In this issue of Blood, Aranguren et al1 provide new insights into the molecular mechanisms that determine the identity of human endothelial cells: ie, will they line arteries or veins? The findings have implications in our understanding of vascular disease and in the design of vascular-specific therapies and tissue engineering.
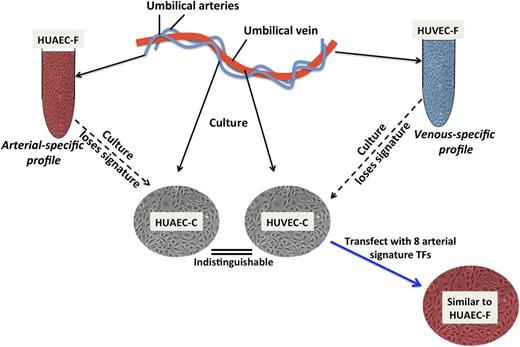
Manipulating AV endothelial cell specification. When endothelial cells from a human umbilical vein (HUVEC) or artery (HUAEC) are freshly isolated (HUVEC-F, HUAEC-F), their gene expression profiles can be readily distinguished. Culture of the endothelial cells (HUVEC-C and HUAEC-C) erases the differential AV gene expression, partly due to loss of Notch activity. The arterial-specific gene profile can, however, be induced in HUVEC-C by combined overexpression of 8 transcription factors (TF) (Prdm16, MSX1, EMX2, NKX2-3, TOX2, Hey2, SOX17, and Aff3), which together are more effective than any one alone, including HEY2, a Notch effector (not shown).
Manipulating AV endothelial cell specification. When endothelial cells from a human umbilical vein (HUVEC) or artery (HUAEC) are freshly isolated (HUVEC-F, HUAEC-F), their gene expression profiles can be readily distinguished. Culture of the endothelial cells (HUVEC-C and HUAEC-C) erases the differential AV gene expression, partly due to loss of Notch activity. The arterial-specific gene profile can, however, be induced in HUVEC-C by combined overexpression of 8 transcription factors (TF) (Prdm16, MSX1, EMX2, NKX2-3, TOX2, Hey2, SOX17, and Aff3), which together are more effective than any one alone, including HEY2, a Notch effector (not shown).
Endothelial cells lining arteries and veins arise from mesoderm-derived angioblasts in the embryo.2 The common origin of these endothelial cell populations belies the fact that arteries and veins have distinct structural features, properties, and functions. The Greek anatomist Erasistratus is credited with being the first to distinguish these 2 vessel types during the third century BCE. Even though he believed that they carry air, his insights have spurred generations of scientists, including Galen (second century AD), Harvey (17th century AD), and, most recently, Aranguren et al,1 to attempt to delineate the unique “signatures” of arteries and veins and how they evolve.
What dictates whether an angioblast will emerge as an artery or vein? The complex molecular mechanisms underlying arteriovenous (AV) specification, particularly in the embryo, have been studied in many species. Although there are differences, all support the notion that AV specification is driven primarily by a cell-intrinsic program that initially occurs independent of blood flow.3 Mesoderm-derived angioblasts that form the primitive vascular plexus are already fated to be either arterial or venous. Notch and Wnt/β-catenin pathways4 are currently believed to mainly drive AV-fate decision making. Through tightly regulated signaling events involving, among others, sonic hedgehog and vascular endothelial growth factor,5 the pathways converge with induction of Notch signaling and consequent upregulation of transcription factor effectors, such as HEY2. These promote arterial endothelial specification, with increased expression of neuropilin-1 (Nrp-1) and ephrinB2, suppression of venous endothelial markers EphB4 and Nrp-2, and release of factors that encourage growth, differentiation, and recruitment of vascular smooth muscle cells. This apparent default pathway to an arterial fate is checked by COUP-TFII, which suppresses Nrp-1 and Notch, thereby promoting and/or maintaining a venous endothelial identity.6
In spite of these insights, the genetic, epigenetic, and environmental factors that determine AV fate remain incompletely understood. Efforts to more readily decipher the underlying molecular mechanisms increased as it became easier to culture human endothelial cells. Although this technology is attractive, the question of whether cultured cells can recapitulate the in vivo situation has been examined only to a limited extent.7
Aranguren et al1 are the first to use an unbiased genome-wide approach to discriminate endothelial cells that are freshly isolated from arteries vs veins, in conjunction with an analysis of the impact of in vitro cell culture on AV specification. Using cultured endothelial cells from multiple adult vascular beds, they first showed that gene expression profiles could not be distinguished based on arterial or venous origin. They then demonstrated that freshly isolated human umbilical vein and arterial endothelial cells yielded an “AV-fresh” gene profile that could be used to reliably separate venous from arterial endothelial cells. This distinction was entirely erased when the cells were cultured for even a short time (see figure).
From the AV-fresh gene profile, Aranguren et al1 went on to characterize the function of 8 arterial endothelial-specific transcription factors, 6 of which have not been implicated previously in AV specification. Collectively, these factors were more effective than any single factor, including HEY2, at restoring and sustaining the arterial endothelial fingerprint of cultured cells. Even though this novel combination of transcription factors could only restore ∼75% of the arterial fingerprint in vitro, it did impart an arterial phenotype to human umbilical vein endothelial cells in an in vivo model.
There are several novel and important implications of these studies. The findings highlight the importance of considering how rapidly and dramatically in vitro culture alters the genetic and functional properties of cells. As implied in this report,1 it is likely that the molecular signatures of freshly isolated endothelial cells from a vascular bed are closer to what exists in vivo, particularly as compared with samples after days or weeks in culture. It should be expected that there will be considerable heterogeneity and plasticity in these signatures—even within the “families” of arteries and veins—that is dependent on multiple factors, such as age, the specific vascular bed, blood flow, and the state of health or disease.8 Nonetheless, the value in obtaining an almost in situ endothelial signature, as achieved by Aranguren et al,1 is relevant for the ultimate design of endothelial cell–focused tissue-specific therapies.
It is interesting that Notch signaling did not predominate in AV specification of these umbilical vessel endothelial cells. This does not detract from the large literature that emphasizes the important role of Notch signaling, particularly during embryonic vasculogenesis. Aranguren et al1 focused on endothelial cells from umbilical vessels, and thus their findings cannot necessarily be generally applied. However, the authors did discover novel pathways by which AV specification is regulated in part by transcription factors that have hitherto not been shown to participate in vascular biology (eg, Prdm16, EMX2). It will be important to characterize these signaling pathways to determine whether they have broader effects in vascular function in health and disease.
Most intriguing was the finding that combinations of transcription factors were needed to optimally provoke a sustained “switch” in endothelial cellular phenotype. This strategy, well known in the field of inducible pluripotent stem cells,9 is an exciting development in vascular biology and may influence the design of therapeutic cell lines for the repair of damaged vasculature that may occur during atherosclerosis or other inflammatory disorders, for tissue engineering and regenerative medicine.
In a 1966 address10 referring to the endothelium, Nobel laureate Lord Florey stated, “our knowledge is still far from being definitive, and I should expect to see the next ten years yield a rich harvest of new knowledge about the cells which stand between the blood and lymph streams and the cells of the tissue.” With new insights provided by Aranguren et al,1 there is no doubt that the next 10 years will be revealing.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal