Key Points
The MllPTD/wt:Flt3ITD/wt mouse is a relevant AML model in which the miR-29b–mediated epigenetics-kinome crosstalk is targetable by bortezomib.
An original liposomal formulation of bortezomib eradicates AML and yields curative therapy for MllPTD/wt:Flt3ITD/wt AML.
Abstract
The coexpression of the MLL partial tandem duplication (PTD) and the FLT3 internal tandem duplication (ITD) mutations associate with a poor outcome in cytogenetically normal acute myeloid leukemia (AML). In mice, a double knock-in (dKI) of MllPTD/wt and Flt3ITD/wt mutations induces spontaneous AML with an increase in DNA methyltransferases (Dnmt1, 3a, and 3b) and global DNA methylation index, thereby recapitulating its human AML counterpart. We determined that a regulator of Dnmts, miR-29b, is downregulated in bone marrow of dKI AML mice. Bortezomib exerted a dose-dependent increase in miR-29b expression in AML blasts ex vivo, followed by decreased Dnmts, reduced proliferation, and increased apoptosis. In vivo, bortezomib was not active against dKI AML, yet liposomal-encapsulated bortezomib, as a single agent, reversed downregulation of miR-29b in vivo and induced a long-term (90-day) disease-free remission in 80% of dKI AML mice that exhibited high leukemic burden at the start of therapy, yet showed no signs of relapse at autopsy. Taken together, these data support that liposomal bortezomib, as a single agent, eradicates MllPTD/wt:Flt3ITD/wt AML in mouse and may represent a powerful and potentially curative approach to high-risk human disease.
Introduction
Acute myeloid leukemia (AML), one of the most common types of adult acute leukemia, is a clinically and genetically heterogeneous disease1 with a poor prognosis in the majority of the patients. Only approximately 40% of younger (<60 years) patients and 10% of older (>60 years) patients achieve long-term survival, supporting the need for novel therapeutic strategies.
The prognosis of AML patients depends largely on cytogenetic and molecular aberrations that are incorporated into classification of AML and used for treatment guidance.2 Importantly, several of these genetic markers may serve not only as prognosticators but also as therapeutic targets. Among the common recurrent genetic aberrations in AML, our group identified a partial tandem duplication (PTD) in the Mixed Lineage Leukemia (MLL) gene. MLL-PTD is present in approximately 5% to 10% of cytogenetically normal AML and is predictive of unfavorable outcome.3 MLL encodes for a histone 3 lysine 4 methyltransferase that participates in the epigenetic regulation of target genes’ expression. MLL-PTD is likely a gain-of-function mutation that encodes a mutant protein with increased histone 3 lysine 4 methyltransferase activity, driving the expression of oncogenes such as HoxA9.4-6 In addition to the histone changes, the MLL-PTD presence in AML blasts coincides with DNA hypermethylation and silencing of tumor suppressor genes,7,8 thereby supporting the contribution of multiple epigenetic mechanisms to MLL-PTD–driven leukemia.
The epigenetic function of MLL-PTD alone, however, is not sufficient to produce a leukemia phenotype. In fact, a murine knock-in mutation of MllPTD under the control of its own promoter demonstrates increased self-renewal of hematopoietic progenitors but no acute leukemia in either primary or transplant models.9,10 This finding is consistent with previous AML models that implicate at least 2 classes of mutations for development of leukemia phenotype (ie, “2-hit” model11 ), suggesting that additional genetic “hits” are required for the development of the leukemia phenotype.
Consistent with this model, patients harboring MLL-PTD often present with other mutations, including the internal tandem duplication of FLT3 (FLT3-ITD).12 FLT3 encodes a member of the receptor tyrosine kinase family that controls survival and proliferation of hematopoietic cells. FLT3-ITD is a gain-of-function mutation that occurs in approximately 25% of the cytogenetically normal AML, causes hyperleukocytosis, and confers poor prognosis.13 However, when Flt3ITD is knocked into the murine Flt3 locus, mice display clonal myeloproliferation but not AML.14 In a 2-hit model, FLT3-ITD is representative of class I mutations (ie, mutations in genes that activate signal transduction pathways), whereas MLL-PTD is representative of class II mutations (ie, those that regulate transcription).
MLL-PTD and FLT3-ITD are concurrently present in approximately 30% of the MLL-PTD AML patients and have poor prognosis.12 We have recently proven that FLT3-ITD and MLL-PTD cooperate in leukemia induction by crossing the MllPTD/wt mice with the Flt3ITD/wt mice. The resulting MllPTD/wt:Flt3ITD/wt double knock-in (dKI) mice spontaneously developed AML with a median survival of 50-60 weeks.15 This murine model recapitulates the cytogenetic and molecular characteristics of human MLL-PTD and FLT3-ITD AML. In fact, as with the MLL-PTD/FLT3-ITD AML patients, the dKI mice have normal cytogenetics, a decrease in the ratio of Mll-WT-to-Mll-PTD alleles, a loss of the Flt3-WT allele, and a gain in total Flt3 expression.15
The cooperative roles of MllPTD/wt and Flt3ITD/wt in the development of an AML phenotype, not only validate a 2-hit model, but also suggest that mechanistic crosstalk occurs between the aberrant Flt3-dependent kinase signaling pathways and the Mll-dependent epigenetic mechanisms. This may be mediated by miR-29b, a microRNA that we previously reported to be abnormally downregulated in human AML through mechanisms that are driven by the aberrantly functioning receptor tyrosine kinases (ie, KIT and FLT3) and involve a Sp1/nuclear factor (NF) κB/histone deacetylase (HDAC) repressor complex.16-18 Indeed, replacing endogenous miR-29b resulted in antileukemic activity.19 miR-29b targets DNA methyltransferases (DNMTs), which regulate epigenetic gene silencing via DNA hypermethylation.17,20 Therefore, kinase-dependent miR-29b downregulation may be involved in the increased DNMT expression and DNA hypermethylation associated with MLL-PTD and may represent a novel target for MLL-PTD/FLT3-ITD AML.7,8
Relevant to the present study, we have shown recently that miR-29b can be restored pharmacologically using bortezomib, a proteasome inhibitor already in the clinic that interferes with the Sp1/NFκB/HDAC repressor complex in other subsets of AML (ie, core binding factor).16-18 Therefore, we postulated here that bortezomib may have activity in dKI AML.
Methods
Reagents
The following antibodies were used for immunoblot experiments: Dnmt1 and 3b (Abcam, Cambridge, MA), Dnmt3a and actin (Santa Cruz, Dallas, TX).
Generation of leukemic MllPTD/wt:Flt3ITD/wt dKI transplant mice
All animal studies were performed under approved protocols following the Ohio State University Institutional Animal Care and Use Committee. Mice were housed in a barrier facility. Transplant experiments used whole spleen cells from secondary transplants of dKI AML that had been stored in liquid nitrogen. After thawing, 2-5 million dKI Ly5.2+ cells were injected intravenously into sublethally irradiated Ly5.1+ syngeneic mice that were 5-6 weeks old. Leukemia progression was monitored by white blood cell (WBC) count analysis.
Ex vivo cell treatment
Mice transplanted with dKI AML as described previously were euthanized when the WBC count was found to be >30 k/µL. Bone marrow (BM) cells were isolated, and any remaining red blood cells were lysed with ammonium chloride. To purify the leukemic Ly5.2 blasts from any remaining wild-type Ly5.1 cells, we performed Ly5.1 negative selection using magnetic beads (Miltenyi Biotec Inc., Auburn, CA). Purity after selection was >95%. Leukemic blasts were then cultured in RPMI + 20% fetal calf serum, 100 ng/mL rat recombinant stem cell factor and 10 ng/mL mouse interleukin-3 for up to 24 hours in vehicle or varying concentrations of bortezomib. For an individual experiment, the results at each dose were normalized to the vehicle-treated control for the same time point. Therefore, the vehicle-treated control was always set as equal to 1, and the relative fold change at a particular dose was calculated by comparing bortezomib-treated cells to the vehicle-treated control. Thus, although each graph represents a composite of at least 3 independent experiments, no variation could be shown for the vehicle-treated control because relative rather than absolute quantification was performed.
Quantitative real-time RT-PCR
Trizol (Life Technologies, Grand Island, NY) RNA extraction and quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) assays were performed as previously reported.15 Normalization was performed using β-2 microglobulin as an internal control. Fold changes in expression between leukemia cells treated with different conditions or at different time points were calculated using the cycle threshold (ΔΔCT) method as previously described.15
MTS assay
Freshly isolated dKI leukemic blasts were cultured in 96 well plates at a concentration of 100 000 cells/100 µL medium with vehicle control or bortezomib (range 0.3 nM-30 nM). After 24 hours, 3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent (Promega, Madison, WI) was added and incubated at 37°C until significant color change occurred (1-24 hours). The absorbance at 490 nm was read with a Thermo Multiskan Spectrophotometer. Background absorbance was subtracted, and % growth was calculated as the absorbance at 490 nm of bortezomib-treated samples divided by the absorbance at 490 nm of vehicle-treated samples.
Apoptosis
Leukemic blasts were stained with Annexin V and 7AAD using BD Biosciences (San Jose, CA) apoptosis kit following the manufacturer’s protocol. Flow cytometry was performed on the LSRII (BD Biosciences, San Jose, CA). Cells undergoing apoptosis were identified as Annexin V+/7AAD−, whereas dead cells were identified as Annexin V+/7AAD+.
In vivo liposomal bortezomib treatment
The procedure for preparing liposomal bortezomib was previously described.21 Free (nonliposomal) bortezomib is commercially available. After generating leukemic transplant mice as described previously, treatment began 2.5 weeks posttransplant, when mice were identified as having high WBC (>11 K/µL). Free (nonliposomal) bortezomib (n = 5 mice), empty liposomes (n = 5 mice), or liposomal bortezomib (n = 5 mice) was intravenously injected at 1 mg/kg twice a week for 2 weeks followed by 2.5 mg/kg twice a week for 2 weeks. Mice were monitored daily.
Statistics
Unpaired 2-tailed t tests were used for real-time RT-PCR on primary mouse BM. For treatments with bortezomib ex vivo, linear mixed effects models were used for analysis to take account of the correlations among the observations from the same subject (MTS, apoptosis, real-time RT-PCR). A log-rank test was used for the mouse survival analysis and Kaplan-Meier survival curve was used to display the results. Holm’s procedure was used to control for multiple end points or multiple comparisons. SAS 9.2 (SAS Institute Inc., Cary, NC) was used for analysis.
Results
miR-29b is downregulated in murine Mll3PTD/wt:Flt3ITD/wt dKI AML
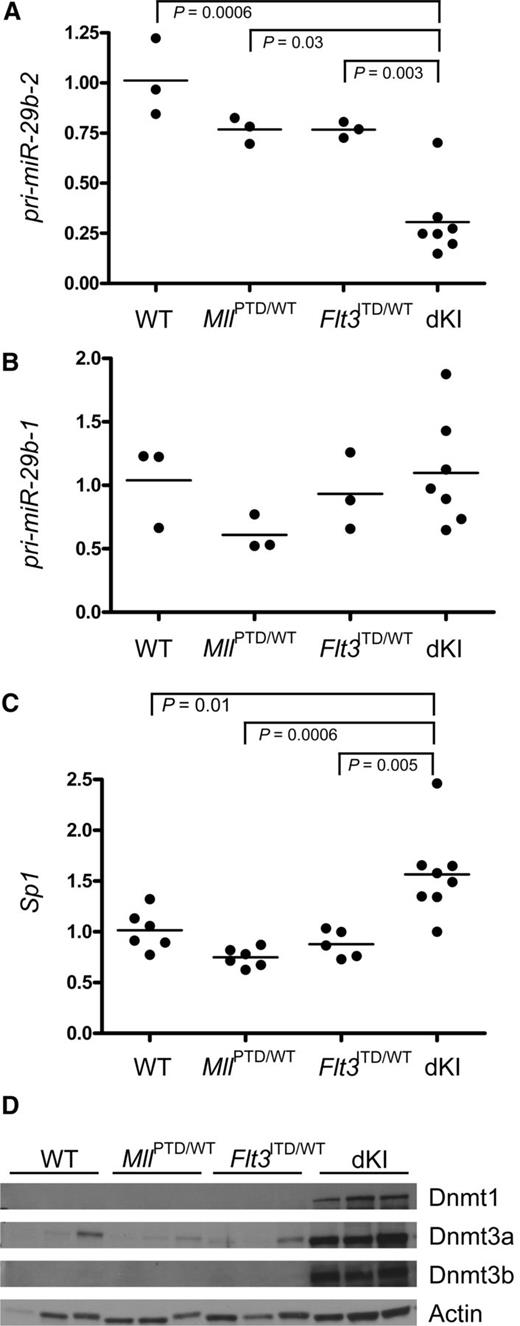
We have previously reported that miR-29b is downregulated in human AML through mechanisms that involved aberrant kinase signaling and may represent a novel, suitable therapeutic target.16-18 Therefore, we hypothesized that miR-29b was also downregulated in MllPTD/wt:Flt3ITD/wt dKI AML. We sought to validate our hypothesis by measuring miR-29b in leukemic dKI BM and comparing it with nonleukemic, age-matched wild-type or single-mutant MllPTD/wt or Flt3ITD/wt controls. Because the sequence of mature miR-29b is low in GC content, which may interfere with accurate RT-PCR–based quantification,22 we measured the levels of the premature form, pri-miR-29b. Genes encoding miR-29b comprise 2 clusters: pri-miR-29b-1/29a is located on murine chromosome 6 and human chromosome 7; pri-miR-29b-2/29c is located on chromosome 1 in both mice and humans. We showed that pri-miR29b-2 and not pri-miR29b-1 was significantly downregulated in BM from dKI AML mice compared with wild-type, MllPTD/wt, or Flt3ITD/wt nonleukemic controls (Figure 1A, P < .0006 and Figure 1B, not significant). This is consistent with our previous results indicating that in AML models, there is a preferential downregulation of the miR-29b cluster located on chromosome 1, where the enhancing region includes a binding site for the Sp1/NFkB/HDAC repressor complex.16 A feedback loop exists between miR-29b and Sp1, with the 2 molecules downregulating each other.16 Consistent with these results, in the dKI leukemic BM where miR-29b was found to be reduced, we observed a 1.6-fold increase in expression of Sp1 (Figure 1C, P = .01). Likewise, other miR-29b targets, namely Dnmt1, 3a, and 3b, were upregulated at both the RNA (not shown here, but previously reported)8 and protein level (Figure 1D).
Dysregulation of miR-29b and Sp1 expression in MllPTD/wt:Flt3ITD/wtdKI leukemia. Real-time quantitative RT-PCR was performed on 3 to 8 bone marrow samples obtained from leukemic MllPTD/wt:Flt3ITD/wt dKI mice, single mutant MllPTD/wt mice, single mutant Flt3ITD/wt mice, or wild-type control, age-matched mice. (A) pri-miR-29b-2. (B) pri-miR-29b-1. (C) Sp1. (D) Immunoblotting for Dnmt1, 3a, and 3b was performed on whole cell lysates from bone marrow (n = 3 for each genotype). Actin was used as a loading control.
Dysregulation of miR-29b and Sp1 expression in MllPTD/wt:Flt3ITD/wtdKI leukemia. Real-time quantitative RT-PCR was performed on 3 to 8 bone marrow samples obtained from leukemic MllPTD/wt:Flt3ITD/wt dKI mice, single mutant MllPTD/wt mice, single mutant Flt3ITD/wt mice, or wild-type control, age-matched mice. (A) pri-miR-29b-2. (B) pri-miR-29b-1. (C) Sp1. (D) Immunoblotting for Dnmt1, 3a, and 3b was performed on whole cell lysates from bone marrow (n = 3 for each genotype). Actin was used as a loading control.
Bortezomib reverses miR-29b
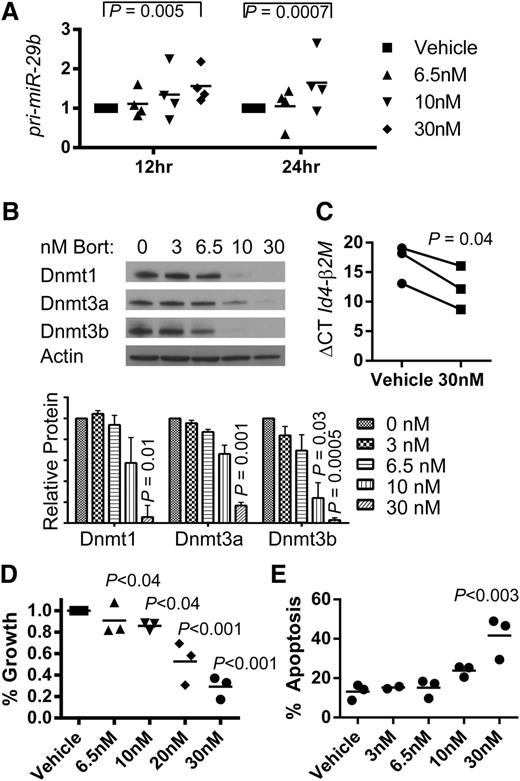
We have shown previously that bortezomib reduces DNA methylation by inducing miR-29b reexpression in AML cell lines.23 Thus, we tested whether bortezomib could upregulate miR-29b in dKI murine AML blasts. AML blasts were harvested from BM of dKI leukemic mice and treated ex vivo with vehicle or varying concentrations of bortezomib (3-30 nM). We observed a dose-dependent increase in miR-29b (Figure 2A) and a corresponding dose-dependent decrease in miR-29b targets Dnmt1, 3a, and 3b protein levels after 12 and 24 hours of bortezomib treatment, respectively (Figure 2B). We did not observe a statistically significant change in Sp1 RNA expression. The tumor suppressor Id4, previously reported to be hypermethylated and silenced in leukemia murine models,21 was found to be upregulated following bortezomib treatment, as supported by a decrease in the normalized ΔΔC values (Figure 2C). By 24 hours, 10-30 nM bortezomib showed antileukemic activity with a decrease in cell proliferation (Figure 2D) and an increased trend in apoptosis (Figure 2E). Taken together, these data support that bortezomib restored expression of the endogenous miR-29b, resulting in miR-29b target downregulation, Id4 upregulation, and antileukemic activity in dKI AML blasts.
Effective targeting of the miR-29b and Dnmts in the dKI AML. Whole spleen cells isolated from secondary transplants of leukemic Ly5.2+ dKI mice were intravenously injected into sublethally irradiated Ly5.1+ syngeneic C57Bl/6 mice. Upon development of AML, Ly5.1 negative selection was performed on bone marrow cells to achieve >95% purity of the Ly5.2 AML blast population. Blasts were then cultured ex vivo in the presence of vehicle or bortezomib up to 24 hours. Each experiment was performed 3 or 4 times as independent replicates. (A) Real-time RT-PCR quantification of pri-miR-29b-2 after 12 and 24 hours of culture in phosphate-buffered saline (vehicle) or various concentrations of bortezomib as indicated in the key. (B) Representative immunoblot of Dnmt1, Dnmt3a, and Dnmt3b on whole cell lysates collected after 24 hours of treatment with vehicle (0), 3, 6.5, 10, or 30 nM bortezomib. Graph shows quantification of protein/actin ratios between vehicle and bortezomib-treated cells calculated from 3 independent experiments. (C) Real-time RT-PCR quantification of Id4 gene expression in murine AML. A decrease in the ΔCT for Id4 was calculated using β-2 microglobulin as an internal control and signifies an increase in Id4 gene expression following 24 hours of culture in 30 nM of bortezomib. (D) Changes in cell growth measured by MTS assays. (E) Changes in apoptosis measured by Annexin V and 7-AAD staining and flow cytometry. (D-E) P values indicate significance of each treatment group compared with vehicle.
Effective targeting of the miR-29b and Dnmts in the dKI AML. Whole spleen cells isolated from secondary transplants of leukemic Ly5.2+ dKI mice were intravenously injected into sublethally irradiated Ly5.1+ syngeneic C57Bl/6 mice. Upon development of AML, Ly5.1 negative selection was performed on bone marrow cells to achieve >95% purity of the Ly5.2 AML blast population. Blasts were then cultured ex vivo in the presence of vehicle or bortezomib up to 24 hours. Each experiment was performed 3 or 4 times as independent replicates. (A) Real-time RT-PCR quantification of pri-miR-29b-2 after 12 and 24 hours of culture in phosphate-buffered saline (vehicle) or various concentrations of bortezomib as indicated in the key. (B) Representative immunoblot of Dnmt1, Dnmt3a, and Dnmt3b on whole cell lysates collected after 24 hours of treatment with vehicle (0), 3, 6.5, 10, or 30 nM bortezomib. Graph shows quantification of protein/actin ratios between vehicle and bortezomib-treated cells calculated from 3 independent experiments. (C) Real-time RT-PCR quantification of Id4 gene expression in murine AML. A decrease in the ΔCT for Id4 was calculated using β-2 microglobulin as an internal control and signifies an increase in Id4 gene expression following 24 hours of culture in 30 nM of bortezomib. (D) Changes in cell growth measured by MTS assays. (E) Changes in apoptosis measured by Annexin V and 7-AAD staining and flow cytometry. (D-E) P values indicate significance of each treatment group compared with vehicle.
Efficacy of liposomal bortezomib in vivo
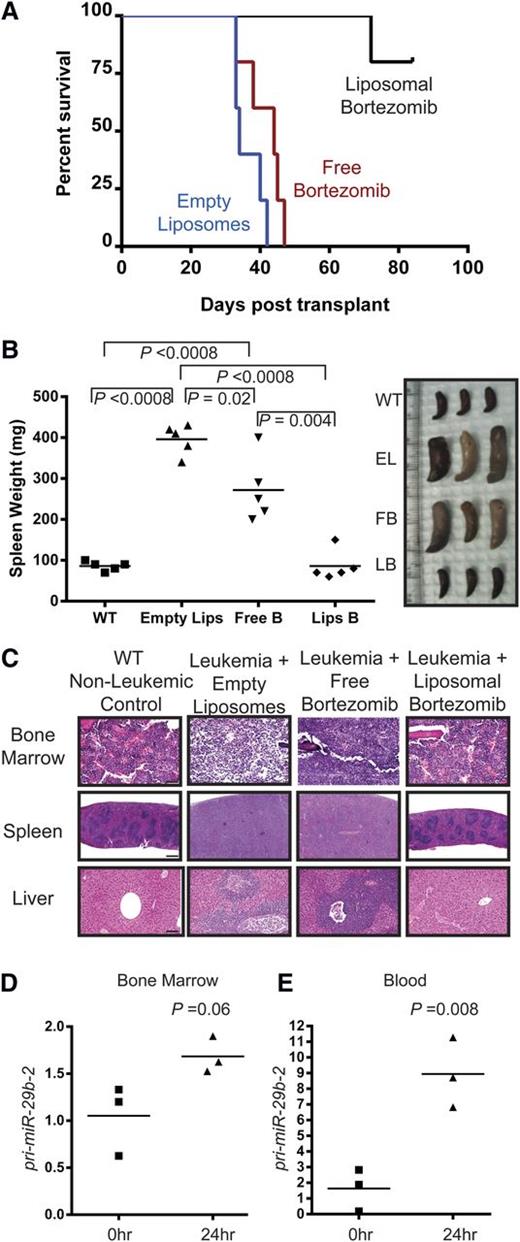
We next tested the activity of bortezomib in vivo. We have previously reported that a liposomal formulation of bortezomib has a better therapeutic index than free bortezomib in a murine model of large granular lymphocytic leukemia.21 Syngeneic Ly5.1 C57Bl/6 mice were transplanted with 5 × 106 million MllPTD/wt:Flt3ITD/wt dKI blasts. Upon detection of significant leukemic burden, as evidenced by increased WBC count, the mice received IV injection of empty liposomes (n = 5), 1 mg/kg free bortezomib (n = 5), or 1 mg/kg liposomal bortezomib (n = 5) twice a week for 2 weeks. After observing no toxicity at this dose, we increased the dose to 2.5 mg/kg while maintaining the same schedule of administration for the following 2 weeks to potentially maximize clinical outcome while evaluating dose-escalation toxicity. Again we did not observe clinical toxicity. Future work on the project may focus on determining a true maximum tolerated dose or maximum effective dose for leukemic mice. Leukemic mice treated with empty liposomes exhibited median survival of 34 days posttransplant. Free bortezomib did not significantly increase median survival compared with vehicle (median survival of 45 days; P = .14). Strikingly, however, leukemic mice treated with liposomal bortezomib achieved longer survival (median survival not reached within the timeframe of the experiment) and 80% were alive and well at 90 days posttransplant (Figure 3A, P = .0027 vs empty liposomes, P = .0018 vs free bortezomib).
In vivo bortezomib treatment of MllPTD/wt:Flt3ITD/wtdKI mice. Whole spleen cells isolated from secondary transplants of leukemic Ly5.2+ dKI mice were intravenously injected into sublethally irradiated Ly5.1+ syngeneic C57Bl/6 mice. Upon development of leukemia, as measured by WBC counts >11 K/µL, treatment was initiated as the following: 1 mg/kg IV twice per week for 2 weeks followed by 2.5 mg/kg IV twice per week for 2 weeks. (A) Kaplan-Meier survival curve. n = 5 for each group. Median survival times: 34 days vehicle, 45 days free bortezomib, not reached with liposomal bortezomib. At 90 days posttransplant, remaining liposomal bortezomib–treated mice were euthanized. (B) Spleen images and weights of mice treated with vehicle, empty liposomes, free bortezomib, or liposomal bortezomib at time of euthanization. n = 5 for each group. (C) Bone marrow, spleen, and liver were stained with hematoxylin and eosin to show normal morphology and lack of infiltrating leukemic blasts in the liposomal bortezomib–treated mice. Slides from 3 individual mice from each treatment group were read in a blinded manner by a pathologist. A representative example is shown for each group. Empty liposomes or free bortezomib–treated mice showed significant blast infiltration in all tissues and loss of normal morphology. Bars represent 50 µm for BM, 500 µm for spleen, and 100 µm for liver. (D-E) RNA was isolated from whole bone marrow (D) or blood (E) taken 0 (n = 3) or 24 (n = 3) hours after a single 1 mg/kg dose of bortezomib on leukemic mice. After complementary DNA preparation, real-time RT-PCR was performed for pri-miR-29b-2.
In vivo bortezomib treatment of MllPTD/wt:Flt3ITD/wtdKI mice. Whole spleen cells isolated from secondary transplants of leukemic Ly5.2+ dKI mice were intravenously injected into sublethally irradiated Ly5.1+ syngeneic C57Bl/6 mice. Upon development of leukemia, as measured by WBC counts >11 K/µL, treatment was initiated as the following: 1 mg/kg IV twice per week for 2 weeks followed by 2.5 mg/kg IV twice per week for 2 weeks. (A) Kaplan-Meier survival curve. n = 5 for each group. Median survival times: 34 days vehicle, 45 days free bortezomib, not reached with liposomal bortezomib. At 90 days posttransplant, remaining liposomal bortezomib–treated mice were euthanized. (B) Spleen images and weights of mice treated with vehicle, empty liposomes, free bortezomib, or liposomal bortezomib at time of euthanization. n = 5 for each group. (C) Bone marrow, spleen, and liver were stained with hematoxylin and eosin to show normal morphology and lack of infiltrating leukemic blasts in the liposomal bortezomib–treated mice. Slides from 3 individual mice from each treatment group were read in a blinded manner by a pathologist. A representative example is shown for each group. Empty liposomes or free bortezomib–treated mice showed significant blast infiltration in all tissues and loss of normal morphology. Bars represent 50 µm for BM, 500 µm for spleen, and 100 µm for liver. (D-E) RNA was isolated from whole bone marrow (D) or blood (E) taken 0 (n = 3) or 24 (n = 3) hours after a single 1 mg/kg dose of bortezomib on leukemic mice. After complementary DNA preparation, real-time RT-PCR was performed for pri-miR-29b-2.
To determine whether these liposomal bortezomib–treated mice showed any evidence of residual disease, they were euthanized at 90 days posttransplant and compared with vehicle-treated or free bortezomib–treated mice whose tissues were collected at time of moribund state. In comparison with spleens taken from age-matched wild-type mice (mean = 86 mg), spleens from mice treated with either empty liposomes (mean = 396 mg, P < .0001 vs wild-type) or free bortezomib (mean 272 mg, P ≤ .0008 vs wild-type) exhibited significant splenomegaly. In contrast, spleen size of liposomal bortezomib–treated mice (mean 86 mg) was indistinguishable from those of age-matched wild-type mice (Figure 3B). Furthermore, histopathological analysis of BM, spleen, and liver showed that both empty liposome-treated mice and free bortezomib–treated mice exhibited significant infiltration of leukemic blasts and a loss of normal architecture in all three organs. In contrast, no infiltration of leukemic blasts was observed in organs from liposomal bortezomib–treated mice. A board-certified mouse pathologist read slides containing BM, spleen, and liver from 3 individual mice within each treatment group in a blinded fashion and could not identify any blasts in either the wild-type nonleukemic control or the liposomal bortezomib–treated mice. Furthermore, the architecture of the organs was indistinguishable from age-matched wild-type control mice (Figure 3C). Together, these data show that liposomal bortezomib produces a significant effect on survival of dKI AML mice that is potentially curative.
To test the pharmacodynamic activity in vivo, we also measured changes of pri-miR-29b-2 levels in Ly5.1 C57Bl/6 mice (n = 6) that had been transplanted with 1.5 × 106 million dKI blasts. The level of pri-miR-29b-2 was measured once mice became leukemic as defined by WBC counts >11 K/µL and received a single dose of bortezomib. Mice were euthanized and hematopoietic tissues obtained 24 hours after treatment. Consistent with our in vitro data, pri-miR-29b-2 was upregulated in BM and blood (Figure 3D-E).
Discussion
We and others have previously demonstrated that miR-29b is downregulated in AML, thereby contributing to leukemogenesis through aberrantly activated mechanisms of cell survival, proliferation, and gene silencing.16,20,24 The likelihood that miR-29b may play a key role in myeloid leukemogenesis is also supported by recent data showing that its expression level is likely to be predictive of clinical response to decitabine, a hypomethylating agent.25 Decitabine-treated patients with lower pretreatment levels of miR-29b have decreased odds of achieving remission. In turn, we showed that replacing endogenous miR-29b with a synthetic counterpart has resulted in preclinical antileukemic activity both in vitro and in vivo and, if given preemptively, potentiates the antileukemic activity of decitabine.19,20 Consistent with these results, we observed no reexpression of miR-29b and no antileukemia activity in blasts from our murine model of AML treated with decitabine alone (data not shown).8 Thus, these results suggest that miR-29b may represent a novel therapeutic target in AML, yet to date, this target remains to be fully validated in mouse models of relevant molecular subsets of human AML or in patients with AML.
In this work, we demonstrated that miR-29b is also downregulated in a murine model of MllPTD/wt:Flt3ITD/wt AML. The miR-29b downregulation was reversed by treatment with bortezomib, leading to higher miR-29b expression and lower levels of the miR-29b–targeted Sp1 and Dnmts. This was followed by antileukemic activity, as evidenced by decreased growth and increased apoptosis of primary mouse AML blasts treated ex vivo. Most importantly, although bortezomib as an unconjugated single agent had no antileukemic activity in vivo, we have demonstrated that a liposomal formulation of bortezomib exerts a striking antileukemic effect in vivo. The majority of the mice treated with liposomal bortezomib (80%) remained alive and well at day 90, suggesting that the disease was eradicated. Interestingly, unconjugated bortezomib upregulated miR-29b in blood, but not nearly as significantly in BM. This led us to speculate that liposomal bortezomib may provide a greater upregulation of miR-29b in BM. In fact, preliminary unpublished pharmacokinetic data suggest that liposomal bortezomib produces a higher sustained concentration of bortezomib in the BM when compared with free bortezomib (M. Phelps, personal communication).
The next question that arises from our results is whether the excellent preclinical activity of liposomal bortezomib is restricted to MllPTD/wt:Flt3ITD/wt AML or whether its activity can be extended to other molecular subtypes of AML or even other types of leukemias. In fact, liposomal bortezomib cured an otherwise fatal murine acute large granular lymphocytic leukemia through a mechanism that at least in part involves the reexpression of miR-29b and subsequent reduction of Dnmt expression and consequent reexpression of the tumor suppressor gene Id4.21 These data, and the data presented in the current report, suggest that liposomal formulated bortezomib may offer a potentially curative therapy for MLL-PTD/FLT3-ITD AML patients.
Several groups have previously studied the efficacy of free bortezomib (Velcade [R]) in AML. As a single agent, however, free bortezomib provided limited clinical activity in early clinical trials,26 but showed more promise in combination with cytotoxic agents.18,27,28 Here, for the first time, we report what is a seemingly curative effect of bortezomib as a single agent in preclinical model of a very high-risk molecular subtype of AML (ie, MllPTD/wt:Flt3ITD/wt). That the antileukemic activity and the potential disease eradication occurred only with the novel liposomal formulation of bortezomib, and not with the free drug that is currently used in the clinic, led us to speculate that distinct pharmacokinetic and/or molecular mechanisms are responsible for these differences. Our preliminary results support an increased area-under-the-curve exposure time for liposomal bortezomib compared with free bortezomib (data not shown). Guzman et al have previously reported that NF-κB is constitutively activated in leukemia-initiating cells.29 Bortezomib may interfere with NF-κB activity; therefore, it is possible that the liposomal formulation may facilitate drug uptake into the leukemia-initiating cells and in turn result in disease eradication. However, demonstration of a differential pharmacologic effect of the 2 drug formulations in such a minute and relatively undefined subpopulation of leukemia cells is difficult to demonstrate and will require an extensive amount of additional preclinical experimentation.
In conclusion, we report here that liposomal bortezomib, delivered as a single agent, may represent a novel therapeutic approach for MLL-PTD/FLT3-ITD AML patients. The activity of this newly formulated agent may be mediated by the pharmacologic increase in miR-29b, as reported previously in acute large granular lymphocytic leukemia. Whether differences in pharmacokinetic profiles are sufficient to fully explain the difference in antileukemic activity of the 2 formulations is currently unknown and will require additional preclinical work. Our results support further development and investigation with liposomal bortezomib, both in preclinical models of AML and eventually in patients with relapsed or refractory AML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge The Ohio State University (OSU) Leukemia Specialized Program of Research Excellence and the OSU Comprehensive Cancer Center Shared Resources: Flow Cytometry, Comparative Pathology and Mouse Phenotyping, and Biostatistics.
This work was supported by grants from the National Cancer Institute (CA089341 and CA009338) (M.A.C.), (CA102031) (G.M.), (P50CA140158) (W.B., M.A.C., and G.M.), (T32 CA009338) (K.M.B. and B.L.M.-B.); OSU Arts and Sciences Undergraduate Research Scholarship (J.N.), National Institutes of Health (T32 GM12453) (N.A.Z.), OSU College of Medicine Fellowship (N.A.Z.), Pelotonia Graduate Fellowship (N.A.Z.), American Society of Hematology Minority Medical Student Award Association (M.M.), American Society of Hematology Scholar (R.F.S.), Barry M. Goldwater Fellowship (R.F.S.), and Pelotonia Undergraduate Fellowship (J.S.N., D.L.B., and R.F.S.).
Authorship
Contribution: K.M.B., R.S., S.L., R.J.L., W.B., M.A.C., and G.M. designed the experiments; M.Z., X.Y., and R.J.L. prepared the liposomal bortezomib; K.M.B., J.S.N., R.S., S.L., N.A.Z., K.E.D., K.K.M., E.H.A., M.R.M., R.F.S., G.G.M., B.L.M.-B., D.L.B., S.G., and A.M.D. performed the experiments; K.M.B., J.S.N., R.S., S.L., N.A.Z., S.P.W., X.Z., J.Z., W.B., M.A.C., and G.M. analyzed the data; and K.M.B., G.G.M., M.A.C., and S.P.W. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
Correspondence: Guido Marcucci, 410 Biomedical Research Tower, 460 West 12th Ave, Columbus, OH 43210; e-mail: guido.marcucci@osumc.edu; and Michael A. Caligiuri, 300 W. 10th Ave, Suite 519, Columbus, OH 43210; e-mail: michael.caligiuri@osumc.edu.
References
Author notes
M.A.C. and G.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal