Abstract
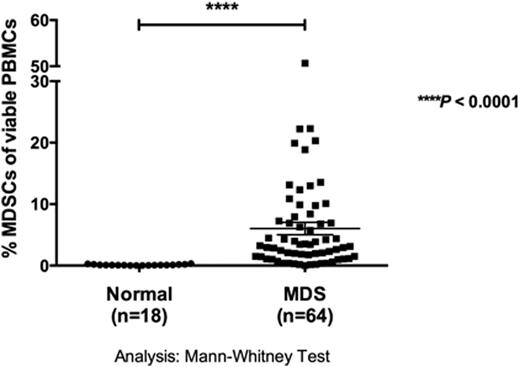
MDS is a clonal heterogeneous stem cell disorder identified by pancytopenia that frequently progresses to acute myeloid leukemia (AML). The only curative treatment is hematopoietic cell transplantation (HCT), but many patients are ineligible due to advanced age and comorbidities. New therapeutic strategies are urgently needed to reduce disease burden and improve overall survival. Studies of NK cell function from MDS patients have shown diminished natural cytotoxicity against K562 targets. These NK cell defects have been ascribed to abnormalities in expression and function of the NK cell activating receptors NKG2D, NKp30, NKp46 and DNAM-1. However, the properties of the potent activating receptor CD16 (FcgRIII) and its potential for mediating targeted immunotherapy remain unexplored in MDS. Frozen pre-transplant samples from 67 MDS patients (age 19-74 years, Median=47) receiving unrelated HCT were obtained from the research sample repository of the National Marrow Donor Program and were compared to normal volunteers. NK cell degranulation, cytotoxicity and cytokine production were evaluated using published methods. We focused on CD16-induced NK cell function using a bispecific killer cell engager generated to target CD16 along with the myeloid differentiation antigen CD33 (CD16xCD33 BiKE). CD16 expression on total CD56+ MDS-NK cells was significantly lower compared to normal donors (MDS vs. normal: 63 ± 3% (n=67) vs. 89 ± 2% (n=20); p < 0.0001). However, reverse-antibody dependent cell-mediated cytotoxicity (R-ADCC) assays revealed no significant differences in CD107a (46 ± 5% (n=16) vs. 46 ± 3% (n=12)), IFN-g (6 ± 1% vs. 10 ± 2%) or TNF-a (9 ± 2% vs. 6% ± 1%) production when resting MDS-NK cells were triggered with an agonistic CD16 mAb, indicating MDS-NK cell function triggered through CD16 is intact. To evaluate the therapeutic potential of targeting CD16 in MDS patients, resting MDS-mononuclear cells were coated with 10mg/mL of the CD16xCD33 BiKE and co-cultured for 4 hours with the CD33+ target cell line HL-60. MDS-NK cell CD107a (CD16xCD33 BiKE vs. no reagent: 42 ± 2% (n=62) vs. 22 ± 1% (n=20); p < 0.0001), IFN-g (15 ± 1% vs. 3 ± 1%; p < 0.0001) and TNF-a (13 ± 1% vs. 3 ± 1%; p < 0.0001) production was significantly increased compared to the no reagent and scFv CD16 controls. Moreover, in the absence of HL-60 targets, MDS-NK cells treated with the CD16xCD33 BiKE targeted endogenous CD33+ targets, significantly enhancing degranulation (37 ± 2% vs. 17 ± 1%; p < 0.0001), IFN-g (4 ± 1% vs. 2% ± 1%; p < 0.01) and TNF-a (5 ± 1% vs. 2 ± 1%; p < 0.01) production. Recent data have shown an increase in the frequency of MDSCs, phenotypically defined as CD33+/CD11b+/CD14-/HLA-DRlo/-, during oncogenesis and in the presence of inflammatory cytokines. Indeed, in our MDS patient cohort, the frequency of MDSCs of total viable mononuclear cells was significantly higher compared to normal donors (6 ± 1% (n=64) vs. 0.1 ± 0.02% (n=18), respectively; p < 0.0001) (figure). Furthermore, CD16xCD33 BiKE-treated resting NK cells from normal donors co-cultured with cytokine-induced MDSCs generated from normal PBMCs in a 51Cr-release cytotoxicity assay successfully targeted and lysed MDSCs (CD16xCD33 BiKE vs. no reagent (E:T = 20:1): 63 ± 5% vs. 7 ± 2% [p < 0.0001, n=4]; (E:T = 6.6:1): 35 ± 3% vs. 4 ± 1% [p < 0.0001]). CD107a (48 ± 2% (n=6) vs. 4 ± 1% (n=3); p < 0.0001), IFN-g (8 ± 1% vs. 1 ± 1%; p < 0.0001) and TNF-a (8 ± 1% vs. 1 ± 1%; p < 0.0001) production was also significantly increased against MDSC targets in the presence of the CD16xCD33 BiKE, demonstrating its ability to facilitate killing of both the CD33+ MDS clone and an immune suppressor cell population. Lastly, analysis of MDS patient karyotypes in correlation with CD16xCD33 BiKE-induced MDS-NK cell CD107a, IFN-g and TNF-a production did not reveal any significant differences between favorable, intermediate or poor international prognostic scoring system (IPSS) cytogenetic scores, further demonstrating the therapeutic potential of the CD16xCD33 BiKE to induce optimal MDS-NK cell function against CD33+ targets despite disease heterogeneity. In summary, our data suggest that the CD16xCD33 BiKE may function not only against CD33+ MDS targets themselves, but also against MDSCs. This double hit approach may protect against MDS progression/transformation to AML through immune targeting and may improve blood counts by targeting MDS-MDSCs that contribute to ineffective hematopoiesis.
No relesvant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal