Key Points
Lineage-inappropriate expression of the B-cell master regulator PAX5 in t(8;21) AML depends on aberrant MAP kinase signaling.
MAP kinase signaling by a mutated growth factor receptor leads to the dissociation of polycomb-repressive complexes from PAX5 chromatin.
Abstract
The activation of B-cell–specific genes, such as CD19 and PAX5, is a hallmark of t(8;21) acute myeloid leukemia (AML) which expresses the translocation product RUNX1/ETO. PAX5 is an important regulator of B-lymphoid development and blocks myeloid differentiation when ectopically expressed. To understand the molecular mechanism of PAX5 deregulation, we examined its chromatin structure and regulation in t(8;21) AML cells, non-t(8;21) myeloid precursor control cells, and pre-B cells. In non-t(8;21) myeloid precursors, PAX5 is poised for transcription, but is repressed by polycomb complexes. In t(8;21) AML, PAX5 is not directly activated by RUNX1/ETO, but expression requires constitutive mitogen-activated protein (MAP) kinase signaling. Using a model of t(8;21) carrying an activating KIT mutation, we demonstrate that deregulated MAP kinase signaling in t(8;21) AML abrogates the association of polycomb complexes to PAX5 and leads to aberrant gene activation. Our findings therefore suggest a novel role of activating tyrosine kinase mutations in lineage-inappropriate gene expression in AML.
Introduction
The t(8;21) is one of the most widely studied chromosomal translocations associated with the core-binding factor (CBF) complex in human myeloid leukemia.1 The CBF complex consists of a member of the RUNX family of transcription factors along with its non-DNA–binding partner CBFβ.2 In t(8;21) acute myeloid leukemia (AML), the N-terminal domain of RUNX1 is fused to the transcriptional repressor ETO (also called RUNX1T1 or MTG8),3 generating the fusion protein RUNX1/ETO. RUNX1/ETO expression initiates extensive reprogramming of the epigenome and deregulates the normal myeloid gene expression program.4,5 Heterozygous RUNX1/ETO knock-in mice fail to establish hematopoiesis and die at embryonic day 11.5,6 but conditional expression of the full-length RUNX1/ETO alone in precursor cells is unable to establish AML.7-10 t(8;21) AML patients present additional mutations, such as activating mutations in growth factor receptors. This includes the KIT N822K or FLT3-length mutations [FLT3-LM]) which, in cooperation with RUNX1/ETO, cause fatal leukemia in mice.11,12 Activating growth factor receptor mutations provide a proliferative advantage to leukemic cells; however, little is known of how aberrant signaling impacts on gene expression in t(8;21) AML.
One of the defining features of t(8;21) AML is a mixed lineage phenotype indicated by the coexpression of myeloid and B-cell–specific genes such as CD19 and PAX5, an expression pattern that is only rarely seen in other types of AML.13-15 Within the hematopoietic system, PAX5 is normally expressed exclusively in the B-lymphoid lineage16 where it regulates B-cell commitment by upregulating B-cell–specific genes (such as CD19)13 and downregulating genes required to adopt other cell fates.17-19 We showed previously that PAX5 is directly responsible for the aberrant upregulation of CD19 in t(8;21) cells.13 Both RUNX1/ETO and PAX5 expression impact on the activity of genes important for myeloid differentiation (such as PU.1 and CSF1R).20 This was further substantiated by experiments ectopically expressing PAX5 in mouse bone marrow stem/progenitor cells which leads to the formation of biphenotypic B220+GR1/MAC1+ cells displaying sustained growth and expressing both myeloid and B-lineage genes at the single-cell level.21 Expression of PAX5 in human CD34+ cord-blood cells results in the generation of small myeloid progenitors that coexpress CD33 and CD19 with blocked myeloid differentiation.22 These studies suggest that aberrant expression of PAX5 in t(8;21) AML accounts for its biphenotypic features and contributes to the block in myeloid differentiation. However, a definite mechanism of PAX5 deregulation has not so far been described. We therefore examined messenger RNA expression, chromatin structure, histone modifications, and RNA polymerase II occupancy at the PAX5 locus in human pre-B cells and compared that to t(8;21) cells as well as non-t(8;21) myeloid precursor cells. We show that PAX5 is not a direct target of RUNX1/ETO, but instead is activated by a signaling-dependent alleviation of polycomb repression.
Methods
Cell culture and inhibitor treatment
Ramos, Nalm-6, Kasumi-1, SKNO-1 (provided by Health Science Research Resources Bank), and HeLa cells were maintained in RPMI 1640 (Sigma-Aldrich); HL-60 and KG-1 were maintained in Iscove modified Dulbecco media (Sigma-Aldrich). All media were supplemented with 10% heat-inactivated fetal calf serum (Gibco; Life Technologies), penicillin/streptomycin (Gibco; Life Technologies), and l-glutamine (Gibco; Life Technologies). Patient materials used are same as in a recent study.5 The method used to purify these leukemic cells is described in detail in supplemental Methods (available on the Blood website). Frozen cells (2 × 106) isolated from the bone marrow of a t(8;21) patient who had relapsed were thawed in RPMI 1640 media and treated with c-Jun N-terminal kinase (JNK), mitogen-activated protein (MAP) kinase kinase (MEK), and p38 inhibitors.
Cells were treated with 10µM AKT inhibitor (Akt inhibitor IV; Santa Cruz Biotechnology), JNK inhibitor (P600125; Sigma-Aldrich), p38 inhibitor (SB20219; Sigma-Aldrich), MEK inhibitor (PD98059; Sigma-Aldrich), signal transducer and activator of transcription 3 (STAT3) inhibitor (Static; Santa Cruz Biotechnology), or MSK1 inhibitor (H89 dihydrochloride hydrate; Sigma-Aldrich). Kasumi-1 cells were treated with 20 ng/mL Trichostatin A (TSA; Sigma-Aldrich) for 2 hours.
Our study was approved by our local National Health Service and research and development ethics committee, and was also conducted in accordance with the Declaration of Helsinki.
siRNA-mediated knockdown
Small-interfering RNA (siRNA) knockdowns of genes were performed as previously described.5 The siRNAs used were: KIT siRNA (h) (Santa Cruz Biotechnology) and 3pK siRNA (h) (Santa Cruz Biotechnology) The sequence for negative control siRNA was 5′-GGCCUCAGCUGCGCGACGC-3′ (Eurofins MWG Operon).
Annexin V/PI staining
Annexin/propidium iodide (PI) staining was performed using the Annexin V (FL) kit (Santa Cruz Biotechnology) according to the manufacturer’s protocol.
Gene expression analysis
Total RNA was extracted using TRIzol solution (Invitrogen) and complementary DNA was synthesized from using oligo(dT) (50µM; Invitrogen) and Moloney–murine leukemia virus reverse transcriptase (Promega) according to the manufacturer’s protocols. Real-time polymerase chain reaction (PCR) was performed using 2× SYBR Green PCR master mix (Applied Biosystems), 100nM primers (final concentration) under standard conditions in an ABI 7500 Real-Time PCR system (Applied Biosystems). Custom primers were designed using Primer Express (supplemental Table 1). Expression of each gene was calculated relative to the expression of TBP or GAPDH. Amplification efficiencies of primers were determined by serial dilution of complementary DNA (for gene expression analysis) or genomic DNA templates (for chromatin immunoprecipitation [ChIP] analysis).
DNAse I hypersensitive site mapping
DNAse I hypersensitive site (DHS) mapping was performed as previously described.23 Sequences of primers used to prepare probes for Southern blotting are listed in supplemental Table 2.
ChIP assays
ChIP assays were performed as previously described.5 For anti-H2Aub1, precleared chromatin was immunoprecipitated overnight at 4°C with anti-H2Aub1, followed by the addition of anti-mouse IgMμ/Dynabeads Protein G mix and incubated for 2 hours at 4°C. Antibodies used are listed in supplemental Table 3, and the primers used to analyze the immunoprecipitated DNA are listed in supplemental Table 4.
DNAse I in vivo footprinting
DNAse I footprinting was performed according to the protocol previously described.24 The primers used are listed in supplemental Table 5.
Results
PAX5 is organized in an open chromatin conformation in t(8;21) and non-t(8;21) myeloid precursors
To establish experimental models to investigate human PAX5 regulation, we compared PAX5 expression in human cell lines representing B cells (Nalm-6, Ramos), t(8;21) AML (Kasumi-1, SKNO-1), and non-t(8;21) myeloid precursor cells (HL-60, KG-1) as well as purified CD34+ primary t(8;21) AML blasts. The HeLa epithelial cell line served as a nonhematopoietic control. As expected, PAX5 was highly expressed in B-cell lines and was undetectable in non-t(8;21) myelobasts (HL-60 and KG-1) and in HeLa cells. t(8;21) AML cell lines and primary t(8;21) cells showed aberrant PAX5 expression albeit at a level lower compared with B cells (Figure 1A).
PAX5 is organized in an open chromatin conformation in t(8;21) AML and non-t(8;21) myeloid precursors. (A) Quantitative real-time PCR analysis showing PAX5 expression relative to TBP expression in Ramos (B-cell line), Nalm-6 (pre-B cell line), Kasumi-1, and SKNO-1 (t(8;21) AML cell lines), primary cells from 2 t(8;21) AML patients [t(8;21)#1-CD34+ cells from the peripheral blood of a t(8;21) patient, t(8;21)#2-CD34+ cells from the bone marrow of a t(8;21) AML patient after relapse], KG1, HL-60 [myeloid cell lines without t(8;21) translocation], HL-60+PMA (HL-60 cell line differentiated to macrophage like cells by treatment with PMA) and HeLa (epithelial cell line). The y-axis represents average relative PAX5 expression from 3 independent experiments; the error bars represent SD between 3 independent experiments. For the patient samples, the error bars represent variability between qPCR measurements. (B-C) Schematic diagram showing the mammalian sequence conservation from the UCSC genome browser. The black bars represent the positions of the DHSs identified at the (B) PAX5 promoter region and (C) enhancer, respectively, in Nalm-6, Kasumi-1, SKNO-1, HL-60, KG-1, and HeLa cell lines. (D-E) DNase I footprinting experiments examining the distal promoter element HS 8 showing that the transcription factor binding pattern and the chromatin fine structure at HS 8 is identical in Nalm-6, t(8;21) AML cells and HL-60. In panel D, samples from the different cell lines digested with different concentrations of DNase I were separated by electrophoresis on the same gel; thereafter, the samples showing equal DNAse I digestion (as shown by the digestion pattern at the TBP locus) were digitally cut and realigned to prepare the figure. PMA, phorbol myristate acetate.
PAX5 is organized in an open chromatin conformation in t(8;21) AML and non-t(8;21) myeloid precursors. (A) Quantitative real-time PCR analysis showing PAX5 expression relative to TBP expression in Ramos (B-cell line), Nalm-6 (pre-B cell line), Kasumi-1, and SKNO-1 (t(8;21) AML cell lines), primary cells from 2 t(8;21) AML patients [t(8;21)#1-CD34+ cells from the peripheral blood of a t(8;21) patient, t(8;21)#2-CD34+ cells from the bone marrow of a t(8;21) AML patient after relapse], KG1, HL-60 [myeloid cell lines without t(8;21) translocation], HL-60+PMA (HL-60 cell line differentiated to macrophage like cells by treatment with PMA) and HeLa (epithelial cell line). The y-axis represents average relative PAX5 expression from 3 independent experiments; the error bars represent SD between 3 independent experiments. For the patient samples, the error bars represent variability between qPCR measurements. (B-C) Schematic diagram showing the mammalian sequence conservation from the UCSC genome browser. The black bars represent the positions of the DHSs identified at the (B) PAX5 promoter region and (C) enhancer, respectively, in Nalm-6, Kasumi-1, SKNO-1, HL-60, KG-1, and HeLa cell lines. (D-E) DNase I footprinting experiments examining the distal promoter element HS 8 showing that the transcription factor binding pattern and the chromatin fine structure at HS 8 is identical in Nalm-6, t(8;21) AML cells and HL-60. In panel D, samples from the different cell lines digested with different concentrations of DNase I were separated by electrophoresis on the same gel; thereafter, the samples showing equal DNAse I digestion (as shown by the digestion pattern at the TBP locus) were digitally cut and realigned to prepare the figure. PMA, phorbol myristate acetate.
In mouse B cells, the Pax5 locus is regulated by 2 transcriptional start sites and an intronic enhancer region.23 Here we show that the same elements are involved in regulating human PAX5 expression by mapping DHSs in B-lymphoid cells, t(8;21) AML, myeloid precursor, and control cells described above (summarized in Figure 1B-C and in more detail in supplemental Figures 1A-B, 2, and 3), including the 2 minimal promoters (HS 4 and HS 8) which are highly conserved between human and mouse. The intronic enhancer region as defined in mouse Pax523 is marked by 4 DHSs, HS A, B, C, and D (supplemental Figures 1B and 2C). Of the enhancer hypersensitive sites, HS B is the best-characterized element in mouse.23 Also in human B cells, HS B formed the most prominent DHS and is most likely to be the main enhancer element of PAX5. The distribution of active cis elements was verified by performing ChIP assays measuring the distribution of histone modifications as well as RNA polymerase II (supplemental Figure 1C-G).
DHS mapping in myeloid cells showed that HL-60 and KG-1 displayed the same pattern at the 2 PAX5 promoters as t(8;21) AML (Figure 1B and supplemental Figure 3A-C). In all myeloid cells (but not in control cells), the regions encompassing the promoter sites HS 4 and HS 8 were DNase I accessible (Figure 1B and supplemental Figure 3A-C). This was confirmed by genome-wide DNase I sequencing in cells from 2 t(8;21) AML patients and in Kasumi-1 (supplemental Figure 3F).5 At the enhancer region, HS A and C were weakly hypersensitive in all tested cell types (Figure 1C and supplemental Figure 3D-E), but the main enhancer site HS B was inaccessible in all myeloid cells, indicating that this element is B-cell specific and inactive in t(8;21) AML. Our DHS mapping experiments were unable to differentiate between myeloid cells with or without t(8;21).
To identify differences in transcription factor occupancy at the PAX5 distal promoter, we performed high-resolution in vivo footprinting using DNase I (Figure 1D-E) in cell lines and primary cells from t(8;21) AML patients. All hematopoietic cells showed a strong footprint upstream of the distal transcription start site which indicated the presence of a transcription factor complex; however, the pattern was indistinguishable between B cells and myeloid cells. Taken together, these data show that the differential expression of PAX5 in t(8;21) and non-t(8;21) myeloid cells is not reflected in its pattern of chromatin accessibility and chromatin fine structure.
PAX5 is poised for transcription in non-t(8;21) myeloid precursors and is repressed by polycomb-repressive complexes
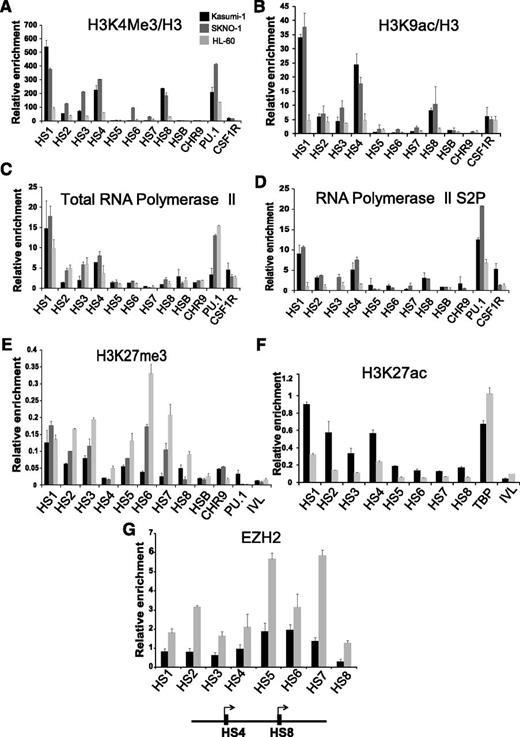
The presence of a DHS at the promoter is not always an indication for active transcription. We therefore tested the distribution of histone modifications at these elements on the PAX5 locus using ChIP–quantitative PCR (qPCR) assays for the active histone marks H3K4me3 and H3K9ac (Figure 2A-B) as well as the distribution of total RNA polymerase II (Pol II) and its elongating phosphoserine 2 form (Figure 2C-D). The PAX5 promoter DHSs carried high levels of H3K4me3 and H3K9ac in Kasumi-1 and SKNO-1 cells but not in HL-60 cells. Although PAX5 promoters carried Pol II in HL-60 cells (Figure 2C), only Kasumi-1 and SKNO-1 contained elevated levels of elongating Pol II (Figure 2D) at these elements. Interestingly, we also observed elongating Pol II recruitment to promoter upstream DHS in t(8;21) AML and B-cell lines (Figure 2D and supplemental Figure 1G) which may indicate noncoding RNA transcription whose role is so far unclear.
PAX5 is poised for transcription in non-t(8;21) myeloid precursors and is repressed by polycomb-repressive complexes. ChIP-qPCR experiments measuring relative enrichment of (A) H3K4me3, (B) H3K9ac, (C) total RNA Pol II, (D) elongating form of RNA Pol II, (E) H3K27me3, (F) H3K27ac, and (G) EZH2 at the PAX5 promoter hypersensitive sites. Relative enrichment is calculated over the inactive IVL locus in panels A, B, C, and D and relative to input in E and F and relative to TBP in panel G. Each bar graph is representative of at least 2 independent experiments analyzed in duplicate. The error bars represent variability in qPCR measurements.
PAX5 is poised for transcription in non-t(8;21) myeloid precursors and is repressed by polycomb-repressive complexes. ChIP-qPCR experiments measuring relative enrichment of (A) H3K4me3, (B) H3K9ac, (C) total RNA Pol II, (D) elongating form of RNA Pol II, (E) H3K27me3, (F) H3K27ac, and (G) EZH2 at the PAX5 promoter hypersensitive sites. Relative enrichment is calculated over the inactive IVL locus in panels A, B, C, and D and relative to input in E and F and relative to TBP in panel G. Each bar graph is representative of at least 2 independent experiments analyzed in duplicate. The error bars represent variability in qPCR measurements.
Murine Pax5 is repressed by polycomb complexes in pluripotent stem cells and myeloid precursor cells.25 This was also the case in non-t(8;21) myeloid precursors as shown by ChIP assays for H3K27me3, H3K27ac, and the PRC2 component EZH2. HL-60 cells displayed high levels of H3K27me3 and EZH2, especially at the distal promoter (Figure 2E,G) and no H3K27ac (Figure 2F), whereas the opposite was true for Kasumi-1 and SKNO-1 cells (Figure 2E,G). In summary, in myeloid precursors, human PAX5 is in an open chromatin conformation carrying paused Pol II, but is a target of polycomb-repressing complexes.
PAX5 is neither a direct nor an indirect target of RUNX1/ETO
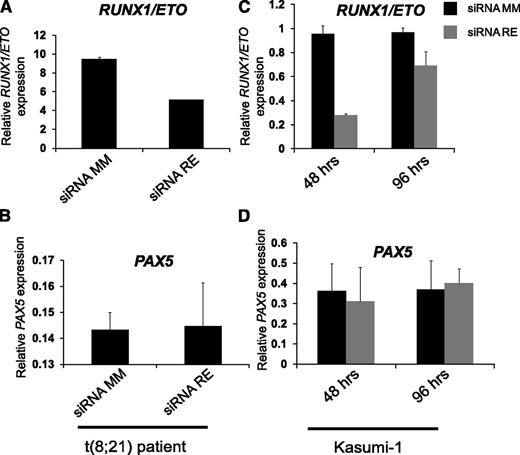
An obvious cause of aberrant activation of PAX5 in t(8;21) AML could be RUNX1/ETO expression. We therefore tested whether PAX5 was one of the genes that were directly or indirectly activated by RUNX1/ETO. However, inspection of the PAX5 locus for RUNX1 or RUNX1/ETO peaks in published RUNX1 and RUNX1/ETO ChIP-sequencing data in t(8;21) AML patient cells and in Kasumi-1 cells5 showed that PAX5 was not a target of RUNX1 or RUNX1/ETO (data not shown). We therefore investigated whether RUNX1/ETO indirectly affected the expression of PAX5 in t(8;21) AML and measured PAX5 in siRNA-mediated RUNX1/ETO-depleted primary cells from a t(8;21) patient and Kasumi-1 cells.5 However, RUNX1/ETO knockdown had no effect on PAX5 expression in Kasumi-1 cells or in patient cells (Figure 3). Therefore, we conclude that RUNX1/ETO alone is not sufficient to influence PAX5 expression.
PAX5 is neither a direct nor an indirect target of RUNX1/ETO. (A) qRT-PCR showing (A,C) RUNX1/ETO and (B,D) PAX5 expression after transfecting (left) a primary t(8;21) sample and (right) Kasumi-1 cell line with siRNA mismatch (siRNA MM) and siRNA RUNX1/ETO (siRNA RE) to knock-down RUNX/ETO. Materials were from a previous study.5 qRT-PCR, quantitative reverse transcription PCR.
PAX5 is neither a direct nor an indirect target of RUNX1/ETO. (A) qRT-PCR showing (A,C) RUNX1/ETO and (B,D) PAX5 expression after transfecting (left) a primary t(8;21) sample and (right) Kasumi-1 cell line with siRNA mismatch (siRNA MM) and siRNA RUNX1/ETO (siRNA RE) to knock-down RUNX/ETO. Materials were from a previous study.5 qRT-PCR, quantitative reverse transcription PCR.
Aberrant expression of PAX5 in t(8;21) requires MAP kinase signaling
We next addressed the question of what caused the lack of polycomb repression in t(8;21) AML. About half of t(8;21) AML patients carry secondary mutations in genes known to control growth factor receptor pathways (FLT3, KIT, NRAS, and KRAS)11,12,26 leading to constitutive activation of AKT, Janus kinase (JAK)/STAT3, and MAP kinase pathways27,28 (schematically depicted in Figure 4A). A large body of literature supports the idea that cellular signaling directly impacts on polycomb group activity (reviewed in Sawarkar and Paro29 ). Moreover, it was previously shown that the posttranslational modification of polycomb proteins in response to signaling regulates their association with chromatin.29 Signaling via MSK regulates polycomb group association with its targets via the phosphorylation of histones,30 and AKT has been shown to phosphorylate the PRC1 member BMI1 disrupting its binding to chromatin.31 Phosphorylation of BMI1 by MAPKAPK3 (3pK) which is downstream of JNK, MEK, and p38 signaling has the same effect.32 Because PAX5 is not activated by RUNX1/ETO alone, and t(8;21) AML cells carry different types of growth factor receptor mutations, we tested the hypothesis that the abrogation of polycomb repression in t(8;21) AML was caused by aberrant signaling.
Aberrant signaling is required for PAX5 deregulation in t(8;21) AML. (A) Schematic diagram showing chronic AKT, JNK, JAK/STAT, and MAP kinase signaling in t(8;21) AML downstream of an activating mutation (indicated by a star) in a tyrosine kinase (growth factor) receptor or activated rat sarcoma (RAS) signaling. The circles indicate the signaling components targeted by small-molecule inhibitors. (B) Table listing the signaling components targeted, small-molecule inhibitors used, and the effect of the inhibitors on PAX5 expression in Kasumi-1 cells. For detailed data, see supplemental Figure 4A. (C-F) Quantitative reverse transcription PCR experiment measuring expression of PAX5, INK4/ARF, TBP, and RPL13A, respectively, after simultaneous treatment with JNK, MEK, and p38 inhibitors. Each bar graph is representative of 3 independent experiments. The error bars represent the variability in qPCR measurements.
Aberrant signaling is required for PAX5 deregulation in t(8;21) AML. (A) Schematic diagram showing chronic AKT, JNK, JAK/STAT, and MAP kinase signaling in t(8;21) AML downstream of an activating mutation (indicated by a star) in a tyrosine kinase (growth factor) receptor or activated rat sarcoma (RAS) signaling. The circles indicate the signaling components targeted by small-molecule inhibitors. (B) Table listing the signaling components targeted, small-molecule inhibitors used, and the effect of the inhibitors on PAX5 expression in Kasumi-1 cells. For detailed data, see supplemental Figure 4A. (C-F) Quantitative reverse transcription PCR experiment measuring expression of PAX5, INK4/ARF, TBP, and RPL13A, respectively, after simultaneous treatment with JNK, MEK, and p38 inhibitors. Each bar graph is representative of 3 independent experiments. The error bars represent the variability in qPCR measurements.
Kasumi-1 and SKNO-1 cell lines are KIT mutant models of t(8;21) AML and carry the activating N822K mutation. To test the above hypothesis and to test which signaling pathway was responsible, we treated Kasumi-1 cells by using small-molecule inhibitors of different signaling pathways alone or in combination and measured PAX5 expression (Figure 4B and supplemental Figure 4A). Inhibition of AKT, JNK, p38, MEK, and MSK1 signaling pathways individually showed no effect on PAX5 expression over a time course of 12 hours (data not shown). Inhibition of STAT3 signaling caused rapid apoptosis (Figure 4B and supplemental Figure 4D), confirming results demonstrating that this pathway is important for the survival of t(8;21) cell lines.33 After simultaneous treatment of Kasumi-1 cells with JNK, MEK, and p38 inhibitors for 6 hours, expression of the polycomb targets PAX5 and INK4/ARF was fourfold downregulated for a period of at least 12 hours (Figure 4C-D). Simultaneous inhibition of JNK and p38 alone led to downregulation of PAX5, however, maximal PAX5 downregulation was achieved only when JNK, MEK, and P38 were inhibited together (supplemental Figure 4A). PAX5 repression after inhibitor treatment was not due to cell death or cell differentiation, as indicated by the maintenance of CD34 expression (supplemental Figure 4C) and by lack of an overt change in morphology compared with untreated and DMSO-treated Kasumi-1 cells (supplemental Figure 4B). Furthermore, viability was unaffected as measured by Trypan blue staining (supplemental Figure 4E), and there was no increase in the number of apoptotic cells 12 hours posttreatment compared with control cells as indicated by Annexin/PI staining (supplemental Figure 4D). No change in expression of control genes such as TBP and RPL13A was observed after inhibition (Figure 4E-F). Downregulation of PAX5 expression in response to inhibition of JNK, MEK, and P38 could be reproduced in SKNO-1 and also in primary t(8;21) AML cells (supplemental Figure 5A-D) but not in the Nalm-6 pre-B-cell line (supplemental Figure 5E).
In summary, these experiments demonstrate that PAX5 expression in t(8;21) cells is dependent on MAP kinase signaling. In addition, we show that PAX5 is not regulated by a signaling-responsive transcription factor shared with B-lymphoid cells, and repression by kinase inhibition is specific for t(8;21) AML.
Inhibition of MAP kinase signaling in t(8;21) leads to reassociation of polycomb complexes to the PAX5 locus
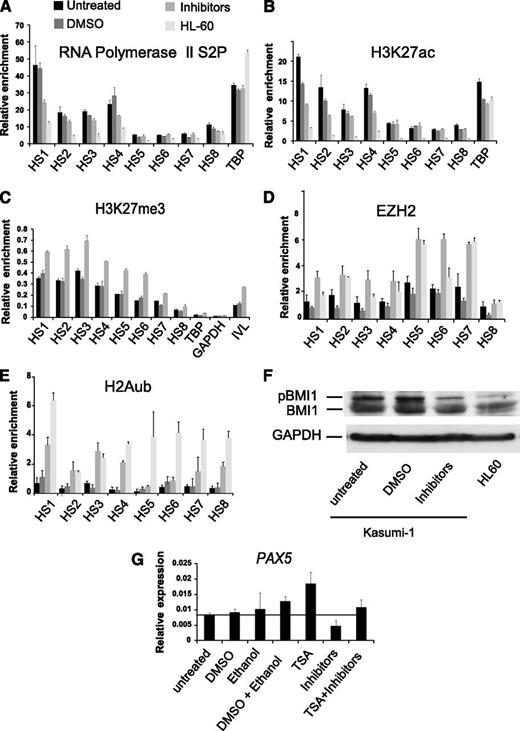
To investigate the molecular mechanism behind the downregulation of PAX5 expression in response to inhibition of MAP kinase signaling, we performed ChIP-qPCR assays for Pol II, H3K27ac, H3K27me3, and EZH2 in MAP kinase inhibitor-treated, DMSO-treated, and untreated Kasumi-1 cells 12 hours after treatment. Inhibitor treatment caused a reduction of Pol II and H3K27ac, particularly at HS 1 and HS 4 (Figure 5A-B) with a concomitant increase in the H3K27me3 mark and EZH2 occupancy (Figure 5C-D). Transcript levels of JMJD3 encoding a H3K27me3 demethylase were not affected by inhibitor treatment suggesting that increased turnover of the methyl mark was not responsible for H3K27me3 loss (supplemental Figure 5F).
Inhibition of chronic signaling re-recruits polycomb to the PAX5 promoter. (A-E) ChIP-qPCR experiments measuring relative enrichment of the elongating form of the RNA Pol II complex as well as H3K27ac, H3K27me3, EZH2, and H2Aub1 at the PAX5 promoter hypersensitive sites in untreated Kasumi-1, DMSO-treated Kasumi-1, JNK, MEK, and p38 inhibitor-treated Kasumi-1 and HL-60 cells. (A,B) Relative enrichment shown is over the inactive IVL locus. (C-E) Enrichment is relative to (C) input, (D) TBP, and (E) Chr18. Each bar graph is a representative of 2 independent experiments and the error bars show variability between qPCR measurements. (F) Western blot showing BMI1 and phospho-BMI1 expression in untreated Kasumi-1, DMSO-treated Kasumi-1, JNK, MEK, and P38-treated Kasumi-1 cells and HL-60 cells. GAPDH expression in each of the above cell lines was used as a loading control. (G) The HDAC inhibitor TSA rescues the inhibition of signaling mediated downregulation of PAX5 expression. qRT-PCR experiment showing PAX5 expression in untreated, DMSO-treated, ethanol-treated, ethanol + DMSO treated, TSA-treated, JNK + MEK + P38 inhibitor–treated, and TSA + JNK + MEK + p38 inhibitor–treated Kasumi-1 cells. The y-axis shows PAX5 expression relative to GAPDH expression. The bar graph shows average values from 2 independent experiments measured in duplicate; error bars show the variability in qPCR measurements between the 2 experiments.
Inhibition of chronic signaling re-recruits polycomb to the PAX5 promoter. (A-E) ChIP-qPCR experiments measuring relative enrichment of the elongating form of the RNA Pol II complex as well as H3K27ac, H3K27me3, EZH2, and H2Aub1 at the PAX5 promoter hypersensitive sites in untreated Kasumi-1, DMSO-treated Kasumi-1, JNK, MEK, and p38 inhibitor-treated Kasumi-1 and HL-60 cells. (A,B) Relative enrichment shown is over the inactive IVL locus. (C-E) Enrichment is relative to (C) input, (D) TBP, and (E) Chr18. Each bar graph is a representative of 2 independent experiments and the error bars show variability between qPCR measurements. (F) Western blot showing BMI1 and phospho-BMI1 expression in untreated Kasumi-1, DMSO-treated Kasumi-1, JNK, MEK, and P38-treated Kasumi-1 cells and HL-60 cells. GAPDH expression in each of the above cell lines was used as a loading control. (G) The HDAC inhibitor TSA rescues the inhibition of signaling mediated downregulation of PAX5 expression. qRT-PCR experiment showing PAX5 expression in untreated, DMSO-treated, ethanol-treated, ethanol + DMSO treated, TSA-treated, JNK + MEK + P38 inhibitor–treated, and TSA + JNK + MEK + p38 inhibitor–treated Kasumi-1 cells. The y-axis shows PAX5 expression relative to GAPDH expression. The bar graph shows average values from 2 independent experiments measured in duplicate; error bars show the variability in qPCR measurements between the 2 experiments.
The PRC1 complex containing BMI1 and the PRC2 complex containing EZH2 cooperate in the silencing of gene expression whereby BMI1 is responsible for the ubiquitination of H2A.34 Together, they regulate transcription elongation at bivalent genes.35 We therefore tested whether the PAX5 promoters carried H2A-ubiquitinated histones in HL60 cells and whether treatment with inhibitors in Kasumi-1 cells restored this feature. Figure 5E shows that this was indeed the case. Moreover, Kasumi-1 cells contained elevated levels of phosphorylated BMI1 as compared with HL-60 cells which were reduced after MAP kinase–inhibitor treatment (Figure 5F).
Polycomb complexes recruit histone deacetylases (HDACs) as one mechanism to mediate repression of their target genes.36 Therefore, we examined whether the HDAC inhibitor TSA counteracted the downregulation of PAX5 after inhibition of MAP kinase signaling and treated Kasumi-1 cells with TSA alone or simultaneously with JNK, MEK, p38 inhibitors. Treatment with TSA alone enhanced the expression of PAX5 in Kasumi-1 compared with control cells. When TSA was added in conjunction with MAP kinase inhibitors, PAX5 expression was rescued up to the levels in untreated Kasumi-1 cells and control cells treated with dimethyl sulfoxide (DMSO) and ethanol (Figure 5G). This result suggests that downregulation of PAX5 due to inhibition of constitutively activated MAP kinase signaling in Kasumi-1 cells involves HDACs.
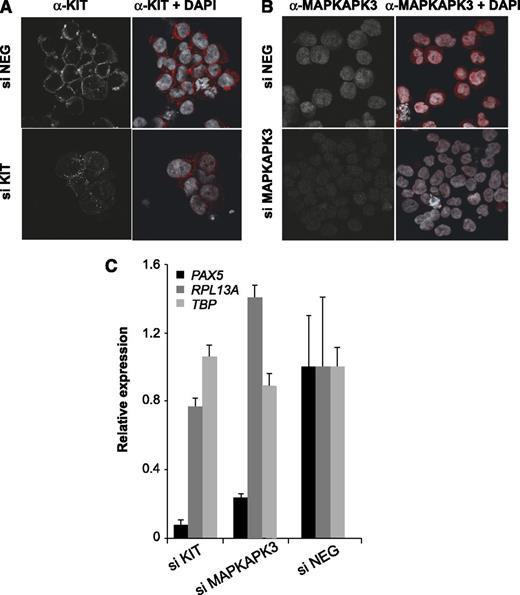
To confirm our inhibitor studies and to directly define which signaling pathway was responsible for the upregulation of PAX5 in t(8;21) cells, we depleted KIT by performing siRNA knockdown (Figure 6) in Kasumi-1 cells. We also depleted MAPKAPK3 (3pK) as an integrated node for MAP kinase signaling by different growth factor stimuli. This kinase is known to phosphorylate BMI1, thereby blocking chromatin association.32 KIT feeds into this node via extracellular signal-regulated kinase (ERK) (Figure 4A). Depletion was confirmed by immunohistochemistry (Figure 6A-B). The depletion of KIT and 3pK led to a downregulation of the expression of PAX5 but not control genes (Figure 6C). Taken together, this demonstrates that growth factor receptor–driven aberrant 3pK activation is required for the aberrant expression of the PAX5 locus in t(8;21) AML.
siRNA-mediated depletion of MAPKAPK3 and KIT signaling molecules downregulates PAX5 expression in Kasumi-1 cells. (A-B) Immunostaining showing KIT and MAPKAPK3 proteins (red), respectively, in Kasumi-1 cells after siRNA-mediated depletion. (siNEG) cells transfected with a control siRNA. (C) qRT-PCR showing expression of PAX5, RPL13A, and TBP relative to GAPDH in Kasumi-1 cells transfected with control siRNA (siNEG), MAPKAPK3 siRNA, and KIT siRNA. Values obtained with siNEG were set as one. The bar graph is representative of 2 independent experiments measured in duplicate; the error bars show variability in qPCR measurements.
siRNA-mediated depletion of MAPKAPK3 and KIT signaling molecules downregulates PAX5 expression in Kasumi-1 cells. (A-B) Immunostaining showing KIT and MAPKAPK3 proteins (red), respectively, in Kasumi-1 cells after siRNA-mediated depletion. (siNEG) cells transfected with a control siRNA. (C) qRT-PCR showing expression of PAX5, RPL13A, and TBP relative to GAPDH in Kasumi-1 cells transfected with control siRNA (siNEG), MAPKAPK3 siRNA, and KIT siRNA. Values obtained with siNEG were set as one. The bar graph is representative of 2 independent experiments measured in duplicate; the error bars show variability in qPCR measurements.
Discussion
Our previous studies have highlighted the important role of PAX5 in aberrant expression of B-cell–specific genes in t(8;21) AML.13 The studies described here demonstrate that MAP kinase signaling is required for PAX5 expression in t(8;21) AML. Moreover, as outlined in the model depicted in Figure 7, using a KIT mutant cell line model of t(8;21), we show that aberrant growth factor receptor signaling via 3pK abrogates polycomb-mediated repression, providing a novel molecular explanation for this phenomenon.
Signaling-mediated abrogation of polycomb silencing activates PAX5 expression in t(8;21) AML. In B cells, the PAX5 promoter is active and robust transcription occurs. In myeloid precursors, the gene is not expressed, but is poised for transcription and repressed by polycomb-repressive complexes. In t(8;21) AML, this polycomb repression is relieved via activated MAP kinase signaling, potentially cooperating with so far uncharacterized transcription factors (TF). This leads to the chronic activation of MAPKAPK3, which in turn leads to the dissociation of polycomb complexes from the PAX5 promoter region, effective transcriptional elongation, and aberrant expression of PAX5.
Signaling-mediated abrogation of polycomb silencing activates PAX5 expression in t(8;21) AML. In B cells, the PAX5 promoter is active and robust transcription occurs. In myeloid precursors, the gene is not expressed, but is poised for transcription and repressed by polycomb-repressive complexes. In t(8;21) AML, this polycomb repression is relieved via activated MAP kinase signaling, potentially cooperating with so far uncharacterized transcription factors (TF). This leads to the chronic activation of MAPKAPK3, which in turn leads to the dissociation of polycomb complexes from the PAX5 promoter region, effective transcriptional elongation, and aberrant expression of PAX5.
The mouse Pax5 locus is repressed by polycomb-repressive complexes in hematopoietic stem cells and multipotent progenitor cells (HSCs and MPPs) and is exclusively activated in the B-lymphoid lineage. Polycomb-mediated repression of Pax5 is required for its correct spatiotemporal regulation as deletion of Bmi1 in mouse HSCs/MPPs leads to its upregulation in these cells.37 This is most likely true also for the human gene. We demonstrate here that PAX5 human and mouse are regulated by a similar set of cis-regulatory elements with similar activity profiles in B cells. t(8;21) cells only activate a subset of these elements, with the B-cell–specific intronic enhancer being inactive. Both our low- as well as high-resolution chromatin structure studies were unable to distinguish between PAX5 expressing or nonexpressing myeloid cells. In addition, we and others showed that the promoter cluster of PAX5 is DNase I hypersensitive in early CD34+ and CD133+ progenitor cells,38 in blasts from non-t(8;21) AML patients5 and in the human promyelocytic leukemia cell line (HL-60). However, PAX5 in non-t(8;21) and t(8;21) myeloid cells differed in its chromatin modification state. Silent PAX5 in non-t(8;21) myeloid cells recruits polycomb-silencing complexes and carries bivalent histone marks and a paused RNA Pol II. Active PAX5 in t(8;21) AML is devoid of polycomb complexes and carries active chromatin marks and elongating RNA Pol II; we describe here for the first time that the maintenance of this active state requires MAP kinase signaling. In Kasumi-1 and SKNO-1 cells, this signal originates from an activated KIT receptor via 3pK.
It should be noted that not all t(8;21) AML patients carry a KIT mutation11,15 but all express PAX5. The patient cells used for our chromatin analysis did not carry a KIT mutation (data not shown). It is therefore likely that FLT3 and RAS mutations, and other, as yet unknown alterations in MAP kinase signaling also impact on lineage-inappropriate gene expression. This notion is supported by experiments demonstrating that FLT3-LM cooperates with RUNX1/ETO to cause AML in mice and, most importantly, leads to an AML with a high frequency of activation of lymphoid genes, including CD19.11 Along similar lines, mutations in FLT3, KIT, and RAS are present in other AML types as well. However, the expression of B-lineage genes is a hallmark of only the t(8;21) and thus RUNX1/ETO is likely to play a role in the upregulation of PAX5, albeit indirectly. It has been shown recently that siRNA-mediated depletion of RUNX1/ETO in primary t(8;21) patient cells together with the overexpression of POU4F1 (BRN3A) downregulates PAX5 expression,39 and it was suggested that POU4F1 and RUNX1 directly activate the PAX5 promoter. Our previous work and the data presented here show that the presence or absence of RUNX1/ETO in both t(8;21) patient cells and cell lines does not influence PAX5 expression and that PAX5 is neither a RUNX1 nor RUNX1/ETO target. Previous expression analyses of human CD34+ hematopoietic progenitor cells transduced with RUNX1/ETO containing retrovirus on its own does not show deregulation of PAX5,40 and RUNX1/ETO-depleted Kasumi-1 cells do not change PAX5 expression.5 However, POU4F1 is specifically upregulated in t(8;21) cells41 and may cooperate with coactivators to upregulate PAX5. It remains to be shown that BRN3A/POU4F1 directly binds to the PAX5 locus and interacts with cell signaling.
Kasumi-1 cells contain constitutively phosphorylated PI3K, AKT, STAT3, and JNK.42 ERK1 and ERK2 were shown to be the first signaling pathways that are deregulated as a result of activating KIT mutation.43,44 A recent study has mechanistically linked AKT signaling to the derepression of polycomb target genes and has implicated this mechanism to affect normal hematopoietic stem cell renewal31 and other recent experiments demonstrated that AKT signaling contributes to the survival of t(8;21) leukemic cells.45,46 However, our studies uncovered that in our cell line models, the main survival signal originates from signaling via STAT3, whereas MAP kinase signaling plays a role in defining the leukemic gene expression phenotype.
Class II mutations in genes encoding for growth factor receptors such as KIT and FLT3 are thought to provide survival and growth advantage to leukemic cells which carry another mutation that blocks differentiation.47 However, this distinction is becoming blurred. Our experiments demonstrate that constitutive MAP kinase signaling can directly regulate gene expression and influence the expression of a lineage-specific gene normally silenced by polycomb proteins in precursor cells. Because leukemic blasts are arrested at an early stage of differentiation they are likely to have a large number of genes under polycomb control. siRNA-mediated depletion of KIT in Sca1+Lin− mouse HSCs transduced with RUNX1/ETO does not reduce the replating ability of these cells, however, its loss leads the colony forming unit–granulocyte macrophage to appear more spread out, indicating an effect on the differentiation status of these cells.48 The mechanism elucidated by our study, that is, deregulation of key genes (in this case PAX5) via abrogation of polycomb-mediated repression by aberrant signaling, may represent an important mechanism by which activating growth factor receptor mutations affect the differentiation of blasts not only in t(8;21) AML, but also in other types of biphenotypic leukemia. Studies along these lines are a subject of current experiments in our laboratory.
A recent clinical study described the treatment of a t(8;21) patient carrying an activating N822K KIT mutation with dasatinib resulted in robust in vivo differentiation of myeloblasts in the peripheral blood,49 suggesting a role of KIT signaling in the differentiation block in this type of leukemia. Our work therefore provides a paradigm for the idea that activating growth factor receptor mutations do not only confer a growth advantage to leukemic cells, but also impact on gene expression patterns and thus have the potential to further subvert cell-fate decisions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Leukemia Lymphoma Research and the University of Birmingham (C.B. and O.H.), and a Cancer Research UK programme grant (O.H.).
Authorship
Contribution: D.R., S.Y.K., and A.P. performed experiments; H.T., A.P., O.H., and C.B. designed the study; and D.R. and C.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.T. is Research Institute of Molecular Pathology, Vienna, Austria.
The current affiliation for D.R. is Cancer Science Institute, National University of Singapore, North Core, Singapore.
Correspondence: Constanze Bonifer, School of Cancer Sciences, Institute of Biomedical Research, University of Birmingham, Birmingham B15 2TT, United Kingdom; e-mail: c.bonifer@bham.ac.uk; and Anetta Ptasinska, School of Cancer Sciences, Institute of Biomedical Research, University of Birmingham, Birmingham B15 2TT, United Kingdom; e-mail: a.ptasinska@bham.ac.uk.

![Figure 1. PAX5 is organized in an open chromatin conformation in t(8;21) AML and non-t(8;21) myeloid precursors. (A) Quantitative real-time PCR analysis showing PAX5 expression relative to TBP expression in Ramos (B-cell line), Nalm-6 (pre-B cell line), Kasumi-1, and SKNO-1 (t(8;21) AML cell lines), primary cells from 2 t(8;21) AML patients [t(8;21)#1-CD34+ cells from the peripheral blood of a t(8;21) patient, t(8;21)#2-CD34+ cells from the bone marrow of a t(8;21) AML patient after relapse], KG1, HL-60 [myeloid cell lines without t(8;21) translocation], HL-60+PMA (HL-60 cell line differentiated to macrophage like cells by treatment with PMA) and HeLa (epithelial cell line). The y-axis represents average relative PAX5 expression from 3 independent experiments; the error bars represent SD between 3 independent experiments. For the patient samples, the error bars represent variability between qPCR measurements. (B-C) Schematic diagram showing the mammalian sequence conservation from the UCSC genome browser. The black bars represent the positions of the DHSs identified at the (B) PAX5 promoter region and (C) enhancer, respectively, in Nalm-6, Kasumi-1, SKNO-1, HL-60, KG-1, and HeLa cell lines. (D-E) DNase I footprinting experiments examining the distal promoter element HS 8 showing that the transcription factor binding pattern and the chromatin fine structure at HS 8 is identical in Nalm-6, t(8;21) AML cells and HL-60. In panel D, samples from the different cell lines digested with different concentrations of DNase I were separated by electrophoresis on the same gel; thereafter, the samples showing equal DNAse I digestion (as shown by the digestion pattern at the TBP locus) were digitally cut and realigned to prepare the figure. PMA, phorbol myristate acetate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/5/10.1182_blood-2013-02-482497/4/m_759f1.jpeg?Expires=1767774138&Signature=Ce4l4BQgdwb8YPf-l19CvHOepwuuHCH9oo5lVZ9R2m5NWuZCzl~PENSCQRdsm17HMCQKYRtbxznO3sMaYdo0kaB-d9vlCMejJz8Pj6uEtqIwrK2QutsOM90qTEegJTBA8KhU8g6HTNom43HFsey2WM5vv4XuwycYhrVIHtWyHCmjHZZJfwYB8B~0G4PLKFQbo1cxa4-tkoc6nCVGJpVaNUUwMVELlyi-bvlAMqElwuKyQ8Lt8iuk~2xl8B083w4EX~SBh2Jf6JqaDaPJybpUT~bzou1I2WLY6kvjMjQPk38CHsv9lFdoyC3znLOmupNReZ~hbKp74ii9uDydcbqyXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal