Key Points
FL TILs have reduced cytokine signaling.
Abstract
Defects in T-cell function in patients with cancer might influence their capacity to mount efficient antitumor immune responses. Here, we identified highly reduced IL-4–, IL-10–, and IL-21–induced phosphorylation of STAT6 and STAT3 in tumor-infiltrating T cells (TILs) in follicular lymphoma (FL) tumors, contrasting other non-Hodgkin lymphoma TILs. By combining phospho-protein–specific flow cytometry with several T-cell markers, we identified that CD4+CD45RO+CD62L− FL TILs were largely nonresponsive to cytokines, in contrast to the corresponding autologous peripheral blood subset. We observed differential expression of the inhibitory receptor PD-1 in FL TILs and peripheral blood T cells. Furthermore, CD4+PD-1hi FL TILs, containing TFH and non-TFH cells, had lost their cytokine responsiveness, whereas PD-1− TILs had normal cytokine signaling. However, this phenomenon was not tumor specific, because tonsil T cells were similar to FL TILs. FL tumor cells were negative for PD-1 ligands, but PD-L1+ histiocytes were found within the T cell–rich zone of the neoplastic follicles. Disruption of the microenvironment and in vitro culture of FL TILs could restore cytokine signaling in the PD-1hi subset. Because FL TILs in vivo probably receive suppressive signals through PD-1, this provides a rationale for testing PD-1 Ab in combination with immunotherapy in patients with FL.
Introduction
Follicular lymphoma (FL) is characterized by the t(14;18) translocation that results in overexpression of BCL2, an antiapoptotic protein. Patient clinical outcomes are markedly heterogeneous, and FL can transform into diffuse large B-cell lymphoma (DLBCL), a more aggressive malignancy.
FL outcome is strongly influenced by the immune cell microenvironment. Gene expression profiling has identified a clinically relevant gene expression signature that possibly represents an immune response to the tumor.1 Furthermore, the immune cell composition of the FL tumor microenvironment is important because high numbers of tissue-infiltrating macrophages correlated with poor outcome in patients receiving chemotherapy regimens,2 but not in patients also receiving the monoclonal antibody rituximab.3,4 Several observations further support the hypothesis of an immune suppressive microenvironment in affected lymph nodes (LNs). These LNs have increased numbers of T regulatory cells (Tregs),5,6 and purified FL lymphoma B cells can induce the conversion of conventional CD4+ T cells into FoxP3+ Tregs.5-8 Most studies have found a positive correlation between the number of infiltrating Tregs and favorable outcome,9-11 although some report opposite findings.12 However, follicular localization of Tregs was then found to be associated with poor overall survival and high risk of transformation.13 A recent study further implied that tumor-infiltrating T cells (TILs) from FL biopsies had impaired immunologic synapse formation.14
Phospho-flow cytometric analysis has emerged as a powerful tool to analyze intracellular signaling events in complex populations of cells, because of its ability to simultaneously discriminate cell types on the basis of surface marker expression and to assess the activation status of intracellular proteins.15-18 We used this method and identified a new lymphoma subset in patients with FL, the lymphoma-negative prognostic subset, with abnormal B-cell antigen receptor signaling.19 Strikingly, the prevalence of this lymphoma cell subset in patient's tumor at the time of diagnosis, before any treatment, was negatively associated both with the response to initial chemotherapy and with overall survival. The patients' T-cell responses were also important, because patients with high IL-7–induced phosphorylation of STAT5 in TILs had a better outcome.19 We therefore expanded on this observation by interrogating the responsiveness of FL TILs to a variety of effector cytokines in comparison with TILs from healthy donors and other B-cell malignancies.
Here, using phospho-flow cytometry, we found that FL TILs had distinctively reduced signaling responses to several cytokines, including IL-4, IL-10, and IL-21. We identified that CD4+CD45RO+CD62L− T cells, the main T-cell subset in FL LNs, was largely unresponsive to cytokines, exemplified by decreased IL-4–induced phosphorylation of STAT6. This was not a general feature of these cells, because most CD4+CD45RO+CD62L− T cells in peripheral blood could respond to IL-4 stimulation. Furthermore, we showed that the nonresponsive FL TILs are characterized by high expression of the inhibitory receptor PD-1, a potential therapeutic target.
Methods
Human samples
All specimens were obtained with informed consent in accordance with the Declaration of Helsinki. Normal human peripheral blood and human non-Hodgkin lymphoma specimens were obtained from patients at the Stanford University Medical Center, Stanford, CA, with informed consent, according to a protocol approved by institutional review board or with informed consent from the Norwegian Radium Hospital, Oslo, Norway, according to a Regional Ethic Committee (REK)–approved protocol (REK no. 2.2007.2949).
Tonsils and autologous peripheral blood samples were obtained from children undergoing tonsillectomy at Stanford Hospital, with informed consent, according to a protocol approved by institutional review board. All samples were processed to mononuclear cells by Ficoll gradient centrifugation (Ficoll-Paque PLUS; GE Healthcare) and cryopreserved in liquid nitrogen. In several cases, FL LN fragments were incubated with collagenase/DNAse solution for 60 minutes at 37°C during preparation of mononuclear cell suspensions. An overview of the non-Hodgkin lymphoma patient samples is given in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and the normal control samples in supplemental Table 2.
Reagents
Recombinant human (rh) IL-4, rh IL-7, rh IL-10, and rh IL-21 were from eBioscience and were used at a final concentration of 20 ng/mL. Antibodies from Becton Dickinson (BD) were used to detect surface expression of CD3 (clone UCHT1), CD4 (clone RPA-T4), CD5 (clone L17F12), CD8 (clone SK1), BTLA/CD272 (clone J168-540.90.22), PD-1/CD279 (clone EH12.1), CTLA4/CD152 (clone BNI3), CD57 (clone NK-1), CD62L (clone Dreg 56), CD274/PD-L1 (clone MIH1), CD273/PD-L2 (clone MIH18), CD69 (clone L78), CD27 (clone L128), CD28 (clone CD28.2), CD45RO (clone UCHL1), CD20 (extracellular, clone L27), and CXCR5 (clone RF8B2). Detection of FoxP3 was performed by staining the samples with antibodies from BD to detect CD25, CD8, CD45RO, CD20, CD4, and CD3, before fixation and permeabilization according to the eBioscience protocol, and then staining with FoxP3 (clone PCH101 from eBioscience). The following antibodies from BD were used to detect phosphorylated STAT3(Y705) (clone 4/P-STAT3), STAT5(Y694) (clone 47), and STAT6(Y641) (clone 18), and these were used in combination with the following antibodies from BD: CD20 cytoplasmic tail (clone H1), BCL2 (clone 6C8), CD5 (clone L17F12), CD3 (clone UCHT1), CD4 (clone RPA-T4), CD8 (clone SK1), CD62L (clone Dreg 56), CD45RO (clone UCHL1), and PD-1/CD279 (clone EH12.1), whereas ICOS/CD278 (clone C398.4A) was from Biolegend. Antibodies from BD were used to detect production of IL-4 (clone 8D4-8) and IL-21 (3A3-N2.1).
Activation of signaling and phospho-flow cytometry
Activation of signaling and detection of phospho-proteins were performed as previously described.16,18,19 Briefly, the samples were thawed, and 1 to 5 million cells were used for flow cytometry–based live/dead discrimination and immunophenotyping. Signaling was analyzed in the remaining sample that were allowed to rest for 30 minutes at 37°C, before redistribution at 200 μL per well into v-bottomed 96-well plates and given another 20 minutes of rest. Signaling was activated by IL-4, IL-7, IL-10, or IL-21 for 15 minutes at 37°C. Paraformaldehyde at a final concentration of 1.6% was added to stop signaling and incubated for 5 minutes at room temperature, followed by permeabilization in > 90% freezer-cold methanol. At this point, the samples could be stored at −80°C, before further processing. After rehydrating the cells by washing by centrifugation 2 times in PBS, the cells were “barcoded” as previously described,20 using the fluorescent esters Pacific Blue and Pacific Orange (Molecular Probes, Life Technologies), see supplemental Methods. After barcoding, all samples from 1 patient were combined into 1 tube and stained with antibodies for 30 minutes in the dark at room temperature. In experiments where phosphorylation in distinct T-cell subsets was measured, more markers were included, and barcoding of cells were skipped. To detect cytokine-induced activation in T follicular helper (TFH) cells, FL LN specimens were stimulated with cytokines, fixed with paraformaldehyde, and then stained with anti-CXCR5 Alexa647 (BD; clone RF8B2). After staining, the cells were washed and permeabilized in > 90% methanol, followed by staining with the other Abs as described earlier. The samples were then collected on a LSR II flow cytometer (Becton Dickinson). Data were analyzed with Cytobank software (www.Cytobank.org).
In vitro culture of FL specimens
FL LN specimens were cultured in vitro at 5 million/mL in the presence of mouse IgG1 anti-PD-1 Ab (Biolegend; clone EH12.2H7; used at a final concentration of 10 μg/mL) or mouse IgG1 isotype control Ab (Biolegend; clone MG1-45; used at a final concentration of 10 μg/mL) for various times as indicated, before the cells were washed 2 times in PBS by centrifugation and then restimulated with or without IL-4 for 15 minutes. IL-4–induced phosphorylation of STAT6 in various T-cell subsets was measured by phospho-flow cytometry as described in the previous section.
Immunohistochemistry
Staining for PD-L1 in paraffin sections was performed with mAb 5H1 (provided by Dr Lieping Chen, Johns Hopkins University) at BioPillar Laboratories with the use of previously described methods.21
Statistics
Mann-Whitney U test or 2-tailed t test for paired or unpaired samples were applied as specified in figure legends, to determine the level of statistical significance, using SPSS 16.0 (SPSS Inc). Data were considered statistical significant at P < .05.
Results
Tumor-infiltrating T cells in FL biopsies have suppressed effector cytokine signaling
Previous studies indicated that TILs in FL patient samples had dysfunctional TCR signaling and impaired capacity for immunologic synapse formation.14 Therefore, we studied their response to effector cytokines in an initial cohort of 14 patients with FL. For comparison, we studied TILs from 12 patients with DLBCL, 19 patients with mantle cell lymphoma (MCL), and 14 patients with chronic lymphocytic leukemia (CLL), as well as blood samples from 6 healthy donors. Lymphoma LN specimens or PBMCs were stimulated with cytokines for 15 minutes, and induced phosphorylation was measured by phospho-flow cytometry as previously described.16,18,19 TILs were identified as CD3+CD5+CD20− cells (Figure 1A). STAT6 was phosphorylated by IL-4 and STAT3 by IL-21 in most of PBMC T cells from a healthy donor. In contrast, a lower fraction of TILs from the FL LN specimen was able to respond (Figure 1A). TILs from most FL specimens had a strikingly low cytokine-induced phosphorylation of STATs, compared with DLBCL, MCL, and CLL T cells and with PBMC T cells from healthy donors (Figure 1B-E). The effect was most prominent for IL-4–induced phosphorylation of STAT6, with a relative median fold change (FC) of p-STAT6 of 0.79 in FL TILs, compared with 1.96, 1.86, 2.39, and 2.18 in TILs from DLBCL, MCL, CLL, and healthy T-cells, respectively (Figure 1B; P < .0003). IL-10– or IL-21–induced p-STAT3 gave similar results (Figure 1C,D). IL-7–induced phosphorylation of STAT5 was more heterogeneous also within each lymphoma type but was significantly lower in FL TILs than in MCL TILs (Figure 1C; P < .04). These results indicate that FL TILs have reduced capacity to respond to important effector cytokines that regulate immune responses such as IL-4, IL-7, IL-10, and IL-21.
Tumor-infiltrating T cells in FL biopsies have suppressed cytokine signaling. Tumor specimens from patients with DLBCL, MCL, FL, or CLL or PBMCs from healthy donors were stimulated with cytokines for 15 minutes. Signaling was then stopped by fixation, followed by permeabilization and detection of cytokine-induced phosphorylation of STATs by phospho-flow cytometry. (A) Representative FACS plots of TILs or normal PBMC T cells were identified on the basis of their coexpression of CD3 and CD5, lack of CD20 expression, and histograms of IL-4–induced p-STAT6 and IL-21–induced p-STAT3 in TILs from a FL tumor sample (FL-J117) and a healthy donor shown as median fold change, relative to unstimulated cells. On the archsinh scale, a FC of 1.75 corresponds to a difference of 1 log10. Scatter plots of cytokine-induced phosphorylation in TILs from DLBCL, MCL, FL, and CLL and PBMC T cells from healthy donors. (B) IL-4–induced p-STAT6, (C) IL-10–induced p-STAT3, (D) IL-21–induced p-STAT3, and (E) IL-7 induced p-STAT5. Each dot represents one patient sample: DLBCL, n = 12; MCL, n = 19; FL, n = 14; CLL, n = 14; and healthy donor PBMCs (normal), n = 6. Significance between groups was determined by unpaired Mann-Whitney U test.
Tumor-infiltrating T cells in FL biopsies have suppressed cytokine signaling. Tumor specimens from patients with DLBCL, MCL, FL, or CLL or PBMCs from healthy donors were stimulated with cytokines for 15 minutes. Signaling was then stopped by fixation, followed by permeabilization and detection of cytokine-induced phosphorylation of STATs by phospho-flow cytometry. (A) Representative FACS plots of TILs or normal PBMC T cells were identified on the basis of their coexpression of CD3 and CD5, lack of CD20 expression, and histograms of IL-4–induced p-STAT6 and IL-21–induced p-STAT3 in TILs from a FL tumor sample (FL-J117) and a healthy donor shown as median fold change, relative to unstimulated cells. On the archsinh scale, a FC of 1.75 corresponds to a difference of 1 log10. Scatter plots of cytokine-induced phosphorylation in TILs from DLBCL, MCL, FL, and CLL and PBMC T cells from healthy donors. (B) IL-4–induced p-STAT6, (C) IL-10–induced p-STAT3, (D) IL-21–induced p-STAT3, and (E) IL-7 induced p-STAT5. Each dot represents one patient sample: DLBCL, n = 12; MCL, n = 19; FL, n = 14; CLL, n = 14; and healthy donor PBMCs (normal), n = 6. Significance between groups was determined by unpaired Mann-Whitney U test.
T-cell composition is skewed toward CD4+CD45RO+ T cells in FL LN biopsies
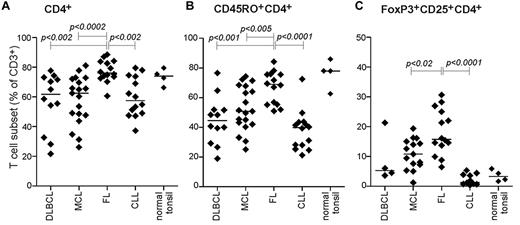
The reduced cytokine responses among FL TILs, compared with those from other B-cell malignancies and normal PBMCs, could be because of different T-cell subset composition. Indeed, immunophenotypic analysis showed a significant higher percentage of CD4+ T cells in FL specimens, with an average of 76% CD4+ T cells of total CD3+ T cells, compared with 62% and 63% in DLBCL and MCL, respectively (Figure 2A). Furthermore, most CD4+ T cells in FL specimens expressed CD45RO (supplemental Figure 1A) and on average accounted for 69% of all TILs in FL specimens, compared with 45% and 51% in DLBCL and MCL specimens, respectively (Figure 2B). However, in addition tonsil T cells showed similar high proportion of CD4+CD45RO+ cells (Figure 2B). Malignant LNs from patients with FL are known to have increased percentages of Tregs compared with healthy controls.5,6 We observed here that FL specimens on average had 15% CD4+CD25+FoxP3+ Tregs of total CD3+ T cells, compared with less than 11% in DLBCL and MCL specimens (Figure 2C).
T-cell composition is skewed toward CD4+CD45RO+ T cells in FL LN biopsies. Immunophenotypic analysis of the lymphoma patient specimens. Shown is scatter plot of percentage positive cells of total CD3+ T cells in the tumor sample of (A) CD4+ T cells, (B) CD4+CD45RO+ memory T cells, and (C) FoxP3+CD25+CD4+ T regulatory cells. DLBCL, n = 12 (n = 4 for FoxP3); MCL, n = 19; FL, n = 14; CLL, n = 14; and tonsil, n = 4. Significance between groups was determined by unpaired Mann-Whitney U test.
T-cell composition is skewed toward CD4+CD45RO+ T cells in FL LN biopsies. Immunophenotypic analysis of the lymphoma patient specimens. Shown is scatter plot of percentage positive cells of total CD3+ T cells in the tumor sample of (A) CD4+ T cells, (B) CD4+CD45RO+ memory T cells, and (C) FoxP3+CD25+CD4+ T regulatory cells. DLBCL, n = 12 (n = 4 for FoxP3); MCL, n = 19; FL, n = 14; CLL, n = 14; and tonsil, n = 4. Significance between groups was determined by unpaired Mann-Whitney U test.
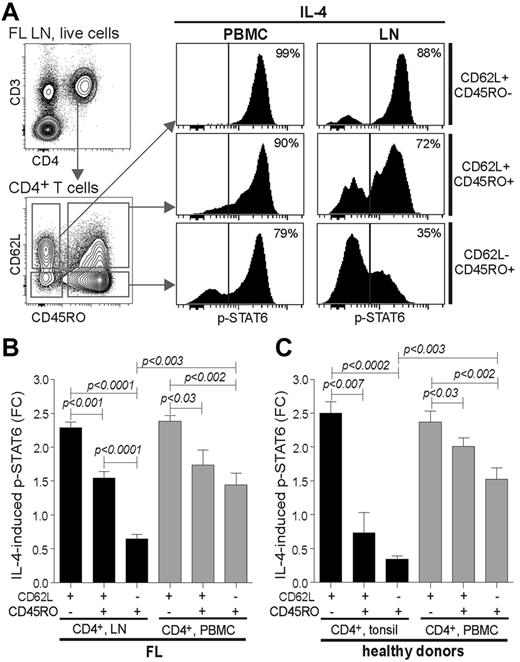
CD4+CD45RO+CD62L− FL TILs, but not the corresponding autologous PBMC T cells, have reduced cytokine signaling capacity
Because Tregs on average were 15% of total T cells in FL specimens, it was unlikely that reduced cytokine signaling was accounted for by this subset alone. This was confirmed when detection of p-STAT3 or p-STAT6 were combined with detection of CD4, CD25, and FoxP3, because CD4+CD25+FoxP3+ Tregs overall responded better than the conventional CD4+ T cells after cytokine stimulation, in particular to IL-10–induced p-STAT3 (supplemental Figure 2). Next, we combined detection of T-cell markers CD45RO and CD62L with p-STATs to measure cytokine-induced signaling in CD62L+CD45RO−, CD62L+CD45RO+, and CD62L−CD45RO+ T cells (gating strategy is shown in Figure 3A). We selected patients with FL whose malignant LN and autologous blood samples were both available. The largest difference in IL-4–induced p-STAT6 between FL TILs and PBMC T cells was observed for the CD4+CD62L−CD45RO+ subset (Figure 3A), with average FC of 0.65 and 1.44 for LNs and PBMCs, respectively (Figure 3B; P < .003). IL-21–induced p-STAT3 was also significantly lower in CD4+CD62L−CD45RO+ FL TILs than in CD4+CD62L+CD45RO− FL TILs, but this was not different from the corresponding PBMC T-cell subset (supplemental Figure 3). We also investigated cytokine-induced signaling in tonsil and autologous PBMC T cells from healthy donors and found that tonsil CD4+CD62L−CD45RO+ and CD4+CD62L−CD45RO+ T cells had significantly reduced IL-4–induced p-STAT6, compared with their blood counterparts, with an average FC of 0.34 and 0.73 compared with 1.53 and 2.01 (Figure 3C). Taken together, the reduced cytokine signaling observed in FL TILs was mainly because of highly reduced signaling capacity in CD4+CD62L−CD45RO+ T cells. Because the frequency of this subset is elevated in FL LNs, this further potentiates the difference in cytokine signaling compared with MCL and DLBCL TILs. However, because of the similarities in reduced cytokine signaling between FL TILs and tonsil T cells, this might represent a physiologic process.
CD4+CD62L−CD45RO+ FL TILs, but not the corresponding autologous PBMC T cells, have reduced cytokine signaling capacity. IL-4–induced phosphorylation of p-STAT6 was analyzed in malignant LN and in autologous PBMC samples from patients with FL by combining CD3-, CD5-, CD4-, CD8-, CD62L-, and CD45RO-specific Abs with p-STAT6 Ab in the phospho-flow cytometric assay. (A) Gating strategy of live cells to identify CD3+CD4+ T cells and then subsequent gating on the basis of expression of CD62L and CD45RO to identify CD62L+CD45RO−, CD62L+CD45RO+, CD62L−CD45RO+ cells, and the corresponding histograms of IL-4–induced p-STAT6 in the various CD4+ T-cell subsets. (A) Representative FACS data from 1 patient with FL are shown and (B) mean FC of IL-4–induced p-STAT6+ cells in CD4+ T subsets from FL LN and autologous PBMCs. Mean ± SEM, n = 5. (C) Mean FC of IL-4–induced p-STAT6 relative to unstimulated cells in CD4+ T subsets from tonsil and autologous blood samples from healthy tonsil donors. Mean ± SEM, n = 3. Significance between groups was determined by unpaired 2-tailed Student t test.
CD4+CD62L−CD45RO+ FL TILs, but not the corresponding autologous PBMC T cells, have reduced cytokine signaling capacity. IL-4–induced phosphorylation of p-STAT6 was analyzed in malignant LN and in autologous PBMC samples from patients with FL by combining CD3-, CD5-, CD4-, CD8-, CD62L-, and CD45RO-specific Abs with p-STAT6 Ab in the phospho-flow cytometric assay. (A) Gating strategy of live cells to identify CD3+CD4+ T cells and then subsequent gating on the basis of expression of CD62L and CD45RO to identify CD62L+CD45RO−, CD62L+CD45RO+, CD62L−CD45RO+ cells, and the corresponding histograms of IL-4–induced p-STAT6 in the various CD4+ T-cell subsets. (A) Representative FACS data from 1 patient with FL are shown and (B) mean FC of IL-4–induced p-STAT6+ cells in CD4+ T subsets from FL LN and autologous PBMCs. Mean ± SEM, n = 5. (C) Mean FC of IL-4–induced p-STAT6 relative to unstimulated cells in CD4+ T subsets from tonsil and autologous blood samples from healthy tonsil donors. Mean ± SEM, n = 3. Significance between groups was determined by unpaired 2-tailed Student t test.
Cytokine signaling deficit in FL TILs is restricted to PD-1hiCD4+ T cells and includes TFH cells and non-TFH cells
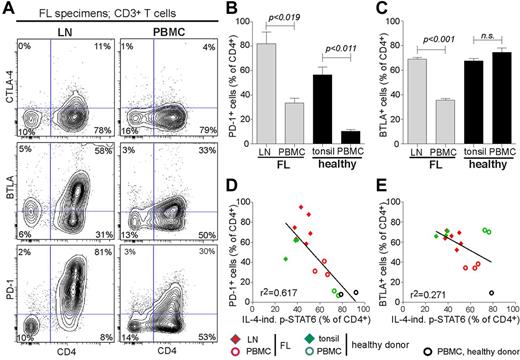
Because CD4+CD62L−CD45RO+ FL TILs had low cytokine signaling capacity compared with their autologous PBMC T-cell counterpart, we next investigated if this difference in cytokine signaling could be explained by differential expression of the inhibitory receptors CTLA-4, BTLA, or PD-1. The greatest difference was found for PD-1, which on average was expressed on 82% of the CD4+ TILs, in comparison with 33% of CD4+ PBMC T cells in patients with FL (Figure 4A-B). A similar difference was found between tonsil and PBMC CD4+ T cells in healthy donors (Figure 4B). In addition BTLA was expressed in a greater fraction of CD4+ FL TILs than in PBMC CD4+ T cells, but the difference was smaller than for PD-1 and did not differ between tonsil and autologous PBMC T cells from healthy donors (Figure 4C). In contrast to the high expression of PD-1 and BTLA in CD4+ TILs, only a few of them expressed CTLA-4 (Figure 4A). Furthermore, the CD4+ TILs were all positive for CD28, and most of them also expressed CD27 and the activation marker CD69 (supplemental Figure 4A; supplemental Table 3). PD-1+CD4+ TILs coexpressed CD45RO, whereas a smaller fraction of them also expressed CD57 (supplemental Figure 4B).
Differential expression of PD-1 in CD4+ FL TILs and autologous PBMC CD4+ T cells and negative association with IL-4–induced p-STAT6. Immunophenotypic analyses of inhibitory receptors CTLA-4, BTLA, and PD-1 were performed in FL LN and autologous blood samples. (A) Representative FACS plot of TILs and peripheral blood T cells from a representative patient with FL. (B) Mean percentage of PD-1+ cells of CD4+ T cells. FL, n = 3; healthy donors, n = 3. (C) Mean percentage of BTLA+ cells of CD4+ T cells. FL, n = 3; normal, n = 3. (D) Scatter plot of IL-4–induced p-STAT6 in CD4+ T cells versus percentage of PD-1+ cells of CD4+ T cells in LNs and PBMCs from patients with FL and in tonsils and PBMCs from healthy donors. (E) Scatter plot of IL-4–induced p-STAT6 in CD4+ T cells versus percentage of BTLA+ cells of CD4+ T cells in LNs and PBMCs from patients with FL and in tonsils and PBMCs from healthy donors. Statistical difference between groups was determined by paired 2-tailed Student t test.
Differential expression of PD-1 in CD4+ FL TILs and autologous PBMC CD4+ T cells and negative association with IL-4–induced p-STAT6. Immunophenotypic analyses of inhibitory receptors CTLA-4, BTLA, and PD-1 were performed in FL LN and autologous blood samples. (A) Representative FACS plot of TILs and peripheral blood T cells from a representative patient with FL. (B) Mean percentage of PD-1+ cells of CD4+ T cells. FL, n = 3; healthy donors, n = 3. (C) Mean percentage of BTLA+ cells of CD4+ T cells. FL, n = 3; normal, n = 3. (D) Scatter plot of IL-4–induced p-STAT6 in CD4+ T cells versus percentage of PD-1+ cells of CD4+ T cells in LNs and PBMCs from patients with FL and in tonsils and PBMCs from healthy donors. (E) Scatter plot of IL-4–induced p-STAT6 in CD4+ T cells versus percentage of BTLA+ cells of CD4+ T cells in LNs and PBMCs from patients with FL and in tonsils and PBMCs from healthy donors. Statistical difference between groups was determined by paired 2-tailed Student t test.
By combining the immune phenotype results for percentage of positive PD-1 cells with IL-4–induced p-STAT6+ cells in CD4+ T cells, we found PD-1 expression to be inversely correlated with IL-4–induced p-STAT6 (Figure 4D; r2 = 0.617, P < .0003), whereas no correlation was found for BTLA expression and p-STAT6 (Figure 4E; r2 = 0.271).
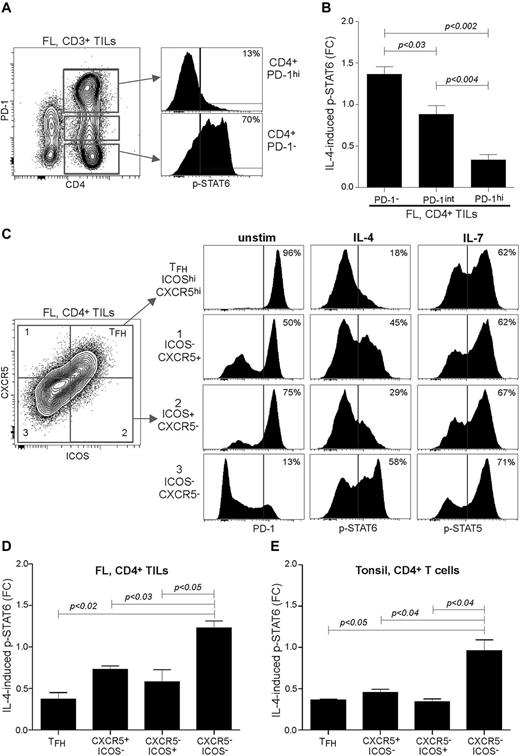
To establish a direct relation between expression of PD-1 and capacity to respond to cytokines, we activated FL LN specimens with IL-4 and then detected expression of PD-1 and p-STAT6 in single cells by phospho-flow cytometry. We found that PD-1−CD4+ TILs had normal levels of IL-4–induced p-STAT6, in contrast to only a few IL-4–induced p-STAT6+ cells in the PD-1hiCD4+ subset (Figure 5A). Furthermore, calculating the FC of IL-4–induced p-STAT6 in CD4+ T cells on the basis of the different PD-1 expression levels, PD-1hi FL TILs had a FC of 0.33 compared with 0.88 and 1.36 in PD-1int and PD-1− TILs, respectively (Figure 5B). Some of these PD-1hi cells could be TFH cells, because they are characterized by high expression of PD-1, as well as high expression of CXCR5 and ICOS.22 TFH cells have emerged as a specialized CD4+ T-cell lineage that provides help to B cells for their selection and differentiation into memory B and plasma cells.23 We first activated FL LN specimens with phorbol 12-myristate 13-acetate and ionomycin and found IL-4 and IL-21 production mainly within the CD4+PD-1hi subset (supplemental Figure 5), suggesting the presence of functional TFH cells. Immunophenotyping showed that the TFH subset in FL LNs on average accounted for 24.4% and 58.5% of CD4+ and CD4+PD-1hi TILs, respectively (supplemental Figure 6). Therefore, to more clearly delineate the identity of the PD-1hi cytokine nonresponsive cells, we next included anti-ICOS and anti-CXCR5 Abs in the phospho-flow assay. Gating of CD4+ TILs into 4 different subsets, based on expression of CXCR5 and ICOS, showed that 96% of TFH cells had high expression of PD-1 (Figure 5C). Note that most of the CXCR5−ICOS+ cells had high expression of PD-1, which contrasted with negative/low PD-1 expression in almost all CXCR5−ICOS− cells. Interestingly, the TFH and CXCR5−ICOS+ subsets had the lowest IL-4–induced p-STAT6 responses with FC of 0.37 and 0.58, respectively, compared with 1.27 in the CXCR5−ICOS− subset (Figure 5C-D). In contrast, these T-cell subsets had similar IL-7–induced p-STAT5 responses in some FL LNs but not in others (Figure 5C; data not shown), suggesting inhibitory mechanisms at play, preventing phosphorylation of STAT6 but not STAT5. Repeating these experiments with tonsil specimens showed that the same signaling features applied to the corresponding T-cell populations, identified by ICOS and CXCR5 (Figure 5E). Taken together, these data indicate that the cytokine signaling deficit observed in FL TILs as well as in tonsil T cells is restricted to PD-1hiCD4+ T cells, which included TFH cells and non-TFH cells.
The cytokine signaling deficit in FL TILs is restricted to PD-1hiCD4+ T cells and includes TFH cells and non-TFH cells. FL LN specimens were cultured with or without IL-4 for 15 minutes and then assayed for IL-4–induced phosphorylation of p-STAT6 by combining CD3-, CD5-, CD4-, and PD-1-specific Abs with p-STAT6 Ab in the phospho-flow cytometric assay. (A) IL-4–induced p-STAT6 was determined in CD4+ TILs gated on different expression levels of PD-1. (B) Data are shown as mean FC ± SEM; FL, n = 7. (C) IL-4–induced p-STAT6 was determined in CD4+ FL TILs, based on expression of ICOS and CXCR5. Shown is 1 representative case, and (D) mean FC ± SEM; FL, n = 3. (E) IL-4–induced p-STAT6 was determined in CD4+ tonsil T cells, based on expression of ICOS and CXCR5. Mean FC ± SEM; tonsil, n = 3. Statistical difference between groups was determined by paired 2-tailed Student t test.
The cytokine signaling deficit in FL TILs is restricted to PD-1hiCD4+ T cells and includes TFH cells and non-TFH cells. FL LN specimens were cultured with or without IL-4 for 15 minutes and then assayed for IL-4–induced phosphorylation of p-STAT6 by combining CD3-, CD5-, CD4-, and PD-1-specific Abs with p-STAT6 Ab in the phospho-flow cytometric assay. (A) IL-4–induced p-STAT6 was determined in CD4+ TILs gated on different expression levels of PD-1. (B) Data are shown as mean FC ± SEM; FL, n = 7. (C) IL-4–induced p-STAT6 was determined in CD4+ FL TILs, based on expression of ICOS and CXCR5. Shown is 1 representative case, and (D) mean FC ± SEM; FL, n = 3. (E) IL-4–induced p-STAT6 was determined in CD4+ tonsil T cells, based on expression of ICOS and CXCR5. Mean FC ± SEM; tonsil, n = 3. Statistical difference between groups was determined by paired 2-tailed Student t test.
Cytokine signaling deficit can be restored in PD-1hi TILs
Although the high PD-1 expression in CD4+ FL TILs and the highly reduced cytokine signaling observed in these T cells were not tumor specific (ie, also seen in tonsil T cells), the high PD-1 expression levels might negatively influence the therapeutic response to immunotherapy for patients with FL. We therefore tested if in vitro culture of FL LN specimens in the presence of neutralizing anti–PD-1 ab could restore the cytokine signaling deficit in PD-1hi TILs. FL LN specimens were precultured with anti–PD-1 Ab or an isotype control Ab for 30 minutes, 24 hours, or 48 hours before the cells were washed twice and restimulated with IL-4 for 15 minutes, followed by phospho-flow analysis. On increasing preculture time in the presence of anti–PD-1 Ab, CD3+ TILs showed improved IL-4–induced p-STAT6 with 68% p-STAT6+ cells compared with 52% p-STAT6+ cells after 48 hours and 30 minutes of preculture time, respectively (Figure 6A). However, the presence of anti–PD-1 Ab did not improve cytokine signaling beyond that of the isotype control Ab (Figure 6A-B), suggesting that disruption of the microenvironment was sufficient to release the negative suppression. The cytokine signaling capacity gradually improved over time, with 48 hours of preculture time being better than 24 hours of preculture time and occurred in CD4+ as well as in CD8+ FL TILs (Figure 6B).
In vitro culture of FL LN specimens over time restores IL-4–induced p-STAT6 in PD-1hiCD4+ T cells but does not require neutralization of PD-1. FL LN specimens were cultured over time as specified in “Methods” with anti–PD-1 neutralizing Ab or with an isotype control Ab, washed twice with PBS, and then restimulated with or without IL-4 for 15 minutes. IL-4–induced p-STAT6 and the expression of PD-1 were measured by phospho-flow cytometry. (A) Representative histograms of IL-4–induced p-STAT6 in FL LN TILs precultured with control Ab or anti-PD-1 Ab for 30 minutes or 48 hours before the cells were washed and restimulated with IL-4 for 15 minutes. (B). Time course of IL-4–induced p-STAT6 is shown in CD4+ or CD8+ T-cell subsets. Results are shown as FC of IL-4–induced p-STAT6, relative to unstimulated cells for 2 different FL donors. (C) FL LN were precultured in the presence of an isotype control Ab for 30 minutes or 48 hours before washing and restimulated with or without IL-4. IL-4–induced p-STAT6 was then determined in PD-1−, PD-1int, or PD-1hi CD4+ TILs. Shown is mean ± SEM; FL, n = 5. (D) Same experiment as described in panel C, using tonsils. Shown is mean ± SEM; tonsil, n = 3. P < .021 as determined by paired 2-tailed t test.
In vitro culture of FL LN specimens over time restores IL-4–induced p-STAT6 in PD-1hiCD4+ T cells but does not require neutralization of PD-1. FL LN specimens were cultured over time as specified in “Methods” with anti–PD-1 neutralizing Ab or with an isotype control Ab, washed twice with PBS, and then restimulated with or without IL-4 for 15 minutes. IL-4–induced p-STAT6 and the expression of PD-1 were measured by phospho-flow cytometry. (A) Representative histograms of IL-4–induced p-STAT6 in FL LN TILs precultured with control Ab or anti-PD-1 Ab for 30 minutes or 48 hours before the cells were washed and restimulated with IL-4 for 15 minutes. (B). Time course of IL-4–induced p-STAT6 is shown in CD4+ or CD8+ T-cell subsets. Results are shown as FC of IL-4–induced p-STAT6, relative to unstimulated cells for 2 different FL donors. (C) FL LN were precultured in the presence of an isotype control Ab for 30 minutes or 48 hours before washing and restimulated with or without IL-4. IL-4–induced p-STAT6 was then determined in PD-1−, PD-1int, or PD-1hi CD4+ TILs. Shown is mean ± SEM; FL, n = 5. (D) Same experiment as described in panel C, using tonsils. Shown is mean ± SEM; tonsil, n = 3. P < .021 as determined by paired 2-tailed t test.
Because the presence of anti–PD-1 Ab during the in vitro preculture time induced down-regulation of PD-1 expression as determined by PD-1 Ab staining (supplemental Figure 7), simultaneous detection of PD-1 and p-STAT6 could only be determined in cells precultured in the presence of control Ab. When dividing the CD4+ TILs from the control Ab preculture condition into 3 different subsets based on PD-1 expression levels, we discovered that IL-4–induced p-STAT6 was improved mainly in the PD-1hi subset (supplemental Figure 8). Although IL-4–induced p-STAT6 slightly decreased after in vitro preculture time for 48 hours in the PD-1− subset, p-STAT6 increased in the PD-1hi subset from 0.33 to 0.86 (Figures 6C; P < .021). After 48 hours of preculture time, a 36% decrease was observed in the frequency of PD-1hi CD4+ T cells (n = 5), as well as of TFH cells (n = 3), but an increase in PD-1− CD4+ T cells, suggesting selective cell death among PD-1hi cells. Repeating the 48-hour preculture experiments with tonsil specimens also showed that cytokine signaling was improved mainly in the PD-1hi subset (Figure 6D), again showing similarities between FL TILs and tonsil T cells. In addition, a trend was observed for improved cytokine signaling in FL TFH, CXCR5hiICOS−, and CXCR5−ICOShi cells, although this did not reach statistical significance (supplemental Figure 9).
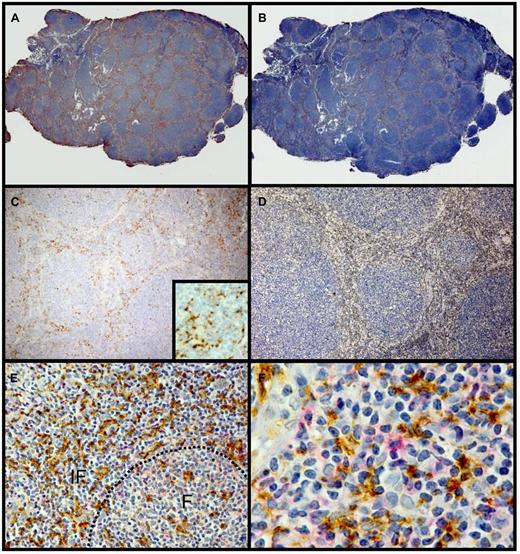
Immunophenotypic analysis of the FL LN specimens before in vitro culture showed that less than 1% of the cells expressed PD-L1 or PD-L2, and CD20+ tumor cells were found to be negative (supplemental Figure 10). Importantly, although PD-L1+ cells were not readily detectable in these cryopreserved tumor cell suspensions used in all the cytokine signaling experiments and in vitro cultures, immunohistochemical staining of 7 cases of FL showed varying numbers of PD-L1+ histiocytes within the T cell–rich zone of the neoplastic follicles (Figure 7). Some of these were colocalized with interfollicular PD-1hi T cells (Figure 7E-F). Reasoning that these PD-L1+ histiocytes might be tightly adhered to the fibrous interfollicular stroma, we attempted to recover these cells from several FL LN specimens by digestion of tumor fragments with collagenase and DNAse during preparation of cell suspensions. However, even under these conditions, PD-L1+ cells were not detectable by flow cytometry in recovered cell suspensions (data not shown), providing an explanation for why the presence of anti–PD-1 blocking Ab had no effect on FL TILs cultured in vitro over time (Figure 6). In conclusion, the highly reduced cytokine signaling responses identified in PD-1hi CD4+ FL TILs could at least partially be restored on disruption of the microenvironment and in vitro coculture when the PD-L1+ histiocytes are no longer present, therefore suggesting that FL TILs in vivo receive suppressive signals through PD-1.
Histiocytes in the T cell–rich zones express PD-L1. Representative follicular lymphoma paraffin sections stained for PD-L1 (5H1) (A,C) and CD3 (B,D). Panels A and C show PD-L1-staining cells predominantly in the T cell–rich zones between the neoplastic follicles. Inset of panel C is a high-power photomicrograph that shows the dendritic process of the 5H1 staining histiocytes. Panels B and D stained for CD3 shown staining predominantly between the neoplastic follicles in the same distribution as the PD-L1-staining cells. Panels E and F show the interfollicular zone stained for PD-L1 (brown) and PD-1 (red). F indicates follicle; IF, interfollicular zone. Hematoxylin counterstain; original magnification ×50 (A-B), ×200 (C-D), ×400 (inset in panel C); ×250 (E), and ×400 (F).
Histiocytes in the T cell–rich zones express PD-L1. Representative follicular lymphoma paraffin sections stained for PD-L1 (5H1) (A,C) and CD3 (B,D). Panels A and C show PD-L1-staining cells predominantly in the T cell–rich zones between the neoplastic follicles. Inset of panel C is a high-power photomicrograph that shows the dendritic process of the 5H1 staining histiocytes. Panels B and D stained for CD3 shown staining predominantly between the neoplastic follicles in the same distribution as the PD-L1-staining cells. Panels E and F show the interfollicular zone stained for PD-L1 (brown) and PD-1 (red). F indicates follicle; IF, interfollicular zone. Hematoxylin counterstain; original magnification ×50 (A-B), ×200 (C-D), ×400 (inset in panel C); ×250 (E), and ×400 (F).
Discussion
An important hallmark of cancer progression is the ability of the malignant cells to evade immune recognition. How the immune microenvironment affects progression and outcome of follicular lymphoma disease outcome is not clearly understood. However, several observations support the hypothesis that T cells infiltrating FL tumors are dysfunctional, possibly mediated by the malignant lymphoma B cells. In this study, we found that effector cytokine-induced activation of signaling pathways, as measured by phosphorylation of STATs, was highly reduced in T cells infiltrating FL tumors. This contrasted normal cytokine-induced signaling in T cells infiltrating MCL and DLBCL tumors. By combined detection of inhibitory receptors, T-cell markers and p-STATs post-cytokine activation, we identified the cytokine signaling deficit to be restricted to PD-1hiCD4+ TILs. Reduced cytokine signaling in PD-1hi TILs were because of the presence of functional TFH cells and the presence of other CD4+ T-cell subsets, identified as CXCR5−ICOS+ cells. The latter subset might be truly exhausted T cells and also showed high expression of PD-1. PD-L1+ histiocytes were found in the T cell–rich areas between follicles, suggesting that these cells may deliver the negative message via PD-L1 to PD-1hi T cells. We also observed a striking similarity in cytokine signaling response between FL TILs and tonsil T cells, suggesting that FL TILs might mimic normality/ongoing immune activation, in contrast to other non-Hodgkin lymphomas such as MCL and DLBCL.
Detection of CXCR5 and ICOS, together with detection of p-STAT6 after IL-4 activation, showed additional heterogeneity within PD-1hi FL TILs, because this included IL-4 low-responsive but otherwise functional TFH cells, in addition to low-responsive CXCR5−ICOS+ cells. Most of the CXCR5−ICOS+ cells also had high expression of PD-1, which contrasted with negative/low PD-1 expression in almost all CXCR5−ICOS− cells with normal IL-4–pSTAT6 response. TILs in FL tumors have previously been shown to be dysfunctional compared with peripheral blood T cells. CD4+ and CD8+ TILs were shown to have impaired T-cell immunologic synapse formation with APCs and subsequent impaired TCR-signaling responses.14 Peripheral blood T cells exhibited this defect only in patients with leukemic-phase disease. However, this T-cell dysfunction was not specific to FL TILs because TILs in DLBCL LN had the same defect.14 This finding contrasted with the highly reduced cytokine signaling responses in FL TILs that we have identified, because this was specific to FL tumors and was not observed in TILs in DLBCL and MCL tumors. On the contrary, reduced cytokine signaling in FL TILs might be physiologic, because we observed similar signaling features in comparable CD4+ T-cell subsets present in tonsils. The features of FL TILs might resemble ongoing immune activation. This is supported by our observation that FL TILs are skewed toward an effector memory (TEM) phenotype and, with the expression of CD69, suggests that these cells are activated antigen-experienced T cells. Therefore, the skewed T-cell subset composition in FL TILs toward CD4+CD45RO+CD62L− T cells could be caused by chronic antigenic stimulation that drives naive T cells into TEM. The identify of such autoantigens are not clear, although microenvironmental lectins can bind to surface immunoglobulins, because of unusual high mannosylation of immunoglobulin in FL.24 Contained within the TEM subset is the specialized TFH subset. TFH cell differentiation is skewed in FL tumors, similar to what is found in reactive LNs as well as in tonsils.25-27 In this respect, viral persistence and prolonged T-cell receptor stimulation was found to redirect CD4+ T-cell differentiation away from TH1 response toward TFH.28 Together, this suggests that these tissues have common inflammatory and immune activation signals which modulate the immune microenvironment.
By the combined detection of p-STAT6 with several T-cell markers and PD-1, we showed that the highly reduced effector cytokine signaling in FL TILs was confined to PD-1hiCD4+ TILs, whereas PD-1−CD4+ T cells within the same samples had normal levels of IL-4–induced p-STAT6. However, the reduced cytokine signaling in PD-1hi FL TILs were not solely because of nonresponsiveness of TFH cells but was also because of low responsiveness of CXCR5−ICOS+ non-TFH cells. This subset also showed high expression of PD-1; thus, it could represent truly exhausted T cells. PD-1, a member of the CD28 family, is an inhibitory receptor involved in maintenance of peripheral tolerance.29 On ligation, PD-1 blocks CD3/CD28-induced activation of PI3K and other signaling events downstream of the TCR by recruiting the protein tyrosine phosphatases SHP-1 and SHP-2.30,31 This culminates in the inhibition of T-cell proliferation, cytokine production, and differentiation. How PD-1 suppresses cytokine-induced signaling in FL TILs remains to be determined, but this could also be mediated via recruitment of phosphatases SHP-1 and SHP-2. SHP-1 is a negative regulator of IL-4–induced signaling by suppressing IL-4–mediated phosphorylation of STAT6 and STAT6-mediated transcription of IL-4–responsive genes.32,33 Of note, we found that in some patients with FL TILs with highly impaired IL-4–, IL-10–, and IL-21–induced signaling showed normal levels of IL-2–, IL-7–, and IL-15–induced p-STAT5.19 Furthermore, we observed that PD-1hiCD4+ T-cell subsets with highly reduced IL-4–induced pSTAT6 responses were capable of mounting normal levels of IL-7–induced p-STAT5. IL-2, IL-7, and IL-15, but not IL-4 and IL-21, have been shown to rescue PD-1 inhibition,34 possibly mediated via STAT5-induced activation of Akt and thereby bypassing the PD-1–induced suppression.34,35 However, PD-1 needs to be ligated to exert its suppressive function. PD-1 has 2 known ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273). Although PD-L1 expression can be induced in a variety of cell types in lymphoid and peripheral tissues, PD-L2 expression is restricted to myeloid cells.29 We found FL tumor cells to be negative for PD-L1 and PD-L2, in agreement with other studies that reported that FL tumor cells do not express PD-L1.36,37 Instead, we found that histiocytes localized to T cell–rich areas between the malignant follicles expressed PD-L1, and some of these were colozalized with PD-1+ T cells. These PD-L1+ histiocytes probably deliver the negative message to PD-1hi FL TILs in vivo, making these T cells anergic to IL-4–, IL-10–, and IL-21–induced cytokine signaling. We were not able to provide direct evidence for this hypothesis, but further support comes from the in vitro culture of PD-1hi TILs, which when cultured in the mix of other FL LN cells, but in the absence of PD-L1+ cells could regain their capacity to respond to cytokines, not seen in PD-1− cells. Furthermore, PD-1 is significantly up-regulated in exhausted virus-specific CD4+ T cells during HIV and CMV infections, and blocking activation of PD-1 was shown to partially restore T-cell function.38-40 Furthermore, a recent study by Yang et al further provides evidence for the existence of exhausted FL TIL subsets, induced by IL-12 via up-regulation of TIM-3.41 The TIM-3+ cells were unresponsive to the cytokines IFN-γ, IL-6, and IL-12 and had intermediate expression of PD-1.41 Further support comes from a recent study that showed the presence of PD-1+–infiltrating T cells in the bone marrow of patients with FL with tumor infiltration, but not in lymphoma-negative marrows,42 suggesting that FL cells alter the local immune microenvironment.
A role of PD-1 as a prognostic marker in FL is currently unclear. High numbers of PD-1+ lymphocytes were associated with improved survival in patients with FL of whom most received combination chemotherapy,43 but with shorter survival in patients with FL of whom most received rituximab-containing regimens.44 However, our finding that most FL TILs expressed PD-1 and that the signaling deficit observed in the PD-1hi subset was reversible on disruption of the microenvironment and subsequent in vitro culture over time has potential clinical significance. PD-1+ TILs in solid tumors have also been found to have dysfunctional TCR signaling, and disrupting the tumor microenvironment followed by in vitro culture over time was sufficient to partially restore TCR signaling.45 In addition, in Hodgkin lymphoma, the PD-1/PD-L1 axis contributes to an immune suppressive environment.46-48 Anti–PD-1 Ab therapy is emerging as one of the new promising ways to modulate the antitumor immune responses.49 Neutralizing anti–PD-1 Ab therapy was found to be safe and was well tolerated with evidence of single-agent clinical beneficial responses in 33% of patients with advanced hematologic malignancies.50 Phase 2 trials are currently ongoing for non-Hodgkin lymphoma, including FL.
A better understanding of how the FL tumor microenvironment affects tumor-infiltrating T cells can facilitate the design of improved immunotherapy of FL. Although the high PD-1 expression and the corresponding reduced cytokine signaling observed in CD4+ FL TILs seemed to be physiologic because this phenomenon also was seen in tonsil T cells, this process might negatively influence therapeutic response to immunotherapy for patients with FL. To overcome this, combination therapies that also take into consideration to neutralize negative regulators of T-cell responses, including PD-1, might offer therapeutic benefit. Our data suggest that anti–PD-1 Ab in combination with immunotherapy might be particularly effective in FL because of the high percentage of PD-1+ TILs in this subtype of non-Hodgkin lymphoma, compared with DLBCL and MCL, and warrants further investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (CA 34233 and CA 33399), the Leukemia & Lymphoma Society, and the Integrative Cancer Biology Program (U56 CA112973). J.H.M. was supported by the Norwegian Cancer Society, the Research Council of Norway, and South East-Regional Health Authorities. J.M.I. was supported as a Leukemia & Lymphoma Society Fellow and by the National Institutes of Health (K99 CA 143231-01). R.L. is a Clinical Research Professor of the American Cancer Society.
National Institutes of Health
Authorship
Contribution: J.H.M. and J.M.I. designed the research, performed experiments, and wrote the paper; J.B. designed the research and provided patient samples; D.K.C., R.H., M.R.G., J.T., and J.S. performed experiments; H.E.K performed experiments and provided tonsil specimens; J.D. provided patient samples and revised histopathologic analysis of patient samples; A.K. provided patient samples and clinical data; A.A.A. provided patient samples and clinical data and performed data analysis; R.L. designed the research, supervised the study, provided patient samples, and wrote the paper; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Levy, Division of Oncology, 269 Campus Dr, CCSR 1105, Stanford University Medical Center, Stanford, CA 94305-5151; e-mail: levy@stanford.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal