In this issue of Blood, Sreeramkumar and colleagues report that E-selectin ligand-1 (ESL-1) is a highly selective ligand for E-selectin on hematopoietic progenitors with unexpected important contributions to their trafficking.1
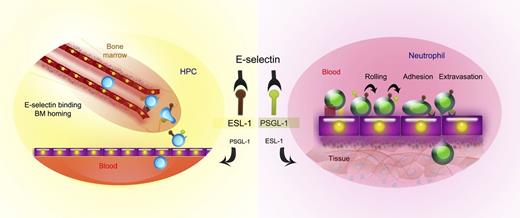
Distinct use of E-selectin ligands between mature and immature leukocytes. Left: Dominant roles for ESL-1 in immature hematopoietic progenitor cells (HPCs) in BM homing. Right: Functional shift toward PSGL-1 dependence in mature neutrophils during their recruitment into inflamed tissues.
Distinct use of E-selectin ligands between mature and immature leukocytes. Left: Dominant roles for ESL-1 in immature hematopoietic progenitor cells (HPCs) in BM homing. Right: Functional shift toward PSGL-1 dependence in mature neutrophils during their recruitment into inflamed tissues.
P- and E-selectins are expressed on endothelium and play a critical role in the recruitment of the appropriate blood cell into specific local sites (see review2 ). Consequently, mice deficient in both P- and E-selectins display defective neutrophil rolling and are immunodeficient.3 Because E-selectin–deficient mice show a very mild phenotype, researchers in the field considered for years that the E-selectin/ligand pathway was a mere backup for P-selectin–mediated interactions. Recent studies, however, have begun to reveal that E-selectin in fact has its own functions. For example, E-selectin engagement on neutrophils triggers β2 integrin activation, thus promoting slow rolling and adhesion.4 The Frenette and Levesque groups have identified important roles for E-selectin during hematopoietic stem/progenitor cell (HSPC) trafficking to the bone marrow (BM), and for the maintenance of their quiescent phenotype in vivo.5,6 Because selectins are also constitutively expressed in the endothelium of certain tissues such as BM even in the absence of inflammatory stimulation, there has been a growing appreciation that these receptors may be important for tissue homeostasis. Notwithstanding these advances, the complex glycosylated structures recognized by E-selectin have prevented the identification of the physiological ligands for E-selectin on hematopoietic cells.2
Genetic-deletion studies have identified P-selectin glycoprotein ligand-1 (PSGL-1) as the major ligand for P-selectin and also as one of several ligands for E-selectin (see review2 ). The promiscuous binding and the lack of function-blocking antibodies, however, prevented at the time the identification of the full repertoire of ligands for E-selectin. Using gene-targeting and gene-silencing strategies, Hidalgo et al reported that 3 glycoproteins, PSGL-1, ESL-1, and CD44, accounted for virtually all E-selectin ligand activity on murine neutrophils, with PSGL-1 and ESL-1 being the most prominent ligand pair.7 Because ESL-1–deficient mice were unavailable at the time, the conclusions of this study could only be extended to the small fraction of circulating cells that were transduced with the ESL-1–silencing vector. Thus, these studies have raised the question of whether ESL-1 was really important for the global trafficking of leukocytes, and whether rare populations of hematopoietic cells, such as HSPCs, used this glycoprotein in vivo.
In this issue of Blood, the Hidalgo group presents the first hematologic and immunologic characterization of mice fully deficient in ESL-1.1 By further generating mice lacking both ESL-1 and PSGL-1, they confirm their previous findings that both receptors cooperate for E-selectin binding, rolling, and recruitment of neutrophils to inflamed tissues.7 Notably, they also find that this pair of ligands is required for the trafficking of myeloid leukocytes (neutrophils and monocytes) under steady-state conditions and generate evidence that their clearance from blood, rather than mobilization from the BM, is mediated by PSGL-1 and ESL-1. These findings partially recapitulate the phenotype of P- and E-selectin–deficient mice3 and formally demonstrate that specific glycoproteins that function as ligands for endothelial selectins on leukocytes control myeloid homeostasis in mammals.
The authors then take advantage of these mice to investigate the specific roles of ESL-1 in the immature hematopoietic compartment. Surprisingly, they report very high levels of ESL-1 on HSPCs compared with fully differentiated leukocytes. These increased levels correlated, notably, with strong reductions in E-selectin binding in hematopoietic precursors in the absence of ESL-1, and a marked impairment in the ability of ESL-1–deficient progenitors to migrate to the BM. In agreement with reports from the Levesque laboratory,6 they find that PSGL-1 is not an important ligand for E-selectin on these cells.
Collectively, the findings in this study strongly indicate the dominant roles for ESL-1 in the immature hematopoietic compartment and a functional shift toward PSGL-1 dependence in mature myeloid cells (see figure).
However, it is puzzling that mice deficient in E-selectin do not reproduce the migratory defects seen in the absence of ESL-1.5 Although the technical approaches used in the 2 studies are different, it will be worth understanding whether ESL-1 controls the trafficking of progenitor cells through mechanisms that are E-selectin independent. This trend is particularly relevant because the majority of ESL-1 is present in the Golgi of many different cell types, and only a minor fraction is exported to the cell membrane.8 In addition, work from the Lee laboratory identified a role for ESL-1 in controlling the production of bioactive transforming growth factor-beta,9 thus arguing for selectin-independent roles of ESL-1 in hematopoietic cells.
There are also several questions that need to be further addressed. For example, because PSGL-1 preserves its P-selectin–binding functions in HSPCs, why does it specifically lose its E-selectin–binding functions in HSPCs? Is this regulated by differential posttranslational modification by glycosyltransferases? It is also striking that among the repertoire of E-selectin ligands functional in mature leukocytes, only ESL-1 retains the capacity to bind E-selectin in the immature compartment despite the expression of same series of molecules. Because ESL-1 is structurally related to members of the fibroblast growth factor receptor family, can this observation be associated to the possible additional signaling functions of ESL-1? The Hidalgo group recently published the clearance of neutrophils in the BM in association with circadian rhythms.10 It would be of interest to dissect whether this machinery is regulated by E-selectin engagement in the BM. Finally, as neither PSGL-1 nor CD44 is required to transduce the quiescence-inducing signals of E-selectin,6 it will be particularly exciting to determine whether ESL-1 contributes to this important feature of HSPCs in the BM niches.
The sophisticated techniques and informative results reported in this important work by Sreeramkumar et al1 pave the way for a direct approach to answer these questions.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal