Key Points
Early clonal dominance may distinguish chronic myelomonocytic leukemia from other chronic myeloid neoplasms with similar gene mutations.
Early dominance of TET2-mutated cells in the hematopoietic tissue promotes myeloid differentiation skewing toward the granulomonocytic line.
Abstract
Genomic studies in chronic myeloid malignancies, including myeloproliferative neoplasms (MPN), myelodysplastic syndromes (MDS), and MPN/MDS, have identified common mutations in genes encoding signaling, epigenetic, transcription, and splicing factors. In the present study, we interrogated the clonal architecture by mutation-specific discrimination analysis of single-cell–derived colonies in 28 patients with chronic myelomonocytic leukemias (CMML), the most frequent MPN/MDS. This analysis reveals a linear acquisition of the studied mutations with limited branching through loss of heterozygosity. Serial analysis of untreated and treated samples demonstrates a dynamic architecture on which most current therapeutic approaches have limited effects. The main disease characteristics are early clonal dominance, arising at the CD34+/CD38− stage of hematopoiesis, and granulomonocytic differentiation skewing of multipotent and common myeloid progenitors. Comparison of clonal expansions of TET2 mutations in MDS, MPN, and CMML, together with functional invalidation of TET2 in sorted progenitors, suggests a causative link between early clonal dominance and skewed granulomonocytic differentiation. Altogether, early clonal dominance may distinguish CMML from other chronic myeloid neoplasms with similar gene mutations.
Introduction
Hematologic malignancies result from evolutionary processes driven by stepwise accumulation of genetic mutations with clonal expansion and selection.1 This model has been refined with the observation of complex, highly subclonal architectures2 and the finding of simultaneous acquisition of genetic lesions.3 Exploration of clonal evolution uses various methods, including deep sequencing of bulk tumors4 and sequencing of single cells or colonies,5 and needs to integrate the tissue-specific cellular hierarchy to understand why different neoplasms emerging from the same tissue and sharing genetic lesions harbor distinct phenotypes and prognoses. Because of the number of associated gene mutations,6,7 chronic myelomonocytic leukemia (CMML) is a unique model to address clonal architecture in chronic myeloid malignancies.
The characteristic feature of CMML is the accumulation of monocytes, together with dysplastic granulocytes, in the peripheral blood, bone marrow (BM), and spleen.8 Mutated genes encode signaling molecules (NRAS, KRAS, CBL, JAK2), transcription factors such as RUNX1, epigenetic regulators (TET2, ASXL1, EZH2, UTX, IDH1, IDH2, DNMT3A), and splicing factors (SF3B1, SRSF2, ZRSF2, U2AF35).6,7,9-15 None of these mutations is specific of the disease, as they were also identified in MPN16 and in MDS.17 In MPN, mutations in epigenetic regulators such as TET2 or ASXL1 can be either late events associated with disease progression, or early events that precede mutations in signaling molecules.5,18,19 In MDS, cell dysplasia in diverse myeloid lineage results from various combinations of gene mutations,17 and knowledge on genotype and phenotype associations is currently limited.20 The role and place of individual mutations regarding clonal emergence and progression of chronic myeloid malignancies remain poorly understood.
The prognosis of CMML is poor, and aside for the few patients eligible for allogeneic stem cell transplantation, there is no curative treatment. Therapeutic options include hydroxyurea to control myeloproliferation, erythropoiesis-stimulating agents to correct anemia, and hypomethylating agents to delay progression.8,21 Novel therapies, such as mitogen-activated protein kinase inhibitors, are in development. The total number of mutated genes seems to be a strong prognostic factor in CMML,7 suggesting a key role for clonal complexity in leukemogenesis and resistance to therapy.
Here, we explored the clonal architecture of 28 CMML by mutation-specific discrimination analysis of single-cell–derived clones. The genetic classification of individual cells allowed a designation of subclones and the assembly of putative evolutionary trees. We established the sequence of mutation acquisition, the dynamics of clonal expansion during hematopoietic differentiation, and its relationship to the disease phenotype and evolution, both in untreated and in treated patients. Our results suggest that early clonal dominance may specify CMML among other chronic myeloid neoplasms.
Patients and methods
Patients
Blood and BM samples from patients with CMML from Groupe Francophone des Myelodysplasies (study group is described in supplemental Methods) and control BM samples from older (>50 years) patients undergoing hip surgery were prospectively collected after informed consent according to the Declaration of Helsinki. Details are provided in supplemental Methods. The noninterventional study and decitabine trials were approved by the Cochin Hospital and Aulnay-sous-Bois ethic committees (Comite Consultatif de Protection des Personnes), respectively.
Flow cytometry and cell sorting or cloning
After immunomagnetic enrichment, BM CD34+ cells were sorted in the following fractions: CD34+CD38−CD90+ (hematopoietic stem cells [HSC]), CD34+CD38−CD90− (multipotent progenitors [MPP]), CD34+CD38+CD45RA−CD123+ (common myeloid progenitors [CMP]), CD34+CD38+CD45RA+CD123+ (granulocyte-monocyte progenitors [GMP]), and CD34+CD38+CD45RA−CD123− (megakaryocyte-erythrocyte progenitors [MEP]). Peripheral blood CD34+ cells were sorted as CD34+CD38− and CD34+CD38+ fractions. Sorted fractions were cloned at 1 cell per well in 96-well plates. Details are provided in supplemental Methods.
Gene mutation analysis
DNA extracted from CD14+ and CD3+ sorted cells (Norgen Biotek, Thorold, ON, Canada) was submitted to whole-genome amplification (Repli-G; QIAGEN, Hilden, Germany) for gene mutation screening of FLT3 (internal tandem duplications and tyrosine kinase domain mutations), NPM1 (exon 12), JAK2 (V617F), KIT (exon 17), DNMT3A (exon 23), IDH1 (exon 4), IDH2 (exon 4), TET2 (exons 3-11), CBL (exons 8-9), RUNX1 (exons 3-8), ASXL1 (exon 12), EZH2 (exons 2-20), SF3B1 (exons 13-16), U2AF35 (exons 2 and 6), ZRSR2 (exons 1-11), and SRSF2 (exon 2). All abnormalities were validated on nonamplified DNA. Details are provided in supplemental Methods.
Whole-exome sequencing and analysis
Exome sequencing was performed on DNA from skin fibroblasts and CD14+ blood cells. Details on sample collection, extraction, library preparation, capture, sequencing, and variant detection (IntegraGen, Evry, France) are provided in supplemental Methods. Indels and nonsynonymous exonic SNP with coverage ≥10×, present in CD14+ cells but not in fibroblasts, were verified by bidirectional sequencing.
Liquid cell culture
Short-term culture of 5 × 104 cells and a single-cell culture of CD34+ fractions were performed for 3 and 12 days, respectively, in minimum essential medium–alpha milieu with 10% fetal bovine serum (FBS) and recombinant human cytokines: stem cell factor (SCF, 50 ng/mL), FLT3-ligand (50 ng/mL), pegylated thrombopoietin (TPO, 10 ng/mL), interleukin-3 (IL-3, 10 ng/mL), interleukin-6 (IL-6, 10 ng/mL), granulocyte-macrophage colony–stimulating factor (GM-CSF, 5 ng/mL), erythropoietin (EPO, 1 IU/mL), and granulocyte colony–stimulating factor (G-CSF, 10 ng/mL). Detailed protocols are provided in supplemental Methods.
Methylcellulose colony–forming cell (CFC) assays
CD34+ fractions were seeded in triplicate at 250 to 1000 cells per 1-mL culture dish in 2% standard methylcellulose supplemented with 37% FCS, 12% bovine serum albumin, 1% l-glutamine, 10−6 M of β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO) and 20 ng/mL of SCF, 10 ng/mL of IL-3, 3 IU/mL of EPO, and 10 ng/mL of G-CSF. Colonies were counted on day 14. Clonogenic assays for TET2-mutated MDS and MPN are described in supplemental Methods.
Cell cycle analysis
CD34+ BM cells were incubated with 10 µg/mL of Hoescht 33342 (Life Technologies Invitrogen, Grand Island, NY) for 1 hour at 37°C in minimum essential medium–alpha milieu with 10% FBS and cytokines (50 ng/mL of SCF, 50 ng/mL of FLT3-ligand, 10 ng/mL of TPO, 10 ng/mL of IL-3, and 10 ng/mL of IL-6). The cells were then stained, acquired, and analyzed as described in supplemental Methods.
Serial replating assays
Clones generated at day 12 in liquid culture in the above-mentioned conditions were individually seeded in 96-well plates in 2% standard methylcellulose with 37% FCS, 12% bovine serum albumin, 1% l-glutamine, 10−6 M of β-mercaptoethanol and 20 ng/mL of SCF, 10 ng/mL of IL-3, and 10 ng/mL of G-CSF. At day 14, wells containing cell clusters (≥20 cells) were counted, picked, washed, and reseeded in 96-well methylcellulose plates in similar conditions. This process was repeated 5 times. Results are a percentage of the initial number of clones.
Genotyping of clones and colonies
DNA from liquid-culture clones or methylcellulose colonies was prepared as described previously.22 Mutational status was analyzed by mutation-specific fluorescent competitive probes with TaqMan real-time polymerase chain reaction (PCR) on an ABI 7500 (Applied Biosystems, Foster City, CA). Primers and probes for each mutation are detailed in supplemental Methods. Equivocal results were validated by sequencing.
Real-time quantitative PCR
Total RNA isolated with Trizol (Life Technologies) was reverse-transcribed with Superscript Vilo (Life Technologies Invitrogen). Real-time PCR was performed on an Applied Biosystems 7500 Fast thermocycler using the SyBrGreen protocol (Applied Biosystems). Primers sequences will be given on request.
TET2 knockdown by lentiviral delivery of shRNA
Sorted human cord blood CD34+/CD38− and CD34+/CD38+ cells were transduced with lentiviruses expressing the green fluorescent protein (GFP) and either shRNA-TET2 (5′-GGGTAAGCCAAGAAAGAAA-3′) or shRNA-scramble (5′-GCCGGCAGCTAGCGACGCCAT-3′) as described previously.23 GFP+ CD34+/CD38− or CD34+/CD38+ were sorted and plated on methylcellulose as described.
Statistical analyses
Statistical tests are indicated in corresponding legends. All tests were 2 tailed. Statistical analyses were performed with StatView (SAS Institute, Cary, NC) or Prism (GraphPad Software, San Diego, CA).
Results
Clonal dominance in the early compartments of hematopoietic differentiation
We first sorted peripheral blood CD14+ cells of 28 patients with CMML and searched for mutations in 18 candidate genes encoding signaling molecules (CBL, NRAS, KRAS, JAK2, FLT3, and KIT), epigenetic regulators (TET2, IDH1, IDH2, DNMT3A, ASXL1, EZH2, RUNX1, and NPM1), and splicing proteins (SRSF2, SF3B1, U2AF35, and ZRSR2). The number of mutations identified was 1, 2, 3, and 4 in 9 patients (32%), 9 patients (32%), 7 patients (25%), and 3 patients (11%), respectively, including 2 patients with 2 concomitant TET2 mutations. TET2, SRSF2, ASXL1, and signaling genes (CBL, NRAS, KRAS, or JAK2) were mutated in 17 patients (61%), 12 patients (43%), 7 patients (25%), and 12 patients (43%), respectively (Table 1). ASXL1 c.1934→p.G646WfsX12 was verified to be a somatic mutation by the TaqMan allele discrimination assay, using skin fibroblasts as germline DNA (supplemental Figure 1).
Clinical and molecular characteristics of the 28 patients studied at the clonal level
| UPN . | Age . | Sex (M/F) . | WHO category . | Karyotype . | Monocytes (g/L) . | No. of mutations . | First mutation . | Second mutation . | Third mutation . | Fourth mutation . | Architecture . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 428 | 74 | F | CMML-1 | Normal | 5.1 | 1 | TET2 (M993VfsX15) | - | |||
| 462 | 83 | M | CMML-2 | Normal | 5.0 | 2 | ASXL1 (E635RfsX15) | RUNX1(G60PfsX52) | Linear | ||
| 495 | 53 | M | CMML-1 | Normal | 1.9 | 2 | CBL (S407P) | SRSF2 (P95H) | Branched (LOH CBL) | ||
| 497 | 59 | M | CMML-1 | Normal | 2.1 | 1 | TET2 (L757TfsX12) | - | |||
| 498 | 84 | M | CMML-1 | Trisomy 13 | 2.2 | 3 | ASXL1 (G646WfsX12)* | ZRSR2* (splice n+1 ex9) | SRSF2 (P95L) | Branched (LOH ZRSR2) | |
| 500 | 86 | F | CMML-2 | Normal | 3.4 | 1 | TET2 (P1619LfsX4) | - | |||
| 501 | 74 | F | CMML-1 | Normal | 1.1 | 1 | TET2 (Q803×) | - | |||
| 507 | 70 | M | CMML-2 | Normal | 4.9 | 3 | SRSF2 (P95H) | CBL (W408R) | TET2 (S716×) | Branched (LOH CBL&TET2) | |
| 514 | 80 | F | CMML-1 | Normal | 1.8 | 1 | KRAS (G12S) | - | |||
| 516 | 76 | M | CMML-1 | Normal | 7.4 | 2 | TET2 (K450×) | NRAS (G12D) | Linear | ||
| 518 | 51 | M | CMML-1 | Normal | 1.6 | 1 | KRAS (G12C) | - | |||
| 524 | 90 | M | CMML-1 | Normal | 38.9 | 3 | TET2 (Q1702×) | SRSF2 (P95L) | TET2(S1525YfsX53)† | KRAS(A18D) | Linear |
| 531 | 81 | F | CMML-1 | Normal | 3.4 | 3 | TET2 (Q810×)* | SRSF2 (P95L)* | JAK2 (V617F) | Linear | |
| 536 | 79 | F | CMML-1 | Normal | 1.4 | 2 | CBL (R420L) | TET2 (S1630×) | Branched (LOH CBL) | ||
| 537 | 57 | M | CMML-1 | Normal | 3.2 | 2 | TET2 (E852×) | SRSF2 (P95H) | Linear | ||
| 550 | 85 | M | CMML-1 | Normal | 2.7 | 3 | TET2 (D1242TfsX11) | SRSF2 (P95H) | RUNX1(Y376LfsX197) | Branched (LOH TET2) | |
| 586 | 61 | F | CMML-1 | ND | 10.3 | 2 | ASXL1 (G646WfsX12) | TET2 (N275IfsX18) | Linear | ||
| 632 | 64 | M | CMML-2 | Normal | 10.0 | 3 | TET2 (N1156I) | SRSF2 (P95H) | NRAS (A59G) | Linear | |
| 638 | 80 | M | CMML-1 | Normal | 2.6 | 4 | SF3B1 (D586H) | CBL (C404Y) | SRSF2 (P95R) | ASXL1(G642×) | Branched (LOH CBL & SF3B1) |
| 644 | 82 | F | CMML-1 | Normal | 1.1 | 4 | ASXL1 (G646WfsX12)* | SRSF2(P95H)* | TET2 (S1190YfsX3) | TET2(R1214Q) | Linear |
| 654 | 70 | F | CMML-1 | Trisomy 8 | 2.3 | 3 | ASXL1 (G646WfsX12) | SRSF2(P95L) | JAK2 (V617F) | Linear | |
| 658 | 67 | M | CMML-2 | Monosomy 7 | 2.62 | 1 | ASXL1 (G646WfsX12) | - | |||
| 662 | 89 | M | CMML-1 | ND | 3.37 | 1 | TET2 (V1454SfsX4) | - | |||
| 731 | 78 | M | CMML-1 | ND | 11.0 | 2 | SRSF2 (P95L) | JAK2(V617F) | Branched (LOH JAK2) | ||
| 736 | 73 | M | CMML-1 | Normal | 5.1 | 1 | KRAS (T58I) | - | |||
| 743 | 82 | F | CMML-1 | Normal | 2.8 | 2 | TET2 (Q323×) | TET2(Q796TfsX6) | Linear | ||
| 752 | 76 | M | CMML-2 | del5q + tri 8 | 17.3 | 2 | NRAS (G12D)‡ | KRAS (G12A)‡ | Branched (mosaicism) | ||
| 759 | 81 | M | CMML-1 | ND | 1.5 | 3 | TET2 (T1047SfsX9)* | U2AF35 (Q157R)* | KRAS (K117R) | Branched (LOH NRAS) |
| UPN . | Age . | Sex (M/F) . | WHO category . | Karyotype . | Monocytes (g/L) . | No. of mutations . | First mutation . | Second mutation . | Third mutation . | Fourth mutation . | Architecture . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 428 | 74 | F | CMML-1 | Normal | 5.1 | 1 | TET2 (M993VfsX15) | - | |||
| 462 | 83 | M | CMML-2 | Normal | 5.0 | 2 | ASXL1 (E635RfsX15) | RUNX1(G60PfsX52) | Linear | ||
| 495 | 53 | M | CMML-1 | Normal | 1.9 | 2 | CBL (S407P) | SRSF2 (P95H) | Branched (LOH CBL) | ||
| 497 | 59 | M | CMML-1 | Normal | 2.1 | 1 | TET2 (L757TfsX12) | - | |||
| 498 | 84 | M | CMML-1 | Trisomy 13 | 2.2 | 3 | ASXL1 (G646WfsX12)* | ZRSR2* (splice n+1 ex9) | SRSF2 (P95L) | Branched (LOH ZRSR2) | |
| 500 | 86 | F | CMML-2 | Normal | 3.4 | 1 | TET2 (P1619LfsX4) | - | |||
| 501 | 74 | F | CMML-1 | Normal | 1.1 | 1 | TET2 (Q803×) | - | |||
| 507 | 70 | M | CMML-2 | Normal | 4.9 | 3 | SRSF2 (P95H) | CBL (W408R) | TET2 (S716×) | Branched (LOH CBL&TET2) | |
| 514 | 80 | F | CMML-1 | Normal | 1.8 | 1 | KRAS (G12S) | - | |||
| 516 | 76 | M | CMML-1 | Normal | 7.4 | 2 | TET2 (K450×) | NRAS (G12D) | Linear | ||
| 518 | 51 | M | CMML-1 | Normal | 1.6 | 1 | KRAS (G12C) | - | |||
| 524 | 90 | M | CMML-1 | Normal | 38.9 | 3 | TET2 (Q1702×) | SRSF2 (P95L) | TET2(S1525YfsX53)† | KRAS(A18D) | Linear |
| 531 | 81 | F | CMML-1 | Normal | 3.4 | 3 | TET2 (Q810×)* | SRSF2 (P95L)* | JAK2 (V617F) | Linear | |
| 536 | 79 | F | CMML-1 | Normal | 1.4 | 2 | CBL (R420L) | TET2 (S1630×) | Branched (LOH CBL) | ||
| 537 | 57 | M | CMML-1 | Normal | 3.2 | 2 | TET2 (E852×) | SRSF2 (P95H) | Linear | ||
| 550 | 85 | M | CMML-1 | Normal | 2.7 | 3 | TET2 (D1242TfsX11) | SRSF2 (P95H) | RUNX1(Y376LfsX197) | Branched (LOH TET2) | |
| 586 | 61 | F | CMML-1 | ND | 10.3 | 2 | ASXL1 (G646WfsX12) | TET2 (N275IfsX18) | Linear | ||
| 632 | 64 | M | CMML-2 | Normal | 10.0 | 3 | TET2 (N1156I) | SRSF2 (P95H) | NRAS (A59G) | Linear | |
| 638 | 80 | M | CMML-1 | Normal | 2.6 | 4 | SF3B1 (D586H) | CBL (C404Y) | SRSF2 (P95R) | ASXL1(G642×) | Branched (LOH CBL & SF3B1) |
| 644 | 82 | F | CMML-1 | Normal | 1.1 | 4 | ASXL1 (G646WfsX12)* | SRSF2(P95H)* | TET2 (S1190YfsX3) | TET2(R1214Q) | Linear |
| 654 | 70 | F | CMML-1 | Trisomy 8 | 2.3 | 3 | ASXL1 (G646WfsX12) | SRSF2(P95L) | JAK2 (V617F) | Linear | |
| 658 | 67 | M | CMML-2 | Monosomy 7 | 2.62 | 1 | ASXL1 (G646WfsX12) | - | |||
| 662 | 89 | M | CMML-1 | ND | 3.37 | 1 | TET2 (V1454SfsX4) | - | |||
| 731 | 78 | M | CMML-1 | ND | 11.0 | 2 | SRSF2 (P95L) | JAK2(V617F) | Branched (LOH JAK2) | ||
| 736 | 73 | M | CMML-1 | Normal | 5.1 | 1 | KRAS (T58I) | - | |||
| 743 | 82 | F | CMML-1 | Normal | 2.8 | 2 | TET2 (Q323×) | TET2(Q796TfsX6) | Linear | ||
| 752 | 76 | M | CMML-2 | del5q + tri 8 | 17.3 | 2 | NRAS (G12D)‡ | KRAS (G12A)‡ | Branched (mosaicism) | ||
| 759 | 81 | M | CMML-1 | ND | 1.5 | 3 | TET2 (T1047SfsX9)* | U2AF35 (Q157R)* | KRAS (K117R) | Branched (LOH NRAS) |
ex aequo mutations.
2 mutations in TET2.
Clonal mosaicism.
Next, in 11 patients with available BM samples, we sorted CD34+/CD38−/CD90+ (hematopoietic stem cells [HSC]), CD34+/CD38−/CD90− (multipotent progenitors [MPP]), CD34+/CD38+/CD45RA−/CD123+ (common myeloid progenitors [CMP]), and CD34+/CD38+/CD45RA+/CD123+ (granulomonocyte progenitors [GMP]; supplemental Figure 2B) cells. Phenotypic profiles of CD34+ compartments were consistent between CMML and controls (supplemental Figure 2C), and their functional relevance in CMML was validated by testing their clonogenic potential (GMP, CMP) and their serial replating capacity (MPP, HSC; supplemental Figure 3G-H). In the 17 patients for whom only peripheral blood (PB) was available, CD34+ cells were fractionated in CD34+/CD38− and CD34+/CD38+ populations.
These subpopulations were cultured at 1 cell per well in liquid medium for 12 days in the presence of a broad panel of cytokines (SCF, FLT3-L, TPO, IL-3, IL-6, G-CSF, GM-CSF, and EPO) to maximize cloning efficacy and limit biases in clonal representation. We then collected the clones and performed TaqMan discrimination analysis specifically designed for each individual mutation identified initially in PB CD14+ cells (supplemental Figure 2A). This strategy allows a faithful representation of clonal repartition for the following reasons: (1) The cloning efficacy of CD34+ fractions was not influenced by the mutational status (supplemental Figure 3A-B). (2) Comparison of clones generated in these conditions with colonies grown in methylcellulose showed an overrepresentation of mutated clones in methylcellulose (supplemental Figure 3C). (3) For 2 mutations, proportions of mutated clones were similar when assessed by pyrosequencing or next-generation sequencing of bulk CD34+ fractions (supplemental Figure 3D-F).
In all 28 patients, at least one of the mutations was found in more than 75% of PB CD34+/CD38− or BM HSC clones (Figure 1A), without significant difference between CMML-1 and CMML-2 cases. In 9 of 28 patients with sufficient materiel, 7 of 16 studied mutations originally detected in peripheral blood CD14+ cells were also found in sorted CD3+, including 2 of 7 in TET2, 2 of 3 in ASXL1, 1 of 2 in CBL, 1 of 1 in JAK2, and 1 of 1 in KRAS but 0 of 2 in RUNX1 (examples in Figure 1B). Taken together, our results point out an early amplification of the CMML clone at the CD34+/CD38− stage of hematopoiesis.
Early clonal dominance, linear accumulation of mutations, and clonal selection during myeloid differentiation. (A) Clones from sorted BM HSC (CD34+/CD38−/CD90+, noted with an asterisk) or peripheral blood immature cells (CD34+/CD38−) of 28 patients (detailed in Table 1) were cultured at 1 per well in liquid medium for 12 days in the presence of cytokines, then collected, and assessed by allele discrimination analysis of each individual mutation initially identified in peripheral blood CD14+ cells. Proportion of clones with black: wild type, yellow: 1 mutation, orange: 2 mutations, red: 3 mutations, purple: 4 mutations. Numbers on the top of the bars indicate the number of clones analyzed. (B) Examples of Sanger sequences in CD14+ and CD3+ sorted cells for 2 mutations in a representative sample (UPN #524). (C) Putative evolutionary trees of sorted BM CD34+ populations generated by genetic classification of individual cells. Simple linear trees from 2 samples (top: UPN #531: TET2 Q810×; SRSF2 P95H; JAK2 V617F; bottom: UPN #632: TET2 N1156I; SRSF2 P95L; NRAS A59G) with all mutations heterozygous in all clones (white: no mutation, yellow: 1 mutation, orange: 2 mutations, red: 3 mutations). (D) Repartition of 193 CD34+/CD38− clones showing a unique somatic mosaicism in patient UPN #752 harboring 2 independent subclones with NRAS G12D and KRAS G12A genotypes. (E) Proportion of mutated clones in HSC or MPP, CMP, and GMP fractions from 14 patients (10 with 2 or more mutations, 4 with 1 mutation); ns, nonsignificant, *P < .05 (HSC or MPP vs GMP, Wilcoxon matched-pair signed-rank test for 11 and 8 pairs, respectively). Bar: median percentage.
Early clonal dominance, linear accumulation of mutations, and clonal selection during myeloid differentiation. (A) Clones from sorted BM HSC (CD34+/CD38−/CD90+, noted with an asterisk) or peripheral blood immature cells (CD34+/CD38−) of 28 patients (detailed in Table 1) were cultured at 1 per well in liquid medium for 12 days in the presence of cytokines, then collected, and assessed by allele discrimination analysis of each individual mutation initially identified in peripheral blood CD14+ cells. Proportion of clones with black: wild type, yellow: 1 mutation, orange: 2 mutations, red: 3 mutations, purple: 4 mutations. Numbers on the top of the bars indicate the number of clones analyzed. (B) Examples of Sanger sequences in CD14+ and CD3+ sorted cells for 2 mutations in a representative sample (UPN #524). (C) Putative evolutionary trees of sorted BM CD34+ populations generated by genetic classification of individual cells. Simple linear trees from 2 samples (top: UPN #531: TET2 Q810×; SRSF2 P95H; JAK2 V617F; bottom: UPN #632: TET2 N1156I; SRSF2 P95L; NRAS A59G) with all mutations heterozygous in all clones (white: no mutation, yellow: 1 mutation, orange: 2 mutations, red: 3 mutations). (D) Repartition of 193 CD34+/CD38− clones showing a unique somatic mosaicism in patient UPN #752 harboring 2 independent subclones with NRAS G12D and KRAS G12A genotypes. (E) Proportion of mutated clones in HSC or MPP, CMP, and GMP fractions from 14 patients (10 with 2 or more mutations, 4 with 1 mutation); ns, nonsignificant, *P < .05 (HSC or MPP vs GMP, Wilcoxon matched-pair signed-rank test for 11 and 8 pairs, respectively). Bar: median percentage.
Linear accumulation of mutations and clonal selection during myeloid differentiation
We next analyzed the clonal hierarchy of CD34+/CD38− cells or HSC of patients with 2 or more mutations. The genetic classification of individual cells by allelic discrimination assays allowed a designation of subclones and the assembly of putative evolutionary trees. In most patients, mutations were found to accumulate in a linear succession, allowing the reconstitution of their sequential acquisition. Detailed examples for 2 patients with 3 mutations are presented in Figure 1C. Only 1 patient (unique patient number [UPN] #752) displayed somatic mosaicism (ie, acquisition of 2 different mutations, NRAS G12D and KRAS G12A), in distinct subclones (Figure 1D). The resulting succession of mutations in the 28 patients is recapitulated in Table 1. In 4 cases, an order of acquisition could not be assigned to 2 mutations (all involving a splice gene) because they were detected in the same clones. Although the order acquisition of the mutations was not fixed, mutations in TET2 or ASXL1 preceded mutations in signaling genes in most cases.
We then studied the dynamics of clonal architecture during myeloid differentiation by comparing clonal frequencies in sorted HSC, MPP, CMP, and GMP. We found an overrepresentation of subclones with greater number of mutations in GMP compared with HSC/MPP (examples in Figure 1C). In 14 of 19 patients with more than 1 mutation and sufficient numbers of assessable clones, the first event was present in more than 85% of HSC/MPP, without significant change in the frequency in CMP and GMP, underscoring the early dominance of the leukemic clone, whereas the second event was significantly more frequent in GMP than in HSC/MPP (Figure 1E). Altogether, our results show linear acquisition of mutations, clonal dominance of the malignant clone at the CD34+/CD38− stages (HSC and MPP) of hematopoiesis, and further selection of the more mutated subclones during the early steps of myeloid differentiation until the GMP stage. This trend suggests that secondary mutations may provide an advantage to the clone during myeloid differentiation.
Clonal branching through loss of heterozygosity
We next took advantage of information on heterozygosity and homozygosity of mutations to refine the evolutionary trees of the 19 patients with 2 or more mutations. In 10 patients, the architecture was strictly linear and mosaicism was identified in 1 patient. In the remaining 8 patients, we identified subclones with loss of heterozygosity (LOH) of mutations in CBL (n = 3, including 2 with a concomitant LOH in the TET2 or SF3B1 loci, respectively), KRAS, JAK2, TET2, and ZRSR2 (each n = 1), giving rise to single or multiple branching in the clonal architecture (example in Figure 2A, summarized in Table 1). The detection of subclones with reversion of a mutation to wild-type status suggested a mitotic recombination event as the origin of LOH. This hypothesis was assessed by taking advantage of an informative intronic single nucleotide polymorphism (SNP, rs77741206G/A) located 46 base pairs 3′ from SF3B1 c.1756G>C (p.D586H) in 1 patient (UPN #638). The SNP was heterozygous in 9 clones with a heterozygous SF3B1 mutation, whereas symmetric LOH of rs77741206 was detected in 9 clones with a wild-type or homozygous SF3B1 mutation (Figure 2B). The clonal diversity generated by these branched architectures was also submitted to clonal selection during myeloid differentiation to the CMP and GMP stages (example in Figure 2C).
Clonal branching through loss of heterozygosity by mitotic recombination. (A) Putative evolutionary tree generated from the classification of all sorted BM CD34+ clones (n = 106; pooling all subpopulations) for UPN #638 accounting for the heterozygosity status at each locus, showing multiple branching with LOH of the CBL and SF3B1 mutation (italicized: heterozygous, bold & underlined: homozygous). (B) Corresponding Sanger sequences at the SF3B1 locus of CD34+ clones from UPN #638 with wild-type, heterozygous, and homozygous SF3B1 according to the TaqMan allelic discrimination assay showing the mutation region (top panel) and the informative SNP rs77741206 located 46 bp in the 3′ intronic region. Sequences from total CD14+ cells of UPN #638 and from the UT7 cell line as control. (C) Putative evolutionary trees of sorted BM CD34+ populations from UPN #507. White: no mutation, yellow: 1 mutation, orange: 2 mutations, red: 3 mutations, purple: 4 mutations. Only mutated genes are indicated in each subclone (italicized: heterozygous, bold and underlined: homozygous).
Clonal branching through loss of heterozygosity by mitotic recombination. (A) Putative evolutionary tree generated from the classification of all sorted BM CD34+ clones (n = 106; pooling all subpopulations) for UPN #638 accounting for the heterozygosity status at each locus, showing multiple branching with LOH of the CBL and SF3B1 mutation (italicized: heterozygous, bold & underlined: homozygous). (B) Corresponding Sanger sequences at the SF3B1 locus of CD34+ clones from UPN #638 with wild-type, heterozygous, and homozygous SF3B1 according to the TaqMan allelic discrimination assay showing the mutation region (top panel) and the informative SNP rs77741206 located 46 bp in the 3′ intronic region. Sequences from total CD14+ cells of UPN #638 and from the UT7 cell line as control. (C) Putative evolutionary trees of sorted BM CD34+ populations from UPN #507. White: no mutation, yellow: 1 mutation, orange: 2 mutations, red: 3 mutations, purple: 4 mutations. Only mutated genes are indicated in each subclone (italicized: heterozygous, bold and underlined: homozygous).
To confirm that the clonal architecture identified by focusing on 18 frequently mutated genes holds true when analyzing the full spectrum of genetic lesions, we performed whole-exome sequencing of peripheral CD14+ cells in a patient with TET2 and U2AF35 mutations. We identified 14 additional gene mutations confirmed by Sanger sequencing, including a noncanonical KRAS mutation (K117R). All mutations were heterozygous except the homozygous TET2 deletion. Again, mutation-specific discrimination analysis of CD34+ colonies after liquid culture showed the linear accumulation of mutations, with only minor subclones possibly resulting from a mitotic recombination affecting the KRAS locus (supplemental Figure 4). Altogether, the dominant clone mostly results from sequential waves of mutation acquisition and expansion, with minor subclones probably generated by homologous recombination at specific sites.
Clonal architecture is dynamic and is selectively affected by therapy
We analyzed the fate of clonal heterogeneity in the face of temporal evolution of CMML, either in untreated patients or in patients with currently available therapies (detailed in supplemental Table 1). In an untreated and clinically stable patient analyzed twice at a 12-month interval, the proportion of clones with 2 mutations increased significantly in HSC/MPP (UPN #507; Figure 3A). A similar evolution was noted, with the same interval in a patient receiving hydroxyurea (UPN #516; Figure 3B). In a patient with stable disease under hypomethylating therapy (UPN #550), we observed the amplification of the more mutated clone, which harbors an ASXL1 mutation (Figure 3C). It is surprising to note that when UPN #506 achieved erythroid improvement with an erythropoiesis-stimulating agent (after natural evolution depicted in Figure 3B), a re-expansion of wild-type hematopoiesis was observed in the CD34+/CD38− compartment, although the selective advantage of the more mutated clone persisted during myeloid differentiation (Figure 3D). In UPN #752 with a somatic mosaicism between KRAS and NRAS mutations, achievement of a transient control of myeloproliferation and circulating blast counts with an investigational mitogen-activated protein kinase kinase inhibitor only resulted in a shift of the equilibrium between the 2 clones, with a slight expansion of the KRAS-mutated clone at the expanse of the NRAS mutation (supplemental Figure 5). Finally, 1 patient (UPN #632) with proliferative CMML achieved partial response with intensive chemotherapy, converted to complete response after hypomethylating therapy, then underwent allogeneic stem cell transplantation and experienced a relapse 6 months later. Sequential analysis of his clonal architecture showed a reduction of the NRAS-mutated clone by chemotherapy and hypomethylating therapy, allowing the expansion of the ancestral TET2/SRSF2-mutated clone. Nonetheless, the more mutated clone persisted and regained dominance at relapse (Figure 3E).
Clonal architecture is dynamic and selectively affected by therapy. Proportion of mutated clones in the indicated peripheral blood (A) or BM (B-E) CD34+ fractions of matched samples from untreated (A) or treated (B-E) patients. Mutated genes are indicated in their inferred order of apparition from top to bottom (see Table 1 for details). (A) UPN #516: TET2 K450×, NRAS G12D. (B,D) UPN #507: TET2 S716×, CBL W408R, SRSF2 P95H. C. UPN #550: TET2 D1242TfsX11, RUNX1 Y376LfsX197, SRSF2 P95H, ASXL1 E635RfsX15.(E) UPN #632: TET2 N1156I; NRAS A59G; SRSF2 P95H. The total number of interrogated clones is indicated on the top of the bars. Missing fractions represent phenotypes underrepresented in posttreatment samples. Note that in UPN #632, showing RAEB-2 rapidly evolving to AML at relapse, the LMPP and GMP fractions were dominant, as previously described.47 12 mo, 12 months untreated evolution; ASCT, Allogeneic Stem Cell Transplantation; ESA, Erythropoiesis-Stimulating Agent; HMA, Hypomethylating Agent; HY, Hydroxyurea; IC, Intensive Chemotherapy; LMPP, Lymphoid-primed multipotent progenitors; Re, AML Relapse.
Clonal architecture is dynamic and selectively affected by therapy. Proportion of mutated clones in the indicated peripheral blood (A) or BM (B-E) CD34+ fractions of matched samples from untreated (A) or treated (B-E) patients. Mutated genes are indicated in their inferred order of apparition from top to bottom (see Table 1 for details). (A) UPN #516: TET2 K450×, NRAS G12D. (B,D) UPN #507: TET2 S716×, CBL W408R, SRSF2 P95H. C. UPN #550: TET2 D1242TfsX11, RUNX1 Y376LfsX197, SRSF2 P95H, ASXL1 E635RfsX15.(E) UPN #632: TET2 N1156I; NRAS A59G; SRSF2 P95H. The total number of interrogated clones is indicated on the top of the bars. Missing fractions represent phenotypes underrepresented in posttreatment samples. Note that in UPN #632, showing RAEB-2 rapidly evolving to AML at relapse, the LMPP and GMP fractions were dominant, as previously described.47 12 mo, 12 months untreated evolution; ASCT, Allogeneic Stem Cell Transplantation; ESA, Erythropoiesis-Stimulating Agent; HMA, Hypomethylating Agent; HY, Hydroxyurea; IC, Intensive Chemotherapy; LMPP, Lymphoid-primed multipotent progenitors; Re, AML Relapse.
GMP are quantitatively and qualitatively normal in CMML
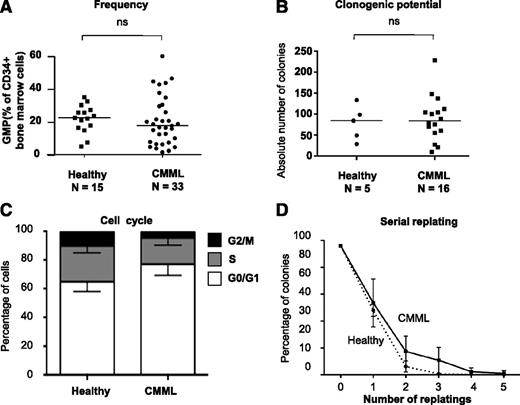
To understand the relationship between the clonal architecture and the phenotype of CMML, we sought to analyze the cellular mechanisms driving the emergence from the CD34+ compartment of the granulomonocytic expansion of CMML. Because clonal dominance, notably regarding secondary mutations, is achieved at the GMP stage (Figure 1E), we quantitatively and qualitatively analyzed this compartment. The differentiation potential of GMP was assessed with the gold-standard 14-day methylcellulose assay, in the presence of FBS and a restricted panel of cytokines (SCF, IL-3, G-CSF, and EPO) compared with previous experiments (Figure 1-3). This panel allowed comparison of the granulomonocytic and potential for erythroid differentiation of all CD34+ fractions, and avoided the use of GM-CSF whose activity is heterogeneous in CMML.24-26 The potential for self-renewal was assessed by serial replating in similar conditions without EPO to focus on granulomonocytic progenitors. By flow cytometry, the percentage of GMP among BM CD34+ cells was not significantly different in 33 patients with CMML (with a mutation spectrum representative of the entire population, not shown) and 15 age-matched healthy participants (Figure 4A). The ability to form granulomonocytic colonies in methylcellulose was similar in patients and healthy control participants (Figure 4B). Cell cycle analysis did not identify any significant change in patient cells (Figure 4C), and serial replating did not demonstrate a strong increase in their self-renewal capability (Figure 4D). Overall, no systematic quantitative or qualitative alteration of GMP was identified in CMML, suggesting that the granulomonocytic expansion characteristic of this disease may have appeared at earlier stages of myeloid differentiation.
GMP are quantitatively and qualitatively normal in CMML. (A) Proportion of GMP (CD34+/CD38+/CD45RA+/CD123+) by flow cytometry among total BM CD34+ cells from CMML or aged-matched healthy control participants. Bar: median. (B) Total number of colonies (all granulomonocytic) per 1000 GMP grown in triplicate in methylcellulose for 14 days with 30% FBS and cytokines (SCF, IL-3, G-CSF, and EPO) from 5 control participants and 16 patients with CMML. (C) Fractions of GMP cells from fresh BM samples in G0/G1 (white), S (gray), and G2/M (black) phase after 1-hour incubation in Hoescht 33342 in the presence of 10% FBS and cytokines; mean and SD from independent samples. (D) Serial replating in methylcellulose of individual clones from sorted GMP of patients with CMML (solid) or control participants (dashed). Results: percentage of colonies relative to the initial number of clones seeded. Mean and SD from 3 independent samples for each group (*P < .05, Mann-Whitney U test); ns, nonsignificant.
GMP are quantitatively and qualitatively normal in CMML. (A) Proportion of GMP (CD34+/CD38+/CD45RA+/CD123+) by flow cytometry among total BM CD34+ cells from CMML or aged-matched healthy control participants. Bar: median. (B) Total number of colonies (all granulomonocytic) per 1000 GMP grown in triplicate in methylcellulose for 14 days with 30% FBS and cytokines (SCF, IL-3, G-CSF, and EPO) from 5 control participants and 16 patients with CMML. (C) Fractions of GMP cells from fresh BM samples in G0/G1 (white), S (gray), and G2/M (black) phase after 1-hour incubation in Hoescht 33342 in the presence of 10% FBS and cytokines; mean and SD from independent samples. (D) Serial replating in methylcellulose of individual clones from sorted GMP of patients with CMML (solid) or control participants (dashed). Results: percentage of colonies relative to the initial number of clones seeded. Mean and SD from 3 independent samples for each group (*P < .05, Mann-Whitney U test); ns, nonsignificant.
CMP and MPP undergo premature granulomonocytic differentiation in CMML
Contrasting with the normal GMP fraction, the percentages of CMP and MPP were higher in patients with CMML than in healthy control participants (Figure 5A). When cultured in methylcellulose in similar conditions as GMP, both CMP and, to a lesser extent, MPP demonstrated an increased ability to form granulomonocytic colonies at the expense of erythroid colonies (Figure 5B). Similar results were found when CMP were cultured as single cells in a liquid medium for 12 days in the presence of a larger panel of cytokines (SCF, FLT3-L, TPO, IL-3, IL-6, G-CSF, GM-CSF, and EPO), with a strong increase in the percentage of granulomonocytes (CD14+ and/or CD15+ and/or CD24+) at the expense of erythroid (GPA+) clones (supplemental Figure 6B), despite unchanged cloning efficacy (supplemental Figure 6A). Analysis of mutations in granulomonocytic and erythroid colonies obtained by culturing CMP from 3 patients showed a similar repartition of the mutated alleles in the 2 types of colonies, demonstrating that the majority of erythroid colonies belong to the most mutated subclone (not shown). Short-term (3-day) culture of CMP in liquid medium followed by immunophenotypic analyses showed their increased ability to mature into GMP at the expense of MEP (Figure 5C). The functional relevance of CD34+ cell immunophenotyping after this 3-day culture was validated by resorting GMP, MEP, and CMP at day 3 and assessing their clonogenic potential (supplemental Figure 6C). Gene expression analysis revealed an increased expression of PU.1 with nonsignificant changes in the level of GATA1, CEBPA, and CEBPB in CMP sorted from CMML compared with those from healthy control participants (Figure 5D; supplemental Figure 6D). Finally, the proportion of granulomonocytic colonies differentiating from CMP at the expense of erythroid colonies correlated inversely with patient hemoglobin level (Figure 5E). Altogether, these results suggest that immature pluripotent progenitors (CMP and, to a lesser extent, MPP) are skewed toward granulomonocytic differentiation in CMML.
CMP and MPP undergo premature granulomonocytic differentiation in CMML. (A) Proportion of MPP (CD34+/CD38−/CD45RA−/CD90−, top panel) and CMP (bottom panel, CD34+/CD38+/CD45RA−/CD123+) by flow cytometry among total BM CD34+ cells from patients with CMML (n = 33) or aged-matched healthy control participants (n = 15). Bar: median; Mann-Whitney U test. (B) Proportion of mixed (CFU-M, white), granulomonocytic (CFU-G/M: total of CFU-G, CFU-M, and CFU-GM; black) and erythroid colonies (BFU-E, gray) colonies from 250 MPP (top) or CMP (bottom) grown in triplicate in methylcellulose for 14 days with 30% FBS and cytokines (SCF, IL-3, G-CSF, and EPO); χ2 test for mean proportions from CMML (CMP: n = 18; MPP: n = 9) and control (CMP: n = 5; MPP: n = 3) samples. (C) 5 × 104 CMP from healthy (n = 4) or CMML (n = 8) samples were cultivated in bulk for 3 days in the presence of 10% FBS and cytokines (SCF, FLT3-L, TPO, IL-3, IL-6, G-CSF, GM-CSF, and EPO) and then were analyzed by flow cytometry for CD34, CD38, CD45RA, and CD123 expression. Representation of the ratio of events with a GMP (CD34+/CD38+/CD45RA+/CD123+) phenotype to cells with an MEP CD34+/CD38+/CD45RA−/CD123−) phenotype; bar: median. (D) Gene expression levels (relative to HPRT expression) of PU.1 (top panel) and GATA1 (bottom) in sorted CMP fractions: mean and SEM from 4 control and 5 CMML samples each analyzed in duplicate. Unpaired t tests. *P < .05; **P < .01; ***P < .001. (E) Correlation between the percentage of granulomonocytic colonies from CMP of 16 CMML samples and the corresponding patient’s hemoglobin level (g/dL). Spearman test, slope from linear regression.
CMP and MPP undergo premature granulomonocytic differentiation in CMML. (A) Proportion of MPP (CD34+/CD38−/CD45RA−/CD90−, top panel) and CMP (bottom panel, CD34+/CD38+/CD45RA−/CD123+) by flow cytometry among total BM CD34+ cells from patients with CMML (n = 33) or aged-matched healthy control participants (n = 15). Bar: median; Mann-Whitney U test. (B) Proportion of mixed (CFU-M, white), granulomonocytic (CFU-G/M: total of CFU-G, CFU-M, and CFU-GM; black) and erythroid colonies (BFU-E, gray) colonies from 250 MPP (top) or CMP (bottom) grown in triplicate in methylcellulose for 14 days with 30% FBS and cytokines (SCF, IL-3, G-CSF, and EPO); χ2 test for mean proportions from CMML (CMP: n = 18; MPP: n = 9) and control (CMP: n = 5; MPP: n = 3) samples. (C) 5 × 104 CMP from healthy (n = 4) or CMML (n = 8) samples were cultivated in bulk for 3 days in the presence of 10% FBS and cytokines (SCF, FLT3-L, TPO, IL-3, IL-6, G-CSF, GM-CSF, and EPO) and then were analyzed by flow cytometry for CD34, CD38, CD45RA, and CD123 expression. Representation of the ratio of events with a GMP (CD34+/CD38+/CD45RA+/CD123+) phenotype to cells with an MEP CD34+/CD38+/CD45RA−/CD123−) phenotype; bar: median. (D) Gene expression levels (relative to HPRT expression) of PU.1 (top panel) and GATA1 (bottom) in sorted CMP fractions: mean and SEM from 4 control and 5 CMML samples each analyzed in duplicate. Unpaired t tests. *P < .05; **P < .01; ***P < .001. (E) Correlation between the percentage of granulomonocytic colonies from CMP of 16 CMML samples and the corresponding patient’s hemoglobin level (g/dL). Spearman test, slope from linear regression.
Early clonal dominance of TET2 mutations leads to granulomonocytic hyperplasia
To unravel a link between early clonal dominance and myeloid differentiation skewing in CMML, we focused on TET2, which is the most frequently6,7,27 and early (Table 1) mutated gene in CMML known to date. TET2 is also mutated in 15% to 25% of cases of MDS and MPN,5,27,28 where granulomonocytic hyperplasia is absent (MDS) or variable (MPN). We interrogated the stage of expansion of the TET2-mutated clone in colonies generated from CD34+/CD38− and CD34+/CD38+ cells collected from TET2-mutated MPN (n = 8) or MDS (n = 5) (supplemental Table 2). We observed a significant correlation between the proportion of cells with mutated TET2 at the CD34+/CD38− level and peripheral monocyte count (Figure 6A, left panel). Such a correlation was not found when the more mature CD34+/CD38+ compartment was considered (Figure 6A, right panel). In 5 patients with CMML, the ability of CMP to form granulomonocytic colonies reflected the size of the TET2-mutated clone in this compartment (Figure 6B). Finally, we recapitulated TET2 invalidation by lentiviral transduction of a previously reported shRNA directed against TET223 in sorted CD34+/CD38− and CD34+/CD38+ normal progenitors, followed by culture in the presence of erythroid and granulomonocytic cytokines. Functional knockdown of TET2 in CD34+/CD38− caused a granulomonocytic expansion that was not observed in CD34+/CD38+ cells (Figure 6C). Altogether, early dominance of the TET2-mutated clone in the immature CD34+/CD38− compartment may participate in the granulomonocytic skewing that defines CMML.
Early dominance of TET2 mutations contributes to granulomonocytic skewing in CMML. (A) Correlation between the proportion of TET2-mutated clones of CD34+/CD38− cells grown in liquid culture with cytokines with (n = 11) or without (n = 2) a stromal layer (top panel), or corresponding CD34+/CD38+ cells grown in methylcellulose (n = 11) or in liquid culture (n = 2; bottom panel) from 13 TET2-mutated samples (8 MPN, 5 MDS). Spearman test and slope from linear regression. (B) Percentage of CMP clones with mutated TET2 assessed in liquid culture (gray bars) compared with the percentage of granulomonocytic colonies after methylcellulose culture of CMP in the presence of SCF, IL-3, G-CSF, and EPO (black bars). Results are from 5 samples. Numbers on top of the gray bars indicate the number of CMP clones assessed. UPN: unique patient number. (C) Sorted CD34+/CD38− and CD34+/CD38+ cord blood cells were transduced with an shRNA directed against TET2 or a scrambled shRNA (as previously described23 ). After infection, GFP+ cells were cultured as in (B). At day 14, cells were washed and stained with PE-anti-GPA and APC-anti-CD33. Ratios of GFP+ granulomonocytic (CD33+/GPA−) to erythroid (CD33±/GPA+) cells from 4 independent experiments are represented (mean ± SEM, Mann-Whitney U test, *P < .05).
Early dominance of TET2 mutations contributes to granulomonocytic skewing in CMML. (A) Correlation between the proportion of TET2-mutated clones of CD34+/CD38− cells grown in liquid culture with cytokines with (n = 11) or without (n = 2) a stromal layer (top panel), or corresponding CD34+/CD38+ cells grown in methylcellulose (n = 11) or in liquid culture (n = 2; bottom panel) from 13 TET2-mutated samples (8 MPN, 5 MDS). Spearman test and slope from linear regression. (B) Percentage of CMP clones with mutated TET2 assessed in liquid culture (gray bars) compared with the percentage of granulomonocytic colonies after methylcellulose culture of CMP in the presence of SCF, IL-3, G-CSF, and EPO (black bars). Results are from 5 samples. Numbers on top of the gray bars indicate the number of CMP clones assessed. UPN: unique patient number. (C) Sorted CD34+/CD38− and CD34+/CD38+ cord blood cells were transduced with an shRNA directed against TET2 or a scrambled shRNA (as previously described23 ). After infection, GFP+ cells were cultured as in (B). At day 14, cells were washed and stained with PE-anti-GPA and APC-anti-CD33. Ratios of GFP+ granulomonocytic (CD33+/GPA−) to erythroid (CD33±/GPA+) cells from 4 independent experiments are represented (mean ± SEM, Mann-Whitney U test, *P < .05).
Discussion
CMML shares with MPN, MDS, and other MDS/MPN several gene mutations. Whereas a driver oncogenic mutation leading to the constitutive activation of intracellular signaling pathways has been identified in most MPN16 and in juvenile myelomonocytic leukemia,29 the driver oncogenic events involved in CMML are less clearly identified. TET2, SRSF2 (which is often associated to TET2),6,13 and ASXL1 are the most frequent genetic events in CMML.6,7,14,15 The number of mutated genes is a strong prognostic factor in CMML.7 This prompted the investigation of the clonal architecture of genetic lesions in 28 cases of CMML, showing that gene mutations are sequentially acquired, leading to a mostly linear clonal architecture, with frequent branching through LOH resulting from mitotic recombinations. Early clonal dominance at the CD34+/CD38− stage of hematopoiesis, especially dominance of TET2-mutated cells, is a key feature of the disease that is accompanied by myeloid differentiation skewing toward the granulomonocytic lineages, whereas additional genetic events rapidly confer a selective advantage to the clone during myeloid differentiation.
Tumor genetic heterogeneity was initially thought to evolve through sequential, “linear” acquisition of collaborating mutations, followed by waves of clonal expansion.30 Recent analyses in acute leukemias revealed more complex, oligoclonal models.2,4,31 Here, we show that the clonal architecture of CMML may reconcile both models (ie, a mostly linear acquisition of the mutations), with a few ramifications likely the result of homologous recombinations.9 LOH occurs at loci-encoding genes from all classes (epigenetic, splicing, and signaling), though homozygous clones achieving clonal dominance are mostly restricted to the TET2 and CBL genes.5,9 Next-generation sequencing identifies an average of 10 somatic exonic mutations in MDS, all of which arise in the same CD34+ cell.4,32 In all but one of the 28 studied cases of CMML, all mutations were present in at least one CD34+/CD38−(/CD90+). Exome sequencing in CD34+ leukemic clones from a representative patient with CMML followed by clonal architecture analysis identified sequential waves of acquisition of mutations, each wave including 1 presumed driver oncogenic lesion, such as TET2, U2AF35, or KRAS mutations. Sequential analyses demonstrated that, aside from allogeneic stem cell transplantation, all current therapeutic strategies modulate, rather than eradicate, the leukemic clone.
The reconstructed phylogenetic trees also allowed ordering of the sequence of acquisition of the studied mutations, except in 4 cases, raising the possibility that 2 mutations were concomitantly gained in those cases. Why certain mutations, such as those in TET2 and SRSF2, are often associated remains uncertain, but the finding that their order of acquisition is biased but not stereotyped suggests that their association arises from functional cooperation.33
Clonal dominance is a heterogeneous feature in myeloid malignancies. For example, clonal expansion of JAK2V617F mutations occurs in earlier progenitors in myelofibrosis34 than in polycythemia vera,22 a difference attributed to the role of additional mutations in TET25 or ASXL1.19 Here, we show that early clonal dominance of initial mutations in CD34+/CD38− immature progenitors is a key feature of CMML. Clones harboring only part of the oncogenic lesions persist in this compartment, whereas the fully mutated leukemic clone harboring secondary lesions is further selected during myeloid differentiation to the GMP stage. Mutations affecting cell signaling such as JAK2, NRAS, or KRAS mutations, or LOH of a heterozygous CBL mutation, may favor this differentiation-associated selection, as JAK2V617F does in MPN.22
Our strategy to establish clonal architecture, based on the tracking of recurrent mutations in single-cell–derived colonies after in vitro culture, had 2 limitations. First, it did not address the full spectrum of genetic aberrations present in the tumor. Founder mutations could have preceded what we detect as “primary” events. If this is the case, early clonal dominance would be of even greater magnitude than reported. In the unique patient studied by whole exome, however, the TET2 “primary” event was present in the founder clone, in accordance with the finding of TET2 mutations in preleukemic HSC in AML35 and in HSC of older patients with clonal, but nonmalignant, hematopoiesis.36 Second, in vitro culture may introduce biases in clonal representation. However, validation of single-cell–derived colonies genotyping has been reported in AML35 and was confirmed in our present study. Next-generation sequencing represents the major alternative strategy to assess clonal architecture, but it is either applied to bulk samples4 with the risk of missing mosaic subclones, or to amplified DNA from single cells, thus introducing other biases and limiting resolution with current techniques.37,38
Because genes mutated in CMML are also found in other myeloid malignancies, we next sought to understand the relationship between the clonal architecture of CMML and the emergence of its distinctive phenotypic trait, namely granulomonocytic hyperplasia. Whereas analysis of sorted progenitors did not identify alterations of the GMP compartment, the CMML BM CD34+ compartment was enriched in CMP and MPP with a skewed myeloid differentiation (ie, an enhanced capability to form granulomonocytic colonies at the expense of erythroid colonies). This skewed myeloid differentiation could result from biased lineage decision and/or differential amplification of granulomonocytic and erythroid progenitors after lineage commitment, a question recently addressed in normal hematopoietic differentiation by single-cell–tracking studies.39,40
Inhibition of TET2 expression in CD34+ cells has been shown to promote granulomonocytic differentiation in vitro and in vivo,23,41,42 yet TET2 mutation allele burden in total BM–nucleated cells (representing differentiated cells) seems comparable in MDS, which lacks monocytosis, and CMML.27 To elucidate this paradox, we analyzed the amplification of TET2 mutations in CD34+ progenitor cells from patients with either MDS, or those with JAK2V617F MPN, where monocyte count is variable.43 In these diseases, we uncovered a specific correlation between amplification of TET2-mutated cells in the immature CD34+/CD38− compartment and higher monocyte counts. This finding could be explained by a role of TET2 in restricting a default granulomonocytic program in immature multipotent progenitors. Such a hypothesis is reinforced by the observation that functional invalidation of TET2 in healthy CD34+/CD38−, but not in CD34+/CD38+, cells, leads to granulomonocytic amplification.
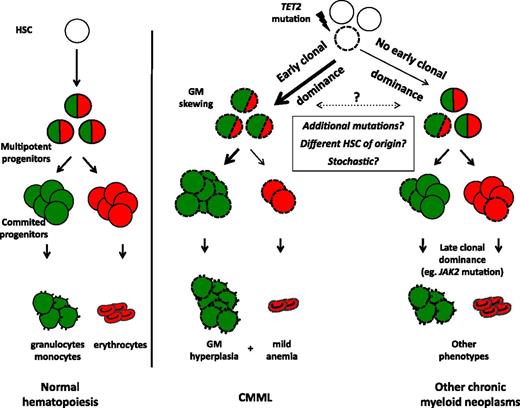
We propose a model linking early clonal dominance with granulomonocytic skewing in CMML (Figure 7). According to this model, early dominance of the mutated clone, in particular, in the presence of a TET2 mutation, accounts for the accumulation of monocytes in CMML. In other chronic myeloid malignancies such as MDS or MPN, TET2 mutations may reach clonal dominance at later stages of myeloid differentiation (eg, because of additional mutations such as JAK2V617F). Differences in the dynamics of clonal amplification of TET2 mutations could be explained by either specific combinations of mutations (eg, TET2/SRSF2 in CMML),6,13 their occurrence in distinct subsets of HSC,44 or a stochastic effect of TET2 in the initially mutated cell (Figure 7). These models could explain why monocytosis can develop in the course of MDS45 or MPN46 without acquisition of novel mutations. In addition to providing hypotheses to explain the disease phenotype, analyses of clonal architecture may allow defining therapeutic strategies that improve disease control and possibly achieve clonal eradication by targeting the first genetic aberrations that accumulate in immature progenitors.
Proposed model linking early clonal dominance with granulomonocytic skewing of immature progenitors in CMML. According to their stage of expansion (early in CMML, later in other chronic myeloid neoplasms), TET2 mutations will or will not give rise to monocytosis. TET2-mutated cells are represented with dashed lines. Granulomonocytic differentiation potential is depicted in green, erythroid potential in red.
Proposed model linking early clonal dominance with granulomonocytic skewing of immature progenitors in CMML. According to their stage of expansion (early in CMML, later in other chronic myeloid neoplasms), TET2 mutations will or will not give rise to monocytosis. TET2-mutated cells are represented with dashed lines. Granulomonocytic differentiation potential is depicted in green, erythroid potential in red.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Fatiha Chermat (GFM administrative officer) and Vladimir Lazar (genomic platform at Institut Gustave Roussy) for their assistance.
This work was supported by grants from the Ligue Nationale Contre le Cancer (Label, E.S.), Agence Nationale de la Recherche (E.S.), Institut National du Cancer (PHRC 2011 to E.S. and support to R.I.), Association Laurette Fugain (N.D.), and Fondation de France (N.D.). The UMR1009, IFR54, and GRC Saint-Antoine equipment was supported by the Association pour la Recherche sur le Cancer and Région Ile-de-France.
Authorship
Contributions: R.I. performed experiments and statistical analyses, analyzed data, and drafted the manuscript. O.K. and A.R. performed genotyping and analyzed data. M.M. performed sample collection, qualification, and nucleic acid extractions. P.R. performed cell sorting. C.O. and G.M. performed and analyzed microarray data. F.D. provided samples and lentiviral vectors. C.B., L.A., P.F., U.P., and O.G. provided samples. C.P., O.A.B., M.F., W.V., and N.D. supervised genotyping and analyzed data. E.S. provided samples, analyzed data, supervised the work, and revised the manuscript. All authors approved the final manuscript.
Conflict-of-Interest disclosure: The authors declare no competing financial interests.
Correspondence: Eric Solary, Inserm UMR 1009, Institut Gustave Roussy, 114, Rue Edouard Vaillant, 94805 Villejuif, France; e-mail: eric.solary@igr.fr.