosis for 3 days. Arginine (100 ng/mL) or NOHA (0.5 mM) and L-NMMA (0.5 mM) were added to cultures to inhibit arginase and iNOS activity, respectively. Data are expressed as a percentage of progenitor proliferation–driven controls.
AML murine xenografts
Nonobese diabetic–severe combined immunodeficiency (NOD-SCID; NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice aged 10-14 weeks were irradiated with 100 cGy twice, 4 hours apart, and given a single dose of intraperitoneal intravenous immunoglobulin 24 hours before leukemia cells were injected into the tail vein or tibia. Bone marrow was harvested from the leg and pelvic bones of mice culled 16-28 weeks after transplantation. AML engraftment was defined by the detection of a single human CD45+CD33+CD19− population that was more than 0.1% of total bone marrow mononuclear cells as assessed by flow cytometry.
Colony-forming assays
CD34+ cells (200) were mixed in 1 mL complete methylcellulose medium H4435 (Stemcell Technologies, Grenoble, France) and plated in 2-mm gridded cell culture dishes. The plates were placed inside a plastic humidity culture box within a 37°C incubator. After 7 days, the culture dishes were placed inside a 60-mm dish to count the colonies, using an inverted microscope.
Enzyme-linked immunosorbent assay
Supernatants of patient-derived AML blasts cultured in RPMI+10% FCS were collected after 24 hours, or plasma was diluted accordingly. Enzyme-linked immunosorbent assay (ELISA) kits were used to measure the concentration of arginase I and arginase II (AntibodiesOnline, Atlanta, GA) according to the manufacturer’s instructions. Concentrations of IL-1β (eBioscience), IL-10 (BD Biosciences), GM-CSF (eBioscience), and transforming growth factor β (TGF-β; eBioscience) were measured by ELISA, according to the manufacturers’ instructions.
Confocal staining
AML blasts (1 × 105) were cytospun (500 rpm) for 5 minutes and then fixed for 30 minutes in 2% paraformaldeyde (Sigma). The cells were washed in phosphate-buffered saline and permeabilized in 0.5% Triton-X100 for 1 minute. After washing, the cells were blocked (5% cold water fish gelatin, 1% bovine serum albumin, 0.5% Tween 20, 0.02% sodium azide) for 1 hour. The cells were incubated with anti-human arginase II (Santa Cruz, TX) antibody (1:50 dilution in blocking buffer) for 2 hours. After washing, secondary antibody was added (donkey-anti-rabbit immunoglobulin G: Alexa Fluor 488, 1:1000 dilution; Jackson Laboratories, Farmington, CT) for 2 hours. After final washes, cells were mounted onto glass slides using 4,6 diamidino-2-phenylindole (DAPI)-Fluromount G (Southern Biotech, AL) and examined using a confocal microscope.
Immunohistochemistry
Paraffin-embedded tissue sections of bone marrow trephines from patients with AML (Oxford University BioBank) at diagnosis (4-μm sections) were deparaffinized and rehydrated. Antigen demasking was performed in 50 mM Tris/2 mM EDTA at pH 9.0, using a Philips Whirlpool Sixth Sense microwave on a steaming program. Staining with anti-human arginase II (Santa Cruz) was carried out, using the Novolink Polymer Detection System (RE7280-K; Leica, Milton Keynes, United Kingdom). Primary antibody incubation was performed overnight in a cold room. Sections were counterstained with Gill Nr 3 hematoxylin (Sigma Aldrich, Dorset, United Kingdom) and mounted in Aquatex (Merck, Feltham, United Kingdom).
Study approval
Patient samples were obtained after written, informed consent before inclusion in the study. Regional Ethics Committee (REC 10/H0501/39) and local hospital trust ethics approval for the study was granted. Mouse experiments were carried out at Oxford University Biomedical Sciences facility in accordance with UK Government Home Office–approved Project License 30/2465.
Results
AML blasts suppress T-cell proliferation via arginase II and iNOS
Nonmalignant immature myeloid cells, called myeloid derived suppressor cells (MDSCs), have been described in cancer, infectious disease, and inflammation and have an ability to suppress T-cell proliferation.10,11 Because AML blasts share several phenotypic markers expressed by MDSCs (supplemental Figure 1A), we examined whether AML blasts also have functional characteristics in common with MDSCs.
To investigate whether AML blasts from patients could directly inhibit T-cell proliferation, we performed an MLR using AML blasts purified from patients’ peripheral blood. Samples from 15 patients were tested for suppressive activity (Figure 1A). Of 15 patients, 12 were able to suppress T-cell proliferation significantly, even at the lowest (1:8) AML blasts:T-cell ratio. No correlation was found between suppressive activity and patient clinical characteristics, including patient age, French-American-British (FAB) classification, or immunophenotype (supplemental Table 1).
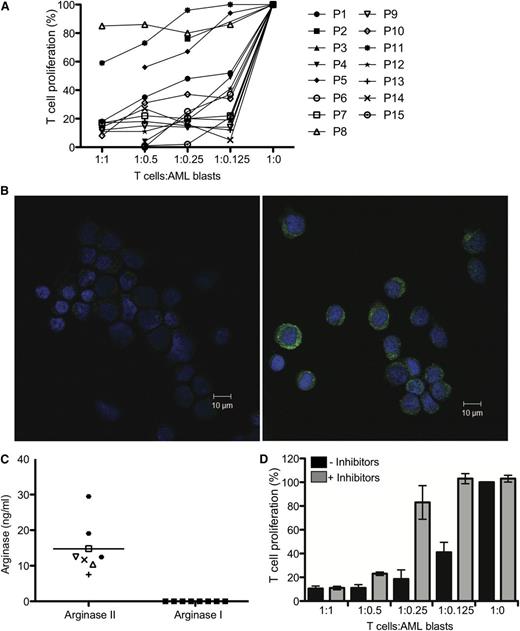
Arginine metabolism regulates the suppressive activity of AML blasts. (A) AML blasts from 15 patients were cultured with allogeneic T cells in a MLR. The ratio of AML cells:T cells ranged from 1:1 to 1:0. T-cell proliferation was measured by 3H-thymidine incorporation after 4 days. AML blasts from 12/15 patients strongly suppressed T-cell proliferation. Patients are identified by unique symbols, which are used consistently throughout the manuscript. (B) Expression of arginase II in blasts from patients with AML was confirmed by confocal microscopy. (Left) Staining with DAPI (nuclear stain) and secondary antibody alone; (right) staining with DAPI, anti-human arginase II antibody, and secondary antibody. Scale bar = 10 μm. (C) AML blasts release arginase II into the microenvironment. Supernatants from cultures (24 hours) of patients’ AML blasts were analyzed by ELISA for arginase II and arginase I (***P = .0001). (D) AML blasts from patients were cultured with allogeneic T cells in a MLR in the presence of the enzyme-specific inhibitors for arginase (NOHA) and iNOS (L-NMMA). The ratio of AML cells:T cells ranged from 1:1 to 1:8. T-cell proliferation was measured by 3H-thymidine incorporation after 4 days. Culture with the enzyme inhibitors restores T-cell proliferation. Data are representative of 5 independent experiments (error bars, SD).
Arginine metabolism regulates the suppressive activity of AML blasts. (A) AML blasts from 15 patients were cultured with allogeneic T cells in a MLR. The ratio of AML cells:T cells ranged from 1:1 to 1:0. T-cell proliferation was measured by 3H-thymidine incorporation after 4 days. AML blasts from 12/15 patients strongly suppressed T-cell proliferation. Patients are identified by unique symbols, which are used consistently throughout the manuscript. (B) Expression of arginase II in blasts from patients with AML was confirmed by confocal microscopy. (Left) Staining with DAPI (nuclear stain) and secondary antibody alone; (right) staining with DAPI, anti-human arginase II antibody, and secondary antibody. Scale bar = 10 μm. (C) AML blasts release arginase II into the microenvironment. Supernatants from cultures (24 hours) of patients’ AML blasts were analyzed by ELISA for arginase II and arginase I (***P = .0001). (D) AML blasts from patients were cultured with allogeneic T cells in a MLR in the presence of the enzyme-specific inhibitors for arginase (NOHA) and iNOS (L-NMMA). The ratio of AML cells:T cells ranged from 1:1 to 1:8. T-cell proliferation was measured by 3H-thymidine incorporation after 4 days. Culture with the enzyme inhibitors restores T-cell proliferation. Data are representative of 5 independent experiments (error bars, SD).
Human MDSCs can suppress T-cell proliferation through a number of mechanisms. The best established is through the metabolism of l-arginine by arginase and iNOS, depleting arginine from the microenvironment.12-14 More recently, cysteine depletion has been identified as an alternate mechanism of suppression used by some subsets of MDSCs.15 MDSCs can also extend their immunosuppressive microenvironment by modulating the surrounding macrophages and dendritic cells.16,17
We investigated whether AML blasts suppress T-cell proliferation by degrading arginine. First, we demonstrated that the majority of blasts in patients with AML expressed arginase II and iNOS (supplemental Figure 1B), whereas arginase I was detected at a low level only in some patients analyzed (data not shown). The significant expression of arginase II was confirmed by confocal microscopy of AML blasts from patients (Figure 1B). We confirmed that the arginase was active by measuring the ability of AML blasts to convert arginine to urea (supplemental Figure 1C). The results showed the presence of high arginase activity in the lysate of AML blasts (range, 4.5-12.4 mU arginase/1 × 106 cells). However, consistent with previously published papers demonstrating that arginase II activity may be modulated by a number of factors, including urea and arginine, we did not observe a correlation between arginase activity and absolute arginase intracellular concentration (P = .29) (supplemental Figure 1D).18,19 In comparison, the arginase activity of monocytes and neutrophils from healthy donors was significantly lower (median of 0 and 0.61 mU of arginase/1 × 106 cells, respectively; supplemental Figure 1C), illustrating that AML blasts have significant arginase activity.
We showed that AML blasts release arginase II, but not arginase I, into culture medium (Figure 1C) and that IL-10, IL-4, GM-CSF, and TGF-β were also not detectable (data not shown). We confirmed that the arginase and iNOS are responsible for patients’ AML suppressive phenotype, as defined by the ability of the arginase and iNOS inhibitors NOHA and L-NMMA, respectively, to rescue T-cell proliferation, which was particularly evident at lower AML blast:T-cell ratios (Figure 1D).
AML enhances the suppressive microenvironment through the release of arginase
The ability of AML blasts to secrete high levels of enzymatically active arginase II in vitro led to our hypothesis that arginase II released from AML blasts could accumulate in the blood of patients and have a significant antiproliferative effect on lymphocytes.
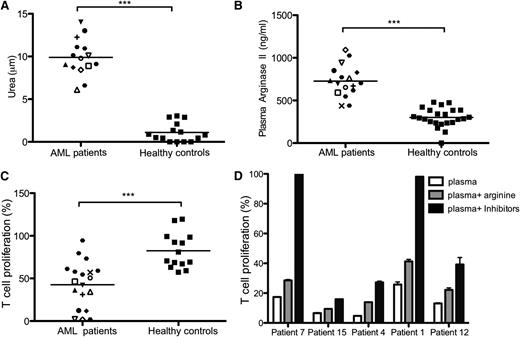
Our results showed, first, that arginase II activity is significantly raised in the plasma of patients with AML compared with healthy controls (mean, 9.9 μmol vs 1.1 μmol urea; P = .0001) (Figure 2A). Second, high levels of arginase II (727 ng/mL vs 314 ng/mL; P = .0001) were seen in the blood of patients with AML at diagnosis (Figure 2B), whereas arginase I levels were not raised in comparison with healthy controls (supplemental Figure 2A). Plasma was available from 2 patients after a single cycle of chemotherapy and showed a significant drop in arginase activity toward the levels found in healthy donors (supplemental Figure 2B). Consistent with the presence of high arginase activity in the plasma of patients with AML, we showed that the plasma of patients with AML significantly inhibits in vitro T-cell proliferation (P = .0001) (Figure 2C) and that the suppression is relieved through the use of L-NMMA and NOHA or by arginine replacement (Figure 2D).
AML blasts extend their suppressive microenvironment through high concentrations of active arginase II in patient plasma. (A) Arginase activity from plasma of 15 patients with AML and 15 healthy donors was analyzed (***P = .0001). Fifty microliters of patient plasma was tested for the ability to convert arginine into urea, using a colorimetric assay. (B) Plasma (50 μL) from 17 patients with AML and 21 healthy donors was analyzed for arginase II concentration by ELISA (***P = .0001). (C) T-cell proliferation of alloreactive T cells stimulated by allogeneic DC in a total volume of 200 μL, with 50 μL of plasma from patients with AML collected at time of diagnosis or from healthy donors (***P = .0001). (D) T cells from healthy donors were cultured in a MLR in the presence of plasma from patients with AML and the enzyme-specific inhibitors for arginase (NOHA) and iNOS (L-NMMA) or arginine. T-cell proliferation was measured by 3H-thymidine incorporation after 4 days. Culture with the enzyme inhibitors or arginine replacement restores T-cell proliferation. Data are representative of 5 independent experiments (error bars, SD).
AML blasts extend their suppressive microenvironment through high concentrations of active arginase II in patient plasma. (A) Arginase activity from plasma of 15 patients with AML and 15 healthy donors was analyzed (***P = .0001). Fifty microliters of patient plasma was tested for the ability to convert arginine into urea, using a colorimetric assay. (B) Plasma (50 μL) from 17 patients with AML and 21 healthy donors was analyzed for arginase II concentration by ELISA (***P = .0001). (C) T-cell proliferation of alloreactive T cells stimulated by allogeneic DC in a total volume of 200 μL, with 50 μL of plasma from patients with AML collected at time of diagnosis or from healthy donors (***P = .0001). (D) T cells from healthy donors were cultured in a MLR in the presence of plasma from patients with AML and the enzyme-specific inhibitors for arginase (NOHA) and iNOS (L-NMMA) or arginine. T-cell proliferation was measured by 3H-thymidine incorporation after 4 days. Culture with the enzyme inhibitors or arginine replacement restores T-cell proliferation. Data are representative of 5 independent experiments (error bars, SD).
AML blasts polarize monocytes to an M2-like phenotype
To determine whether AML blasts create an immunosuppressive niche, we investigated whether AML blasts can induce polarization of monocytes into immunosuppressive M2-like cells, characterized in humans by the expression of CD206 (mannose receptor)20 and by the increase of arginase I expression and IL-10 secretion.21
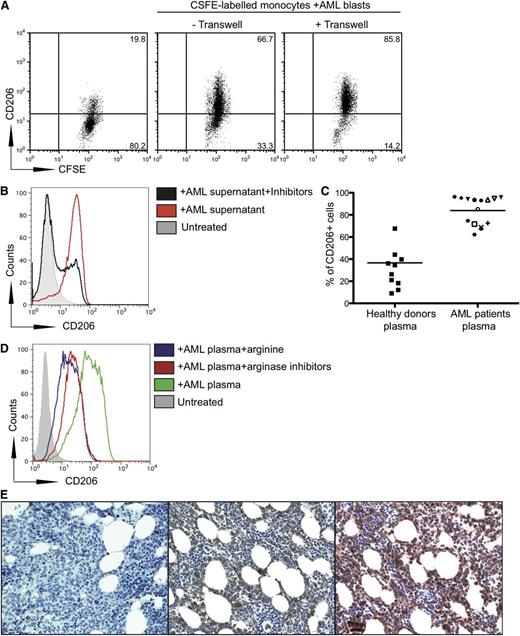
We showed that the expression of CD206 was significantly up-regulated on monocytes cocultured with AML blasts compared with that on untreated monocytes (Figure 3A). This upregulation occurred even if AML blasts and monocytes were separated by a transwell.
AML blasts increase CD206 expression on monocytes. (A) Transwell culture of CFSE-labeled monocytes from healthy donors with AML blasts from patients upregulates CD206 expression on monocytes. Monocytes from healthy donors were placed in the lower well and AML blasts from patients were placed in the upper well of a transwell assay system. Representative flow cytometry plots from 1 patient of 11 tested are shown. (B) Coculture of CFSE-labeled monocytes from healthy patients with supernatants (50% of final volume) from AML blasts upregulates CD206 on monocytes, as analyzed by flow cytometry. (C) Upregulation of CD206 on monocytes cultured with plasma of 15 patients with AML or 15 healthy donors (P < .0001). Percentage of CD206+ cells is shown. (D) Upregulation of CD206 on monocytes cultured with plasma of patients with AML with or without arginine (100 ng/mL) and inhibitors (0.5 mM). Data are representative of 3 independent experiments (E) Staining of bone marrow from patients with AML at diagnosis with anti-human CD206 (center), with DAPI alone (left), and with anti-arginase II (right). Representative marrow from a single patient with AML shown of 6 (samples obtained from University of Oxford Biobank).
AML blasts increase CD206 expression on monocytes. (A) Transwell culture of CFSE-labeled monocytes from healthy donors with AML blasts from patients upregulates CD206 expression on monocytes. Monocytes from healthy donors were placed in the lower well and AML blasts from patients were placed in the upper well of a transwell assay system. Representative flow cytometry plots from 1 patient of 11 tested are shown. (B) Coculture of CFSE-labeled monocytes from healthy patients with supernatants (50% of final volume) from AML blasts upregulates CD206 on monocytes, as analyzed by flow cytometry. (C) Upregulation of CD206 on monocytes cultured with plasma of 15 patients with AML or 15 healthy donors (P < .0001). Percentage of CD206+ cells is shown. (D) Upregulation of CD206 on monocytes cultured with plasma of patients with AML with or without arginine (100 ng/mL) and inhibitors (0.5 mM). Data are representative of 3 independent experiments (E) Staining of bone marrow from patients with AML at diagnosis with anti-human CD206 (center), with DAPI alone (left), and with anti-arginase II (right). Representative marrow from a single patient with AML shown of 6 (samples obtained from University of Oxford Biobank).
It has been demonstrated that IL-10, IL-4, GM-CSF, and TGF-β are able to polarize monocytes to an M2-like phenotype.22,23 Because we have demonstrated that AML blasts do not spontaneously release IL-10, IL-4, GM-CSF, and TGF-β into culture supernatants, we investigated whether the increased arginase II secretion could have a role in CD206 upregulation on healthy donor monocytes. We cocultured monocytes from healthy patients with supernatants from AML blasts with or without NOHA and L-NMMA and demonstrated that the inhibitors significantly reduced the expression of CD206 on monocytes from 70% to 22.5% (Figure 3B).
To further investigate these results, we studied whether the increased arginase activity in plasma from patients with AML increased CD206 expression on healthy monocytes. We demonstrated a significant upregulation of CD206 on monocytes cultured with AML plasma compared with those cultured with plasma from the healthy donors, indicating a switch to an M2-like phenotype (P = .0001) (Figure 3C). The addition of L-NMMA and NOHA or exogenous arginine to the culture media significantly reduced CD206 expression (Figure 3D). ELISAs of patient plasma confirmed that levels of IL-4, IL-10, GM-CSF, and TGF-β were not raised compared with healthy controls, and thus were unlikely to be controlling polarization of the monocytes (data not shown). We confirmed that CD206+ monocytes are suppressive to T-cell proliferation (supplementary Figure 3). Importantly, we found significant expression of CD206+ cells with morphology consistent with that of monocytes (Figure 3E) and expression of arginase II within bone marrow of patients with AML (Figure 3E). Flow cytometry ruled out that CD206 is expressed on AML blasts (data not shown).
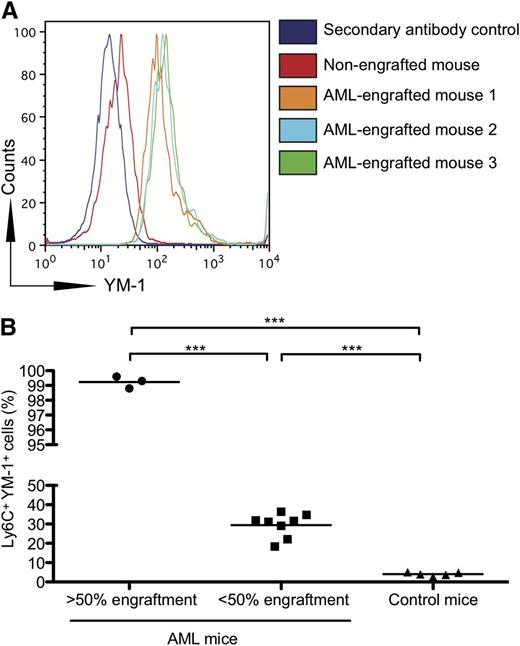
Consistent with the hypothesis that AML blasts have the ability to polarize monocytes to an M2-like phenotype in vivo, we examined the expression of YM-1, a murine M2 marker, on the bone marrow monocytes of NOD-SCID mice engrafted with different percentages of blasts from patients with AML. We found that YM-1 is significantly up-regulated on the Ly6C+ monocytes of mice engrafted with blasts from patients with AML (Figure 4A). Furthermore, the percentage of YM-1 monocytes increased as the percentage of AML engraftment increased (Figure 4B).
Monocytes from NOD-SCID mice engrafted with human AML express increased YM-1. (A) NOD-SCID mice were injected with AML cells from patients. After engraftment, bone marrow was harvested from the femurs, and the expression of YM-1 on murine monocytes (Ly6C+) was assessed by flow cytometry. (B) Increased percentage of YM-1 positive monocytes in NOD-SCID mice engrafted with human AML. NOD-SCID mice were injected with AML cells from patients. After engraftment, bone marrow was harvested from the femurs, and the percentage of YM-1+ Ly6C+ murine monocytes was assessed by flow cytometry (***P = .0001).
Monocytes from NOD-SCID mice engrafted with human AML express increased YM-1. (A) NOD-SCID mice were injected with AML cells from patients. After engraftment, bone marrow was harvested from the femurs, and the expression of YM-1 on murine monocytes (Ly6C+) was assessed by flow cytometry. (B) Increased percentage of YM-1 positive monocytes in NOD-SCID mice engrafted with human AML. NOD-SCID mice were injected with AML cells from patients. After engraftment, bone marrow was harvested from the femurs, and the percentage of YM-1+ Ly6C+ murine monocytes was assessed by flow cytometry (***P = .0001).
The AML microenvironment suppresses the proliferation of bone marrow hematopoietic progenitors
Patients with AML frequently present with pancytopenia, even though the bone marrow is not packed with AML blasts. We hypothesized that the secretion of arginase II from AML described earlier could also inhibit the proliferation of bone marrow hematopoietic progenitors. To address this possibility, we isolated murine GMPs and investigated the effect of AML-derived arginine metabolism on GMPs by incubating murine GMPs in the presence of plasma from patients with AML. Plasma from patients with AML significantly suppressed the proliferation of GMPs compared with plasma from healthy donors (P = .0001), and this effect could be reversed by the addition of NOHA and L-NMMA (P = .0001) (Figure 5A-B).
The AML microenvironment suppresses GMP and CD34+ progenitors. (A) plasma from patients with AML suppresses murine GMP proliferation. CFSE-labeled murine GMPs were isolated and cultured in the presence of plasma from patients with AML and in the presence of L-NMMA (0.5 mM) and NOHA (0.5 mM). A representative histogram plot of 5 patient AML plasma experiments is shown. Independent experiments were performed on 2 separate occasions. (B) AML-induced GMP suppression is overcome by the addition of L-NMMA and NOHA. GMPs cultured in the presence of AML plasma (50% of final volume) have significantly reduced proliferation compared with those cultured in the presence of healthy donor plasma (P = .0001) or AML plasma with L-NMMA (0.5 mM) and NOHA (0.5 mM) (P = .0001). (C) Plasma from patients with AML suppresses human CD34+ HSC proliferation. CFSE-labeled human CD34+ HSCs were isolated and cultured in the presence of plasma from AML patients and in the presence of L-NMMA (0.5 mM) and NOHA (0.5 mM). Data are representative of 5 experiments with plasma from patients with AML. Independent experiments were performed on 2 separate occasions. (D) AML-induced human CD34+ HSC suppression is overcome by the addition of L-NMMA and NOHA. CD34+ HSCs cultured in the presence of AML plasma have significantly reduced proliferation compared with those cultured in the presence of healthy donor plasma (P = .0002) or AML plasma with L-NMMA and NOHA (P = .003). Independent experiments were performed on 2 separate occasions.
The AML microenvironment suppresses GMP and CD34+ progenitors. (A) plasma from patients with AML suppresses murine GMP proliferation. CFSE-labeled murine GMPs were isolated and cultured in the presence of plasma from patients with AML and in the presence of L-NMMA (0.5 mM) and NOHA (0.5 mM). A representative histogram plot of 5 patient AML plasma experiments is shown. Independent experiments were performed on 2 separate occasions. (B) AML-induced GMP suppression is overcome by the addition of L-NMMA and NOHA. GMPs cultured in the presence of AML plasma (50% of final volume) have significantly reduced proliferation compared with those cultured in the presence of healthy donor plasma (P = .0001) or AML plasma with L-NMMA (0.5 mM) and NOHA (0.5 mM) (P = .0001). (C) Plasma from patients with AML suppresses human CD34+ HSC proliferation. CFSE-labeled human CD34+ HSCs were isolated and cultured in the presence of plasma from AML patients and in the presence of L-NMMA (0.5 mM) and NOHA (0.5 mM). Data are representative of 5 experiments with plasma from patients with AML. Independent experiments were performed on 2 separate occasions. (D) AML-induced human CD34+ HSC suppression is overcome by the addition of L-NMMA and NOHA. CD34+ HSCs cultured in the presence of AML plasma have significantly reduced proliferation compared with those cultured in the presence of healthy donor plasma (P = .0002) or AML plasma with L-NMMA and NOHA (P = .003). Independent experiments were performed on 2 separate occasions.
Consistent with these findings, we showed that human CD34+ HSCs had impaired proliferation (P = .0002) (Figure 5C-D) and retained CD34+ expression in the presence of plasma from patients with AML compared with those cultured in the plasma of healthy donors (P = .0007) (supplemental Figure 4B). Inhibition of arginine metabolism through L-NMMA and NOHA restored HSC proliferation (P = .003) (Figure 5C-D).
Although patients with AML present with pancytopenia, treatment with chemotherapy leads to a reduction in AML blast numbers and bone marrow reconstitution. Using colony-forming assays, we investigated whether CD34+ cells retained the ability to proliferate after their removal from the AML microenvironment. HSCs cocultured for 3 days in plasma from either patients with AML or healthy donors were transferred into a colony-forming assay (supplemental Figure 4A). We observed that HSCs previously cultured in healthy donor plasma (P = .0007 compared with AML plasma cultures) or with AML plasma treated with the inhibitors (P = .029 compared with AML plasma cultures) formed limited numbers of colonies. In contrast, HSCs initially cultured in AML plasma alone retained CD34+ expression and had increased colony-forming potential when transferred into a colony-forming assay (supplemental Figure 4A-B). The results of these experiments support the conclusion that CD34+ HSCs incubated with plasma from patients with AML, unlike HSCs incubated with plasma from healthy donors, are kept quiescent, but when removed from this suppressive microenvironment, they can proliferate and differentiate. Therefore, the increased arginase activity of the AML microenvironment not only polarizes T-cell and monocyte differentiation but also prevents the division and differentiation of both early myeloid progenitors and CD34+ HSCs.
Discussion
This study describes, for the first time, several mechanisms by which AML suppresses the immune response. We have shown that AML blasts have, in vitro, the ability to inhibit proliferation of mouse and human hematopoietic precursors and that patient-derived AML blasts suppress T-cell proliferation, an effect mediated by the secretion of arginase II. We have extended these results by demonstrating an arginase-dependent ability of AML blasts to polarize surrounding monocytes into a suppressive M2-like phenotype. In addition, the AML microenvironment can suppress the proliferation and differentiation of hematopoietic progenitor cells. Finally, the study showed that the immunosuppressive activity of AML blasts described earlier can be modulated through the use of small-molecule inhibitors of arginase II and iNOS. Together, the results support the hypothesis that AML blasts can create an immunosuppressive microenvironment both in the bone marrow and in blood, which may account for the pancytopenia often observed in patients with AML.
AML blasts are a malignant expansion of immature myeloid cells and thus are reminiscent of the MDSCs identified in a number of human diseases and tumors.24 Consistent with this, AML blasts express the same immunophenotype described for nonmalignant myeloid cells (CD33+HLADR− CD14+/CD15+), suggestive of their common developmental origin.11,25 It has been shown that T cells and NK cells cocultured in AML blasts supernatants have decreased proliferation, although the mechanisms have not been identified.5,6
One of the most well-characterized mechanisms of myeloid cell immunosuppression is mediated by altered arginine catabolism and the production of reactive nitric species. It is well established that arginase I expression in MDSCs is responsible for the suppressive activity of these cells. In our study, we found that AML blasts express arginase II, another isoform of arginase that has been less well characterized in the context of immunosuppression. We showed that arginase II in the AML blasts is enzymatically active, converting arginine into urea. The 2 isoforms of arginase are likely to have resulted from a gene duplication event during evolution with arginase I located in the cytosol, whereas arginase II is located in the mitochondria.26 The 2 enzymes catalyze the same reaction, converting arginine into ornithine, with urea as a byproduct. In healthy donors, arginase I is expressed predominantly by hepatocytes, whereas arginase II is expressed in a more diverse range of organs. We also show for the first time that AML blasts express and release arginase II and that the arginase II activity suppresses T-cell proliferation. The role of arginase II in inhibiting immune responses has been subject to only a limited study.27 Our study therefore identifies a novel mechanism through which malignancies, and in particular AML, can deplete arginine from the microenvironment, leading to impaired T-cell responses.
The ability of AML blasts to create an immunosuppressive microenvironment is extended through the finding of increased active arginase II in the plasma of patients with AML. It has previously been reported that concentrations of arginase I and increased arginase activity are found in patients with cancer who have increased numbers of suppressive myeloid cells.12 Our study is the first to report increased plasma arginase II levels in patients with a malignant disease. The combination of increased intracellular arginase activity and plasma arginase activity halts T-cell proliferation and may contribute to the lymphopenia found at diagnosis. In addition, arginase II levels and activity may serve as an important biomarker in patients with AML. Plasma levels of other enzymes are already routinely assayed to indicate tissue damage and disease progression (aspartate aminotransferase, alanine aminotransferase). Measurement of arginase II levels may act as a biomarker for minimal residual disease.
Our study identified that AML blasts, and accordingly the supernatants of AML cultures, can polarize surrounding monocytes to an immunosuppressive M2-like phenotype, and this finding was confirmed in vivo. In our model, we found increased numbers of YM-1 expressing monocytes in the bone marrows of NOD-SCID mice engrafted with human AML blasts. Furthermore CD206+ monocytes were found in the bone marrow of patients with AML at diagnosis. The polarization of monocytes to an M2-like phenotype was confirmed to be arginase dependent.
It is widely reported that monocytes/macrophages can play a critical role in tumor progression and can be associated with poor clinical outcome.28 M2 macrophages are associated with the expression of YM-1 in mice and CD206 in humans.29 Macrophages also promote the retention of progenitor cells in the bone marrow niche.30 This mechanism may be particularly important in the early development of AML, in which surrounding monocytes can be coopted in the bone marrow to create an immunosuppressive niche. There is growing evidence that AML can influence normal bone marrow cells, such as osteoclasts, to create a niche even when the disease burden is low.31 Further work is required to understand whether AML stem cells can remain quiescent and immunologically undetected by switching local monocytes to an M2 phenotype.
Alongside lymphopenia, patients with AML frequently present with a more generalized pancytopenia. This is often assumed to be a result of the malignant expansion of AML blasts reducing the stromal niche available to support normal hematopoiesis. Our study provides the first evidence that the AML microenvironment can actively suppress both GMP and CD34+ HSC proliferation through increased arginine metabolism, and further work is required to elucidate the mechanisms through which arginine regulates HSC differentiation and proliferation.
Inhibitors of arginase and iNOS have been used to confirm the roles of these enzymes in mediating immunosuppressive activity in studies of suppressive myeloid cells, and we showed that a combination of the arginase and iNOS inhibitors can relieve the suppressive activity of AML. The requirement for inhibitors of both enzymes for T-cell proliferation to be restored is well-established in the literature and points to a more complex interplay between arginase and iNOS in catabolizing arginine to cause immunosuppression.11
The finding of altered arginase metabolism by AML blasts may have important clinical consequences. One of the difficulties in curing patients of AML is that residual AML stem cells remain in the bone marrow. If the immunosuppression created by AML blasts can be overcome, it is possible that a patient’s own immune system or a graft-vs-leukemia effect (postallogeneic transplant) may enable the killing of any residual blasts. Therapy that targets this immunosuppressive activity of AML could be given as a maintenance course after AML consolidation chemotherapy or after bone marrow transplant. Furthermore, the ability to overcome the AML microenvironment could lead to enhanced hematopoiesis after therapy. However, limitations remain, as NOHA and L-NMMA have not entered clinical trials because of concerns that NOHA may inhibit the urea cycle in healthy cells and because L-NMMA can be toxic to mice in high doses. Alternate strategies are being developed including the use of nitroaspirin and phosphodiesterase-5 inhibitors.32
This study provides strong preclinical evidence for how AML blasts avoid immune detection and prompts further work to understand how the immunosuppressive environment in patients can be overcome to improve overall survival.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and parents who contributed samples to the study and Nicolas Goardon for AML-engrafted murine bone marrow, Marina Samitsch for the human CD34+ differentiation protocol, and Mimi Sheikh, Andrew Innes, and Kate Stringaris for the collection of patient samples. The authors also thank Moira Johnson and John-Paul Jukes for critical reading of the manuscript.
This work was supported by the Amber Phillpott Trust, the Anya Sturdy Trust, the Wellcome Trust and Cancer Research UK (Grant C399/A2291), and the UK Medical Research Council.
Authorship
Contribution: F.M. and C.D.S. designed the study, performed research, analyzed data and wrote the manuscript; F.M. secured ethical approval and was chief investigator of the study; I.A.-D. designed and performed research; S.B. performed research; L.Q. provided patient samples and access to murine xenografts; R.M.M.-S. performed confocal microscopy; A.Q. provided patient samples; F.D. provided patient samples; P.V. provided patient samples and supervised the experimental design; and V.C. designed the study and supervised the research, data analysis, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vincenzo Cerundolo, Medical Research Council Human Immunology Unit, Radcliffe Department of Medicine, Medical Research Council Weatherall Institute of Molecular Medicine, University of Oxford, OX3 9DS Oxford, United Kingdom; e-mail: vincenzo.cerundolo@ndm.ox.ac.uk.
References
Author notes
F.M. and C.D.S. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal