Abstract
Cytokine-induced expansion of hematopoietic stem and progenitor cells (HSPCs) is not fully understood. In the present study, we show that whereas steady-state hematopoiesis is normal in basic fibroblast growth factor (FGF-2)–knockout mice, parathyroid hormone stimulation and myeloablative treatments failed to induce normal HSPC proliferation and recovery. In vivo FGF-2 treatment expanded stromal cells, including perivascular Nestin+ supportive stromal cells, which may facilitate HSPC expansion by increasing SCF and reducing CXCL12 via mir-31 up-regulation. FGF-2 predominantly expanded a heterogeneous population of undifferentiated HSPCs, preserving and increasing durable short- and long-term repopulation potential. Mechanistically, these effects were mediated by c-Kit receptor activation, STAT5 phosphorylation, and reduction of reactive oxygen species levels. Mice harboring defective c-Kit signaling exhibited abrogated HSPC expansion in response to FGF-2 treatment, which was accompanied by elevated reactive oxygen species levels. The results of the present study reveal a novel mechanism underlying FGF-2–mediated in vivo expansion of both HSPCs and their supportive stromal cells, which may be used to improve stem cell engraftment after clinical transplantation.
Introduction
Hematopoietic stem cells (HSCs) are a rare population of primordial cells responsible for the continuous replenishment of mature hematopoietic cells of the different blood and immune lineages. External cues presented by the surrounding microenvironment direct HSC fate between quiescence, proliferation, self-renewal, and differentiation.1 Hematopoietic stem and progenitor cell (HSPC) transplantation is used clinically to treat a wide variety of diseases and HSPC expansion may improve transplantation protocols.2
Basic fibroblast growth factor (FGF-2) belongs to a family of 22 members that bind to 4 different FGF receptors (FGFRs).3 Although displaying a mild defective bone phenotype,4 FGF-2–knockout (KO) mice do not possess any observed deficits in steady-state hematopoiesis.5 However, several lines of evidence suggest that FGF-2 may have an active role in hematopoiesis. First, BM stromal cells from FGF-2 KO mice showed attenuated in vitro support of HSC maintenance.6 Second, FGFRs are present on the surface of long-term repopulating (LTR) murine CD34− HSCs.7 Third, a chromosomal translocation mutation resulting in a constitutively active FGFR1 isoform was identified in some human myeloproliferative disorders.8 Last, an in vitro study showed that the addition of FGF-2 to prolonged BM cultures expands murine LTR-HSCs. This study also revealed that FGF signaling sustained and expanded HSCs only in unfractionated BM cultures and not in sorted HSPC cultures, suggesting an indirect effect mediated by the microenvironment.9
Parathyroid hormone (PTH) treatment stimulates murine HSC expansion coupled with osteoblast expansion,10 suggesting a rapport between these 2 processes. Other studies with PTH indicate that osteoblast proliferation11 and activation12 are impaired in FGF-2 KO mice and that PTH expands directly Nestin+ mesenchymal stem cells (MSCs), which form a niche for HSCs in the murine BM.13
Overall, the published data suggest that FGF-2 plays a role in active hematopoiesis in vitro and in malignant conditions, but no study has yet defined the in vivo role of FGF-2–induced signaling and its impact on HSC fate. Furthermore, there are no published data on the mechanism underlying FGF-2–mediated HSC expansion and how an FGF-2–modified microenvironment supports HSC expansion.
The results of the present study show that FGF-2 mediates in vivo HSPC expansion under stress-induced conditions, but not during steady-state homeostasis. Moreover, our study presents the downstream mechanisms to FGF-2–induced HSPC expansion and the involvement of immature stromal cells in this process.
Methods
Mice and treatment protocols
Six- to 8-week-old mice from the following strains were used: C57BL/6 (Harlan Laboratories); B6.SJL, FVB/N wild-type (WT), or FGF-2 KO4 ; c-Kit mutated on a C57BL/6 background14 (Wv/Wv mice; The Jackson Laboratory); and Nestin-green fluorescent protein (Nestin-GFP) on a C57BL/6 background.13,15 Experiments were approved by the Weizmann Institutional Animal Care and Use Committee. All mice were bred and maintained under defined flora conditions at the Weizmann Institute of Science. Mice were treated with FGF-2 (the treatment protocol was adopted from previously described studies16 ), PTH, SCF, or 5-fluorouracil (5-FU) or were irradiated to study HSPC expansion (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Some mice were also treated with the antioxidants N-acetyl-cysteine and rapamycin (see supplemental Methods). Mice were killed by CO2 inhalation.
Survival assay
FVB/N WT or FGF-2 KO mice were lethally irradiated (1100 cGy, cesium source) and transplanted with a minimal dose of 5 × 104 BM cells from donors. Results were plotted on a Kaplan-Meier survival curve.
Transplantation assays
For competitive LTR, B6.SJL (CD45.1) recipient mice were lethally irradiated and injected 5 hours later with 2 × 105 donor-derived BM cells together with 4 × 105 recipient-derived BM cells. Recipient mice were killed 4, 16, and 24 weeks after transplantation to determine chimerism levels. For calculation of competitive repopulating units (CRUs), recipient mice were transplanted with limiting dilutions of donor-derived cells (2.5 × 104 to 2 × 105). Mice were killed after 4 months and myelolymphoid donor-derived contribution in the peripheral blood was assessed. Myelolymphoid chimerism > 1% was scored as LTR. CRU frequency was determined using L-Calc Version 1.1 software (StemCell Technologies).
In vitro cultures with primary Lin− cells
Lineage-negative (Lin−) cells were enriched from BM cells of C57BL/6 mice using a mouse lineage-depletion kit (BD Biosciences) according to the manufacturer's instructions. A total of 3 × 105 Lin− cells were seeded in a 24-well plate in RPMI 1640 medium (Gibco), supplemented with 10% FCS (Biologic Industries), l-glutamine (Biologic Industries), penicillin, and streptomycin (Invitrogen), together with a cytokine mixture used in our CFU-C assay. Some wells were supplemented with FGFR inhibitor SU5402 (5mM; Calbiochem) dissolved in DMSO (Sigma-Aldrich) or with FGF-2 (50ng/mL; PeproTech). Lin− cells (3 × 104) were transplanted into sublethally irradiated recipient B6.SJL mice and examined 16 weeks later to determine chimerism levels.
CFU-F assay
For details on the colony forming units–fibroblasts (CFU-F) assay, please see supplemental Methods.
Immunofluorescent staining
For details on immunofluorescent staining, please see supplemental Methods.
miRNA in situ hybridization
In situ hybridization to detect miRNA expression in BM paraformaldehyde- and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide–fixated tissue samples was performed as described previously17 with some modifications (see supplemental Methods).
MS-5 treatment protocols
Murine BM stromal cell line MS-5 cells were grown in RPMI 1640 medium (Gibco) supplemented with 10% FCS (Biologic Industries), l-glutamine (Biologic Industries), 50nM β-mercaptoethanol, penicillin, and streptomycin (Invitrogen). All experiments using MS-5 cells were performed when cells reached 50%-60% confluence. MS-5 cells were treated with FGF-2 (50 ng/mL) for 48 hours. All in vitro experiments were repeated at least 3 times with n > 3 per test.
MS-5 transfection protocol
Cells were transfected with miRIDIAN Mimic mmu-mir-31 (40μM; Thermo Scientific), miRIDIAN Mimic nontargeting control (40μM; Thermo Scientific), miRIDIAN hairpin inhibitor for mmu-mir-31 (400μM; Thermo Scientific), or with miRIDIAN hairpin inhibitor nontargeting control (400μM; Thermo Scientific). Cell transfection was according to the manufacturer's instructions using transfection reagent 3 (Thermo Scientific).
CFU-C assay
For details on the colony-forming units–cells (CFU-C) assay, please see supplemental Methods.
HSPC ex vivo expansion protocol and treatments
Ex vivo expansion was performed as described previously9 (see supplemental Methods).
Flow cytometric analysis
Cell phenotype was determined by immunostaining, followed by flow cytometric analysis on a FACSCalibur or FACS LSRII flow cytometer (BD Biosciences; see supplemental Methods).
qRT-PCR
Total RNA was isolated using TRIzol reagent (Sigma-Aldrich) according to the manufacturer's protocol. An aliquot of 2 μg of total RNA was reverse transcribed using MMLV-RT (Promega) and oligo-dT primers (Promega). Quantitative RT-PCR (qRT-PCR) was performed using the ABI 7000 (Applied Biosystems) with SYBR Green PCR Master Mix (Applied Biosystems). Comparative quantification of transcripts was assessed relative to hypoxanthine phosphoribosyl transferase (HPRT) and amplified with appropriate primers (see supplemental Methods). miRNA qRT-PCR was performed with mirScript SYBR Green PCR kit (QIAGEN) using the Light Cycler 480 (Roche). Comparative quantification of transcripts was assessed relative to the levels of U6 small nuclear RNA and amplified with appropriate primers (see supplemental Methods).
Detection of luciferase activity
For details on the detection of luciferase activity, please see supplemental Methods.
ELISA
Detection of CXCL12 and SCF levels was as described previously.18 For details on FGF-2 detection, please see supplemental Methods.
Statistical analysis
Data were analyzed statistically by single-factor ANOVA or the P value was calculated using a 2-tailed Student t test assuming unequal variances by Excel Version 2007 software.
Results
Expansion of HSPCs after PTH treatment or myeloablation is FGF-2 dependent
Previous studies have shown that FGF-2 is essential for PTH-induced bone remodeling.11,12 In the present study, we found that PTH induced an increase in the frequency and number of the Sca-1+/c-Kit+/Lin− (SKL) HSPCs in WT mice (Figure 1A and supplemental Figure 1A), confirming previously described observations.10 In contrast, FGF-2 KO mice did not respond to PTH treatment by increasing the proportion of SKL cells in the BM (Figure 1A and supplemental Figure 1A). Interestingly, SKL examination in the spleen revealed that PTH treatment resulted in increased frequency and numbers of this population in WT mice, but not in FGF-2 KO mice (Figure 1B and supplemental Figure 1B). As expected, PTH stimulation increased FGF-2 levels in BM supernatants of WT mice (Figure 1C). We also examined BM FGF-2 levels after in vivo 5-FU or total body irradiation (TBI) treatments that stimulate HSPC proliferation. Both 5-FU and TBI increased BM FGF-2 levels significantly (Figure 1D). To assess the significance of increased FGF-2 levels after myeloablative treatment, we compared the recovery kinetics of WT versus FGF-2 KO mice after sublethal TBI. Although FGF-2 KO mice exhibited delayed recovery of BM SKL cells and reduced recovery of BM CD34− SKL cells (enriched with LTR-HSCs19 ) compared with WT mice (Figure 1E-F), the peripheral blood counts of WBCs and RBCs were higher in irradiated FGF-2 KO mice (Figure 1G-H). In addition, we examined whether TBI-induced apoptosis is altered in the absence of FGF-2 and found no change in the percentage of apoptotic BM immature SKL cells and mature Lin+ cells collected from WT or FGF-2 KO mice 1 or 4 days after TBI (supplemental Figure 1C-D). These results imply that, in the absence of FGF-2, the recovery and production of mature blood cells is increased at the expense of recovering immature HSPCs.
FGF-2 is required for HSPC expansion under stress-induced conditions, but not during steady-state conditions. FVB/N WT or FGF-2 KO mice were treated with either vehicle or PTH (n > 8). (A-B) Percentage of BM or spleen SKL cells as determined by flow cytometry. (C) FGF-2 protein levels as determined by ELISA in the BM supernatants of WT mice after PTH treatment. (D) FGF-2 protein levels as determined by ELISA in the BM supernatants of WT mice after irradiation or 5-FU treatments (n > 6). FVB/N WT or FGF-2 KO mice were treated with single dose of sublethal irradiation and killed at the indicated time points (n > 4). (E-F) Numbers of BM SKL and BM CD34− SKL per femur of FVB/N WT or FGF-2 KO mice as determined by flow cytometry. (G-H) Numbers of peripheral WBC counts and peripheral RBC counts of FVB/N WT or FGF-2 KO mice. (I) Survival curve for FVB/N WT or FGF-2 KO mice treated once with a lethal dose of TBI followed by transplantation with minimal dose of 5 × 104 BM cells from either WT or FGF-2 KO mice (n > 12). *P < .05; **P < .01. Data shown are means ± SEM.
FGF-2 is required for HSPC expansion under stress-induced conditions, but not during steady-state conditions. FVB/N WT or FGF-2 KO mice were treated with either vehicle or PTH (n > 8). (A-B) Percentage of BM or spleen SKL cells as determined by flow cytometry. (C) FGF-2 protein levels as determined by ELISA in the BM supernatants of WT mice after PTH treatment. (D) FGF-2 protein levels as determined by ELISA in the BM supernatants of WT mice after irradiation or 5-FU treatments (n > 6). FVB/N WT or FGF-2 KO mice were treated with single dose of sublethal irradiation and killed at the indicated time points (n > 4). (E-F) Numbers of BM SKL and BM CD34− SKL per femur of FVB/N WT or FGF-2 KO mice as determined by flow cytometry. (G-H) Numbers of peripheral WBC counts and peripheral RBC counts of FVB/N WT or FGF-2 KO mice. (I) Survival curve for FVB/N WT or FGF-2 KO mice treated once with a lethal dose of TBI followed by transplantation with minimal dose of 5 × 104 BM cells from either WT or FGF-2 KO mice (n > 12). *P < .05; **P < .01. Data shown are means ± SEM.
We also examined the survival of WT versus FGF-2 KO mice after lethal TBI followed by transplantation of a minimal dose of donor cells. Under these conditions, BM recovery and regeneration are dependent on the ability of HSPCs to restore hematopoiesis. FGF-2 KO mice transplanted with FGF-2 KO BM cells had reduced survival compared with WT mice transplanted with WT BM cells (Figure 1I). Interestingly, FGF-2 provided by WT cells of either recipient WT BM microenvironment or by transplanted donor WT BM cells enhanced survival frequency (Figure 1I).
Our results indicate that stress-induced HSPC recovery is dependent on FGF-2.
In vivo FGF-2 treatment expands heterogeneous HSPC subpopulations
Because the effects of FGF-2 on HSPCs were investigated previously only in vitro, we decided to search for a potential in vivo role for FGF-2. We administered FGF-2 to mice for 7 consecutive days and observed a slight decrease in BM cellularity (Figure 2A). In contrast to mature cells, the frequency of committed progenitors in the BM of FGF-2–treated mice was increased by approximately 1.7-fold compared with control mice, as defined by CFU-C (Figure 2B). The effect on the frequency of SKL HSPCs was even stronger, demonstrating a more than a 2-fold increase after FGF-2 treatment (Figure 2C). In addition, spleen cellularity was slightly increased (Figure 2D) and CFU-C and SKL frequency showed an approximately 2-fold increase (Figure 2E-F). We also investigated whether FGF-2 treatment augments LTR-HSC populations, and found that the frequency of CD34− cells among SKL was increased after FGF-2 treatment (Figure 2G-H). In addition, we used SLAM markers to identify another population enriched for LTR-HSCs.20 FGF-2 treatment increased the frequency of SLAM HSPCs by almost 2-fold (supplemental Figure 2A).
FGF-2 signaling mediates expansion of HSPCs. C57BL/6 mice were treated with either PBS or FGF-2 (n > 7). (A,D) BM cellularity per femur and tibia or spleen cellularity. (B,E) Frequency of CFU-Cs in the BM or spleen. The total number of colonies per 1.5 × 104 BM or 2 × 105 spleen seeded cells is presented. (C,F) Representative flow cytometric dot plot analysis of BM or spleen SKL cells, numbers indicate mean percentage ± SEM of BM or spleen SKL cells. (G) Percentage of CD34− SKL cells determined by flow cytometry. (H) Representative flow cytometric analysis of CD34 expression on SKL cells. Numbers indicate mean percentage ± SEM. (I) B6.SJL recipient mice were lethally irradiated (n > 15 per time point per treatment) and transplanted with 2 × 105 BM cells from either PB- or FGF-2–treated donors (n > 5) together with recipient 4 × 105 BM cells. Mice were killed at the indicated time points and the levels of engraftment were determined. (J) CRU frequency and numbers were determined after sacrificing B6.SJL recipient mice transplanted with PBS- or FGF-2–treated donor-derived BM cells in limiting dilution (2.5 × 104 to 2 × 105) and measuring donor-derived myelolymphoid contribution among PB cells. *P < .05; **P < .01. Data shown are means ± SEM.
FGF-2 signaling mediates expansion of HSPCs. C57BL/6 mice were treated with either PBS or FGF-2 (n > 7). (A,D) BM cellularity per femur and tibia or spleen cellularity. (B,E) Frequency of CFU-Cs in the BM or spleen. The total number of colonies per 1.5 × 104 BM or 2 × 105 spleen seeded cells is presented. (C,F) Representative flow cytometric dot plot analysis of BM or spleen SKL cells, numbers indicate mean percentage ± SEM of BM or spleen SKL cells. (G) Percentage of CD34− SKL cells determined by flow cytometry. (H) Representative flow cytometric analysis of CD34 expression on SKL cells. Numbers indicate mean percentage ± SEM. (I) B6.SJL recipient mice were lethally irradiated (n > 15 per time point per treatment) and transplanted with 2 × 105 BM cells from either PB- or FGF-2–treated donors (n > 5) together with recipient 4 × 105 BM cells. Mice were killed at the indicated time points and the levels of engraftment were determined. (J) CRU frequency and numbers were determined after sacrificing B6.SJL recipient mice transplanted with PBS- or FGF-2–treated donor-derived BM cells in limiting dilution (2.5 × 104 to 2 × 105) and measuring donor-derived myelolymphoid contribution among PB cells. *P < .05; **P < .01. Data shown are means ± SEM.
As evident from Figure 2I, donor-derived chimerism levels in the BM were increased functionally in recipients transplanted with BM donor cells from FGF-2–treated mice. This increase was observed already after 1 month and was steadily maintained after 4 and 6 months. Moreover, a limiting dilutions transplantation revealed that FGF-2 could increase both the frequency and total numbers of CRUs capable of multilineage myelolymphoid reconstitution (Figure 2J). The same trend of increased LTR-HSC potential after FGF-2 treatment was also observed in another assay in which donor-derived cells were transplanted into sublethally irradiated mice (supplemental Figure 2B). Because FGF-2 was able to increase LTR-HSCs to the same extent as PTH,10 we compared the ability of both treatments to increase the CD34− SKL HSPC population and HSC short-term repopulation (STR) potential. As evident from our data (supplemental Figure 2C-D), both treatments were capable to increase the CD34− SKL population and STR-HSC potential.
Our data suggest that FGF-2 induces the expansion of a broad variety of HSPCs in different states of commitment, most importantly increasing donor-derived STR- and LTR-HSC capacity.
FGF-2 facilitates LTR-HSC expansion by induction of cell cycling and via down-regulation of intracellular ROS levels
Cell proliferation is tightly regulated during each of the cell-cycle phases. To confirm our hypothesis regarding the capability of FGF-2 to expand HSPCs by inducing cell cycling, we analyzed cycling BM SKL cells. After FGF-2 treatment, a shift was observed in the cell-cycle status of SKL cells, decreasing the quiescent noncycling G0 cells and increasing the portion of cycling cells (Figure 3A-B). The same phenotype was observed in the quiescent SLAM population (Figure 3C-D). LTR-HSCs are evident among SKL cells by their reactive oxygen species (ROS) expression levels. ROShigh SKL cells exhibit poorer LTR capacities in comparison with ROSlow SKL cells and tend to exhaust during serial transplantations.21,22 In addition, ROS promotes the differentiation of HSPCs in Drosophila.23 Indeed, when examining ROS levels in SKL cells, a strong down-regulation was observed after FGF-2 treatment, resulting in a higher frequency of ROSlow SKL cells (Figure 3E-F). Four days in vitro incubation of enriched Lin− progenitors with a cytokine mixture triggering enhanced proliferation and supplemented with FGF-2 or FGFR inhibitor shifted ROS levels in SKL HSPCs. FGF-2 increased SKL frequency, decreased ROS levels in SKL cells, and increased the frequency of ROSlow SKL cells. Concomitantly, the addition of the FGFR-specific inhibitor SU5402 yielded the opposite effects (Figure 3G-I). To test LTR-HSC functionality, cultured Lin− cells were transplanted into sublethally irradiated recipient mice to examine donor-derived contribution after a 4-month period. FGF-2 treatment enhanced donor-derived chimerism in recipient mice, whereas FGFR inhibition resulted in complete repopulation failure (Figure 3J).
FGF signaling regulates HSPC quiescence versus cycling states and HSPC intracellular ROS levels. C57BL/6 mice were treated with either PBS or FGF-2 (n > 7). (A) Percentage of G0 quiescent (Ki67−/7-amino-actinomycin D-low [7AADlow]) SKL cells determined by flow cytometry. (B) Representative flow cytometric analysis of cell-cycle status of SKL cells. Numbers indicate the mean percentage ± SEM of G0 quiescent (Ki67−/7AADlow) SKL cells. (C) Percentage of G0 quiescent (Ki67−) SLAM cells determined by flow cytometry. (D) Representative flow cytometric analysis of Ki67 expression inside SLAM cells. Numbers indicate mean percentage ± SEM of G0 quiescent (Ki67−) SLAM cells. (E) Percentage of ROSlow-expressing SKL cells as determined by flow cytometry. (f) Representative flow cytometric analysis of ROS expression inside SKL cells. SKL were further divided into subpopulations of ROSlow (ROS-L)–, ROSintermediate (ROS-I)–, and ROShigh (ROS-H)–expressing cells. Lin− progenitors were enriched from C57BL/6 mice total BM cells and cultured for 5 days with a proliferation-inducing cytokine mixture (erythropoietin, SCF, GM-CSF, and IL-3) supplemented with FGF-2 or FGFR inhibitor (SU5402; n = 4 experimental repeats). (G) Frequency of cultured SKL cells as measured by flow cytometry. (H) Percentage of ROSlow-expressing cultured SKL cells as determined by flow cytometry. (I) Representative flow cytometric analysis of ROS expression inside cultured SKL cells. (J) B6.SJL recipient mice were sublethally irradiated (n = 15) and transplanted with C57BL/6 3 × 104 Lin− cultured cells from DMSO-, FGF-2-, or SU5402-treated cultures (n = 5). Mice were killed and levels of engraftment were determined by examining the percentage of CD45.2 donor–derived chimerism. *P < .05; **P < .01. Data shown are means ± SEM.
FGF signaling regulates HSPC quiescence versus cycling states and HSPC intracellular ROS levels. C57BL/6 mice were treated with either PBS or FGF-2 (n > 7). (A) Percentage of G0 quiescent (Ki67−/7-amino-actinomycin D-low [7AADlow]) SKL cells determined by flow cytometry. (B) Representative flow cytometric analysis of cell-cycle status of SKL cells. Numbers indicate the mean percentage ± SEM of G0 quiescent (Ki67−/7AADlow) SKL cells. (C) Percentage of G0 quiescent (Ki67−) SLAM cells determined by flow cytometry. (D) Representative flow cytometric analysis of Ki67 expression inside SLAM cells. Numbers indicate mean percentage ± SEM of G0 quiescent (Ki67−) SLAM cells. (E) Percentage of ROSlow-expressing SKL cells as determined by flow cytometry. (f) Representative flow cytometric analysis of ROS expression inside SKL cells. SKL were further divided into subpopulations of ROSlow (ROS-L)–, ROSintermediate (ROS-I)–, and ROShigh (ROS-H)–expressing cells. Lin− progenitors were enriched from C57BL/6 mice total BM cells and cultured for 5 days with a proliferation-inducing cytokine mixture (erythropoietin, SCF, GM-CSF, and IL-3) supplemented with FGF-2 or FGFR inhibitor (SU5402; n = 4 experimental repeats). (G) Frequency of cultured SKL cells as measured by flow cytometry. (H) Percentage of ROSlow-expressing cultured SKL cells as determined by flow cytometry. (I) Representative flow cytometric analysis of ROS expression inside cultured SKL cells. (J) B6.SJL recipient mice were sublethally irradiated (n = 15) and transplanted with C57BL/6 3 × 104 Lin− cultured cells from DMSO-, FGF-2-, or SU5402-treated cultures (n = 5). Mice were killed and levels of engraftment were determined by examining the percentage of CD45.2 donor–derived chimerism. *P < .05; **P < .01. Data shown are means ± SEM.
Our data show that FGF-2 signaling promotes HSPC cycling. Nonetheless, the increased HSPC cycling state does not result in the loss of the LTR-HSC pool. We suggest that FGF-2–induced down-regulation of ROS is part of the mechanism increasing LTR-HSC capacity.
Maintenance of primitive HSPCs during FGF-2–induced proliferation is c-Kit dependent
SCF and its receptor, c-Kit, play a major role in the maintenance of HSPCs.14,24,25 We hypothesized that FGF-2 regulates c-Kit expression on HSPCs and SCF levels in supportive stromal cells. On examining c-Kit, we noticed an increase in the frequency of Lin−/Sca-1+ progenitors and Lin−/Sca-1− myeloid progenitors expressing c-Kit in both the BM and spleens from mice treated with FGF-2 (Figure 2C,F). Further examination of c-Kit levels revealed its up-regulation after FGF-2 treatment, in Lin−/Sca-1+ progenitors, and in SLAM HSPCs (Figure 4A-B and supplemental Figure 3A). Therefore, we pursued our investigation into c-Kit downstream and upstream components to assess their activation.
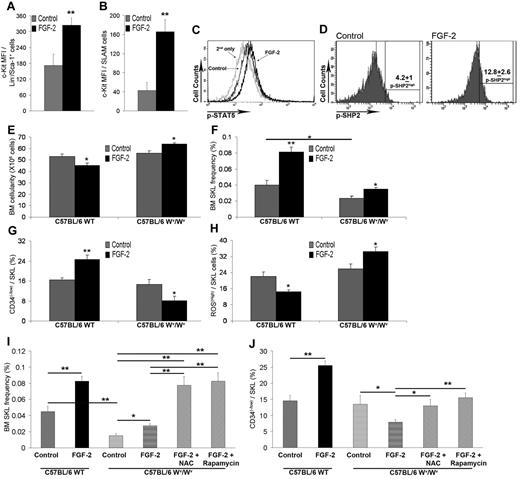
HSPC expansion and increased LTR-HSC capacity is dependent on c-Kit up-regulation and activation by FGF-2. C57BL/6 mice were treated with PBS or FGF-2 (n > 7). (A) c-Kit expression on Lin−/Sca-1+ cells was determined by flow cytometry. (B) c-Kit expression on SLAM cells was determined by flow cytometry. (C) Representative flow cytometric analysis of p-STAT5 levels in SKL cells. (D) Representative flow cytometric analysis of p-SHP2 levels in SKL cells. Numbers indicate the percentage of p-SHP2high–expressing SKL cells. C57BL/6 WT or Wv/Wv mice were treated with either PBS or FGF-2 (n = 4). (E) BM cellularity per femur and tibia. (F) Percentage of BM SKL cells as determined by flow cytometry. (G) Percentage of BM CD34−/SKL as determined by flow cytometry. (H) Percentage of SKL-expressing ROShigh cells as determined by flow cytometry. C57BL/6 WT or Wv/Wv mice were treated with either PBS or FGF-2 or FGF-2 combined with N-acetyl-cysteine (NAC)/rapamycin (n = 4). (I) Percentage of BM SKL cells as determined by flow cytometry. (J) Percentage of BM CD34−/SKL as determined by flow cytometry. *P < .05; **P < .01. Data shown are means ± SEM.
HSPC expansion and increased LTR-HSC capacity is dependent on c-Kit up-regulation and activation by FGF-2. C57BL/6 mice were treated with PBS or FGF-2 (n > 7). (A) c-Kit expression on Lin−/Sca-1+ cells was determined by flow cytometry. (B) c-Kit expression on SLAM cells was determined by flow cytometry. (C) Representative flow cytometric analysis of p-STAT5 levels in SKL cells. (D) Representative flow cytometric analysis of p-SHP2 levels in SKL cells. Numbers indicate the percentage of p-SHP2high–expressing SKL cells. C57BL/6 WT or Wv/Wv mice were treated with either PBS or FGF-2 (n = 4). (E) BM cellularity per femur and tibia. (F) Percentage of BM SKL cells as determined by flow cytometry. (G) Percentage of BM CD34−/SKL as determined by flow cytometry. (H) Percentage of SKL-expressing ROShigh cells as determined by flow cytometry. C57BL/6 WT or Wv/Wv mice were treated with either PBS or FGF-2 or FGF-2 combined with N-acetyl-cysteine (NAC)/rapamycin (n = 4). (I) Percentage of BM SKL cells as determined by flow cytometry. (J) Percentage of BM CD34−/SKL as determined by flow cytometry. *P < .05; **P < .01. Data shown are means ± SEM.
One of the downstream targets of activated c-Kit is STAT5,26 which is involved in normal and leukemic HSPC self-renewal.27,28 We analyzed changes in STAT5 phosphorylation levels as an indication of c-Kit activity. In vivo FGF-2 treatment resulted in increased p-STAT5 levels in BM SKL cells (Figure 4C and supplemental Figure 3B), indicating the possible activation of c-Kit. We also examined activation of SHP2 phosphatase, which was shown recently to regulate c-Kit expression29 and also to mediate STAT5 activation by growth factors in order to regulate human CD34+ progenitor cell proliferation, survival, and differentiation.30 As evident from Figure 4D, FGF-2 treatment increased p-SHP2 expression in BM SKL cells. These results indicate that FGF-2–induced signaling up-regulated c-Kit expression and activity in HSPCs. To validate the dependency of FGF-2 signaling on c-Kit activation, we administered FGF-2 to c-Kit mutated31 (Wv/Wv) mice and to their WT littermates. Phenotypical examination revealed that the Wv/Wv SKL population lacked high c-Kit expression and expressed only intermediate c-Kit levels (supplemental Figure 3C). Examination of mature cells showed a clear difference between Wv/Wv and WT mice in their response to FGF-2 treatment. BM cellularity was reduced after FGF-2 treatment in WT mice and increased in Wv/Wv mice (Figure 4E). In steady-state conditions, SKL frequency was reduced 2-fold in Wv/Wv mice compared with their WT littermates (Figure 4F). Treatment with FGF-2 resulted in a 2-fold increase in SKL frequency in WT mice compared with an approximately 1.5-fold increase in Wv/Wv mice (Figure 4F). Although to lesser extent, FGF-2 could still generate the cycling effect in HSPCs lacking functional c-Kit (data not shown). Because Wv/Wv HSPCs are not transplantable, even in noncompetitive transplantation assays,14,31 we determined the levels of LTR-HSCs phenotypically. SKL cells from Wv/Wv mice had a normal CD34− population frequency (Figure 4G), but after FGF-2 administration, the frequency of CD34− cells was decreased among SKL HSPCs from Wv/Wv mice, whereas the levels were increased in WT treated mice (Figure 4G). The different phenotype was also observed when we examined ROS levels. Treatment with FGF-2 decreased the ROShigh SKL population, which represent STR-HSPCs21 in WT mice but increased the ROShigh SKL population in Wv/Wv mice (Figure 4H). Inspection of p-STAT5 levels at steady state revealed the same basal levels in SKL from Wv/Wv and WT mice (supplemental Figure 3B); however, no increase in p-STAT5 was observed in Wv/Wv SKL cells after FGF-2 treatment (supplemental Figure 3B).
Our results suggest that, after FGF-2 treatment, HSPCs cycle but the LTR-HSC–enriched pool of CD34− cells is reduced because of increased ROS levels in Wv/Wv mice. Pursuing this line of thought, we decided to combine FGF-2 treatment with ROS inhibition in Wv/Wv mice. Indeed, ROS inhibition in vivo using the previously described agents N-acetyl-cysteine or rapamycin21 could restore FGF-2–expanded SKL levels to the same extent as FGF-2–treated WT mice (Figure 4I); however, it could only partially restore the pool of CD34− SKL HSPCs back to steady-state levels in FGF-2–treated Wv/Wv mice without further expansion of this population that was seen in WT mice (Figure 4J). Because ROS inhibition could prevent exhaustion of cycling Wv/Wv HSPCs but could not assist in FGF-2–stimulated expansion, we assume that other c-Kit downstream components (eg, STAT5) need to be activated to promote HSPC expansion.
To strengthen our in vivo observations we used a previously described in vitro HSPC expansion system in which FGF-2 is the only supplemented factor.9 Culture of BM cells supplemented with FGF-2 for 5 weeks increased SKL frequency (supplemental Figure 3D). To determine whether STAT5 activity is indeed crucial for FGF-2–induced HSPC expansion, we applied STAT5 inhibitor (compound 132 ) and a kinase inhibitor (AG490) for inhibition of upstream STAT5 activator kinases. The addition of a STAT5 or kinase inhibitor during the last 2 weeks of culture decreased the frequency of expanded SKL cells at the end of culture (supplemental Figure 3D). We also tested the potential of HSPCs from Wv/Wv mice to expand in vitro. Similar to our in vivo observations, HSPCs from Wv/Wv mice could expand in vitro after FGF-2 stimulation, but not with the same efficiency as HSPCs from WT mice (supplemental Figure 3E).
Our data suggest that FGF-2 activates 2 processes in HSPCs to promote their expansion: (1) HSPC cycling, which is only partially c-Kit dependent; and (2) increased LTR-HSC capacity, which is c-Kit dependent.
In vivo FGF-2 treatment expands Nestin+ MSCs, which in turn present higher SCF levels
Because our results indicated that the stromal compartment plays a role in FGF-2–induced expansion of HSPCs, we examined the influence of FGF-2 on a recently discovered population of Nestin+ MSCs. This population was established as being essential for the support and maintenance of BM hematopoiesis by serving as a reservoir of functional “niche” cells for primitive HSPCs.13 Treatment of Nestin-GFP mice15 with FGF-2 revealed an almost 2-fold increase in the frequency of Nestin-GFP+/CD45− MSCs (Figure 5A-B). To exclude the possibility that FGF-2 increases Nestin expression, we examined bromodeoxyuridine incorporation in Nestin+ MSCs and found that a higher frequency of Nestin-GFP+/CD45− MSCs incorporated bromodeoxyuridine after FGF-2 treatment (Figure 5C-D), indicative of enhanced proliferation of this population. It was shown recently that FGFR1/2 mark human and murine MSCs and that FGFR1/2 are important to maintain MSC stemness.33 In the present study, we found that the Nestin+ MSC population also expresses FGFR1/2, as indicated by colocalization of Nestin and FGFR1/2 in BM sections (Figure 5E) and also by flow cytometry surface staining of FGFR1/2 on Nestin+/CD45− MSCs (Figure 5F).
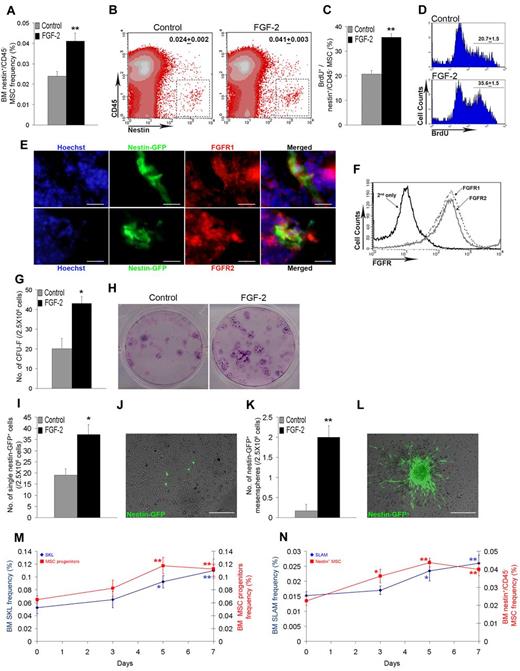
FGF-2 induces expansion of Nestin-expressing MSCs and increases expression level of SCF. C57BL/6 Nestin-GFP mice were treated with PBS or FGF-2 (n = 6). (A) Percentage of BM Nestin+/CD45− MSCs as determined by flow cytometry. (B) Representative flow cytometry density plot analysis of BM Nestin+/CD45− MSCs. Numbers indicate the percentage of BM Nestin+/CD45− MSCs. (C) Percentage of bromodeoxyuridine-positive (BrdU+) cells from BM Nestin+/CD45− MSCs as determined by flow cytometry. (D) Representative flow cytometry histogram analysis of BrdU incorporation in BM Nestin+/CD45− MSCs. Numbers indicate the mean percentage ± SEM of BrdU+ cells from BM Nestin+/CD45− MSCs. (E) Representative tissue expression of Hoechst, Nestin, and FGFR1/2 in C57BL/6 Nestin-GFP mice BM at steady-state conditions (100×). Scale bar represents 20 μm. (F) Representative flow cytometry histogram analysis of FGFR1/2 expression on BM Nestin+/CD45− MSCs. (G) Number of CFU-Fs derived from 2.5 × 106 total BM cells taken from control or FGF-2–treated mice. (H) Representative images of CFU-Fs derived from 2.5 × 106 total BM cells taken from control or FGF-2–treated mice. (I) Number of Nestin-GFP+ cells counted in cultures derived from 2.5 × 106 total BM cells taken from control or FGF-2–treated mice. (J) Representative image of Nestin-GFP+ cells found in cultures in scattered single-cell appearance (10×). Scale bar indicates 200 μm. (K) Number of Nestin-GFP+ adherent mesenspheres counted in cultures derived from 2.5 × 106 total BM cells taken from control or FGF-2–treated mice. (L) Representative image of Nestin-GFP+ adherent mesensphere found in cultures (10×). Scale bar indicates 200 μm. C57BL/6 Nestin-GFP mice were treated with PBS or FGF-2 for the indicated time points (n > 4). (M) Percentage of BM SKL (blue) and BM CD45−/CD11b−/Sca-1+/CD29+ (red) MSC progenitors as determined by flow cytometry. (N) Percentage of BM SLAM (blue) and BM Nestin+/CD45− (red) MSCs as determined by flow cytometry. *P < .05; **P < .01. Data shown are means ± SEM.
FGF-2 induces expansion of Nestin-expressing MSCs and increases expression level of SCF. C57BL/6 Nestin-GFP mice were treated with PBS or FGF-2 (n = 6). (A) Percentage of BM Nestin+/CD45− MSCs as determined by flow cytometry. (B) Representative flow cytometry density plot analysis of BM Nestin+/CD45− MSCs. Numbers indicate the percentage of BM Nestin+/CD45− MSCs. (C) Percentage of bromodeoxyuridine-positive (BrdU+) cells from BM Nestin+/CD45− MSCs as determined by flow cytometry. (D) Representative flow cytometry histogram analysis of BrdU incorporation in BM Nestin+/CD45− MSCs. Numbers indicate the mean percentage ± SEM of BrdU+ cells from BM Nestin+/CD45− MSCs. (E) Representative tissue expression of Hoechst, Nestin, and FGFR1/2 in C57BL/6 Nestin-GFP mice BM at steady-state conditions (100×). Scale bar represents 20 μm. (F) Representative flow cytometry histogram analysis of FGFR1/2 expression on BM Nestin+/CD45− MSCs. (G) Number of CFU-Fs derived from 2.5 × 106 total BM cells taken from control or FGF-2–treated mice. (H) Representative images of CFU-Fs derived from 2.5 × 106 total BM cells taken from control or FGF-2–treated mice. (I) Number of Nestin-GFP+ cells counted in cultures derived from 2.5 × 106 total BM cells taken from control or FGF-2–treated mice. (J) Representative image of Nestin-GFP+ cells found in cultures in scattered single-cell appearance (10×). Scale bar indicates 200 μm. (K) Number of Nestin-GFP+ adherent mesenspheres counted in cultures derived from 2.5 × 106 total BM cells taken from control or FGF-2–treated mice. (L) Representative image of Nestin-GFP+ adherent mesensphere found in cultures (10×). Scale bar indicates 200 μm. C57BL/6 Nestin-GFP mice were treated with PBS or FGF-2 for the indicated time points (n > 4). (M) Percentage of BM SKL (blue) and BM CD45−/CD11b−/Sca-1+/CD29+ (red) MSC progenitors as determined by flow cytometry. (N) Percentage of BM SLAM (blue) and BM Nestin+/CD45− (red) MSCs as determined by flow cytometry. *P < .05; **P < .01. Data shown are means ± SEM.
To verify the functionality of expanded Nestin+/CD45− MSCs, we examined CFU-F activity in the BM of FGF-2–treated mice because it has been shown that CFU-Fs are the progeny of Nestin+ MSCs.13 CFU-F frequency in BM cultures from FGF-2–treated mice was increased by approximately 2-fold (Figure 5G-H). Close examination of cultures before the CFU-F staining assay revealed an approximately 2-fold increased frequency of Nestin+ cells in cultures obtained from FGF-2–treated mice compared with cultures from control mice (Figure 5I-J). We also observed increased formation of Nestin+ adherent mesensphere-like structures in BM cultures from FGF-2–treated mice (Figure 5K-L). Interestingly, in a previous study, the formation of HSCs supporting mesenspheres using sorted Nestin+ BM cells required the addition of FGF-2 as part of the culturing protocol.13 In addition, comparing FGF-2 and PTH treatments in context of bone remodeling, we observed that, like PTH, FGF-2 in vivo treatment increased trabecular bone growth and formation at the epiphyseal area (supplemental Figure 4A-C). To determine how expanded Nestin+/CD45− MSCs contribute to HSPC expansion, we examined expression levels of SCF to assess its potential to activate HSPC-expressed c-Kit. In vivo FGF-2 treatment increased the expression of membrane-bound SCF on Nestin+/CD45− MSCs (supplemental Figure 5F-G), and in vitro FGF-2 treatment increased SCF production in Nestin+ cells (supplemental Figure 5H). To confirm that the SCF/c-Kit axis is located downstream of FGF-2, we treated WT and FGF-2 KO mice with SCF. Applying the protocol of high-dose SCF treatment, which is commonly used for HSPC mobilization, resulted in an unexpected BM HSPC phenotype. High doses of SCF reduced c-Kit expression levels on HSPCs dramatically and decreased the SKL population frequency by 2-fold (supplemental Figure 5A-C). This phenotype resembled the observed phenotype of SKL cells obtained from Wv/Wv mice (supplemental Figure 3C). Because of the possibility that the high dose of SCF treatment resulted in internalization of the c-Kit receptor,34 we also administrated low doses of SCF. Low-dose SCF increased the BM SKL population frequency by approximately 1.5-fold in both WT and FGF-2 KO mice (supplemental Figure 5D). This effect was accompanied by up-regulation of p-STAT5 in BM SKL cells from WT and FGF-2 KO mice (supplemental Figure 5E). Consistent with these results, we observed that SCF transcription and secretion were increased in cultures of a murine BM stromal progenitor MS-5 cell line after in vitro FGF-2 treatment (supplemental Figure 5I-J), as well as in BM cells and supernatants taken from in vivo FGF-2–treated mice (supplemental Figure 5K-L). Because our results indicated that stromal expansion precedes hematopoietic expansion, we examined the kinetics of MSC versus HSPC expansion and observed an increased expansion rate of the stromal progenitor population, reaching a peak 2 days before the SKL population peak (Figure 5M). Complementing this observation, a comparison of the SLAM HSPCs versus the Nestin+ MSCs revealed that Nestin+ MSC levels peaked at day 5, 2 days before the peak of expanded SLAM HSPCs (Figure 5N).
Our data indicate that FGF-2 augments the expansion of stromal supportive cells and accelerates bone growth alongside HSPC expansion. After stimulation with FGF-2, Nestin+ MSCs may provide higher levels of SCF to HSPCs in order to activate c-Kit.
FGF-2 down-regulates CXCL12 mRNA levels via mir-31
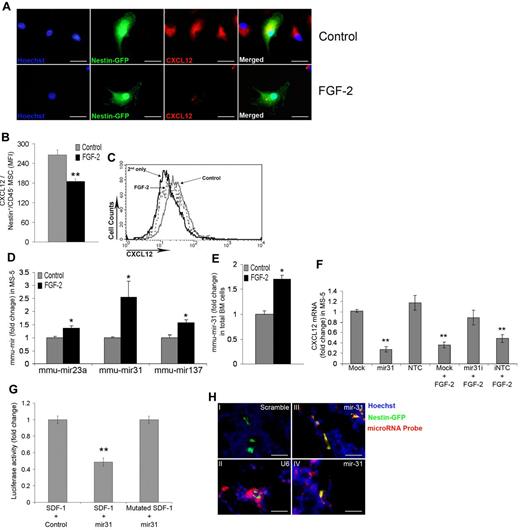
To extend our mechanistic insight as to how FGF-2 modifies stromal cells, we searched for a candidate that would mediate the FGF-2–induced HSPC exit from quiescence and cell cycle reentry. CXCL12 was shown previously to maintain HSPC quiescence via interactions with its receptor, CXCR4. CXCR4 KO mice possess hypercycling HSPCs35,36 and the addition of CXCL12 increases quiescence of HSPCs.35 Moreover, CXCL12 conditional KO results in HSPC hypercycling alongside exhaustion of LTR-HSCs.37,38 In addition, a recent study has demonstrated posttranscriptional regulation of CXCL12 mRNA in total BM stromal cells by FGF-2.39 We confirmed these observations in the present study, showing that FGF-2 in vitro down-regulates both CXCL12 mRNA and protein levels in MS-5 cells (supplemental Figure 6A-B). After 7 days of FGF-2 treatment in vivo, we noticed CXCL12 mRNA down-regulation in BM cells and protein down-regulation in BM supernatants (supplemental Figure 6C-D). Because it was shown previously that Nestin+ MSCs express the highest CXCL12 mRNA levels in the BM,13 we examined herein how FGF-2 would influence CXCL12 production in this population. In vitro FGF-2 treatment of BM cultures reduced intracellular CXCL12 production levels in Nestin+ MSCs markedly (Figure 6A). This was also confirmed in vivo. Because FGF-2 treatment reduced membrane-associated functional CXCL12 expression on Nestin+/CD45− MSCs (Figure 6B-C), we hypothesized that if CXCL12 mRNA levels are regulated posttranscriptionally, then the miRNA regulatory pathway may be a good candidate to act downstream of FGF-2. Accordingly, we applied in silico analysis using Web-free miRNA prediction programs (Microrna.org, PicTar, TargetScan, and miRanda) to search for potential miRNAs that could target CXCL12. We chose 3 predicted miRNAs with high potential to target CXCL12: mir-23a, mir-31, and mir-137. MS-5 treatment with FGF-2 led to increased expression of all 3 predicted miRNAs, with mir-31 showing the strongest up-regulation (Figure 6D). When examining how FGF-2 influenced BM cell expression of these miRNAs in vivo, we were only able to detect mir-31 expression. Expression of mir-31 was up-regulated in total BM cells taken from FGF-2–treated mice (Figure 6E). To confirm that mir-31 regulates CXCL12 mRNA, we transfected MS-5 cells with mirmimic for mir-31 or with mirmimic nontargeting control. Transfection of mirmimic successfully down-regulated CXCL12 mRNA levels in a manner similar to FGF-2 treatment (Figure 6F). We also used a mir-31 inhibitor or the inhibitor mirmimic nontargeting control combined with FGF-2 treatment to ensure that mir-31 acts downstream of FGF-2, decreasing CXCL12 mRNA levels. Combined treatment of a mir-31 inhibitor with FGF-2 prevented the down-regulation of CXCL12 mRNA levels (Figure 6F). Because a previous study determined that, although computationally predicted, mir-31 does not target CXCL12,40 we decided to reassess this observation in the present study and to determine whether mir-31 is capable of targeting CXCL12 directly. Closer computational analysis revealed that the binding site for mir-31–binding “seed” is located, not in the CXCL12 3′-untranslated region, but rather in the last exon of CXCL12 (exon 3; supplemental Figure 6E). Performing a luciferase assay similar to one described previously40 —with the modification of cloning the third exon region of CXCL12 transcript together with CXCL12 3′-untranslated region into the luciferase-reporter construct—showed a significant and strong decrease in luciferase activity, indicating mir-31 binding to its target site (Figure 6G). To further confirm this result, we mutated the binding site inside the third exon of CXCL12 (supplemental Figure 6F) and achieved a complete rescue of luciferase activity (Figure 6G). To demonstrate the significance of this finding in the context of the supporting HSC “niche,” we examined mir-31 expression in BM sections taken from Nestin-GFP mice. Control probes revealed no binding of mir-31 scrambled sequence (negative control; Figure 6Hi) or positive U6 small RNA (positive control) binding at various levels between different types of BM cells (Figure 6Hii). Colocalization of mir-31 with Nestin-GFP–expressing cells was observed in BM sections (Figure 6Hiii-iv).
FGF-2 down-regulates CXCL12 mRNA levels via mir-31 up-regulation. (A) Representative cultured-cell expression of Hoechst, Nestin, and internal CXCL12 in cultures of total BM cells from C57BL/6 Nestin-GFP mice treated with PBS or FGF-2 for 48 hours (100×). Scale bar indicates 20 μm. (B) Membrane-associated functional CXCL12 expression on BM Nestin+/CD45− MSCs as determined by flow cytometry. (C) Representative flow cytometric analysis of membrane-associated functional CXCL12 levels on BM Nestin+/CD45− MSCs. (D) mir-23a, mir-31, and mir-137 levels as determined by qRT-PCR. C57BL/6 mice were treated with either PBS or FGF-2 (n > 7). (E) mir-31 levels in total BM cells as determined by qRT-PCR. Stromal MS-5 cells were transfected with mock transfection, mir-31 mimic, nontargeting control (NTC), mir-31 inhibitor (mir-31i), or the inhibitor nontargeting control (iNTC). In some cases, cultures were treated with FGF-2. (F) CXCL12 mRNA levels as determined by qRT-PCR. HEK293T cells were cotransfected with constructs carrying either luciferase reporter gene with CXCL12 exon 3 plus the CXCL12 3′-untranslated region (UTR) in its 3′-UTR combined with nontargeting control expressing vector or the luciferase reporter gene with CXCL12 exon 3 plus the CXCL12 3′-UTR in its 3′-UTR combined with mir-31–expressing vector or the luciferase reporter gene with muted (mir-31–binding site) CXCL12 exon 3 plus the CXCL12 3′-UTR in its 3′-UTR combined with mir-31–expressing vector. (G) Measured luciferase activity is represented as the fold change relative to the control. (H) Representative tissue expression of Hoechst, Nestin, and miRNA detection probes for mir-31 scramble control (Hi), U6 control (Hii), and (Hiii-iv) mir31 in C57BL/6 Nestin-GFP mice BM (100×). Scale bar indicates 20 μm. *P < .05; **P < .01. Data shown are means ± SEM.
FGF-2 down-regulates CXCL12 mRNA levels via mir-31 up-regulation. (A) Representative cultured-cell expression of Hoechst, Nestin, and internal CXCL12 in cultures of total BM cells from C57BL/6 Nestin-GFP mice treated with PBS or FGF-2 for 48 hours (100×). Scale bar indicates 20 μm. (B) Membrane-associated functional CXCL12 expression on BM Nestin+/CD45− MSCs as determined by flow cytometry. (C) Representative flow cytometric analysis of membrane-associated functional CXCL12 levels on BM Nestin+/CD45− MSCs. (D) mir-23a, mir-31, and mir-137 levels as determined by qRT-PCR. C57BL/6 mice were treated with either PBS or FGF-2 (n > 7). (E) mir-31 levels in total BM cells as determined by qRT-PCR. Stromal MS-5 cells were transfected with mock transfection, mir-31 mimic, nontargeting control (NTC), mir-31 inhibitor (mir-31i), or the inhibitor nontargeting control (iNTC). In some cases, cultures were treated with FGF-2. (F) CXCL12 mRNA levels as determined by qRT-PCR. HEK293T cells were cotransfected with constructs carrying either luciferase reporter gene with CXCL12 exon 3 plus the CXCL12 3′-untranslated region (UTR) in its 3′-UTR combined with nontargeting control expressing vector or the luciferase reporter gene with CXCL12 exon 3 plus the CXCL12 3′-UTR in its 3′-UTR combined with mir-31–expressing vector or the luciferase reporter gene with muted (mir-31–binding site) CXCL12 exon 3 plus the CXCL12 3′-UTR in its 3′-UTR combined with mir-31–expressing vector. (G) Measured luciferase activity is represented as the fold change relative to the control. (H) Representative tissue expression of Hoechst, Nestin, and miRNA detection probes for mir-31 scramble control (Hi), U6 control (Hii), and (Hiii-iv) mir31 in C57BL/6 Nestin-GFP mice BM (100×). Scale bar indicates 20 μm. *P < .05; **P < .01. Data shown are means ± SEM.
These findings reveal a novel, miRNA-mediated pathway by which FGF-2 down-regulates CXCL12 expression in BM-supportive stromal cells, suggesting a mechanism that promotes in vivo HSPC expansion under nonquiescent conditions.
Discussion
In the present study, we reveal dual FGF-2–mediated in vivo effects in which HSPC expansion is accompanied by MSC expansion. We have also elucidated mechanisms that maintain HSPCs in a cycling state while further increasing LTR-HSC capacity (Figure 7). Considering previous studies reporting that increased HSPC cycling results in consequent loss of the stem cell pool,22,41 FGF-2–induced expansion shows promising potential.
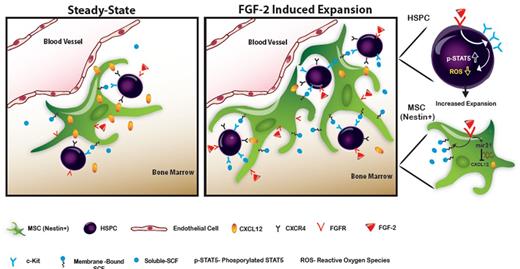
Scheme of the proposed model. At steady-state conditions, HSPCs reside in proximity to “niche” supportive Nestin+ MSCs. FGF-2 is expressed locally in low levels, allowing low-key steady-state maintenance of HSPCs. CXCL12 is expressed at high levels, keeping HSPCs in a quiescent state. After FGF-2 treatment, a dual increase in the Nestin+ MSC and the HSPC pool size occurs. Stromal CXCL12 levels are reduced via mir-31 up-regulation, allowing HSPCs to exit from their quiescent state and begin cycling. Stromal SCF levels are up-regulated, activating c-Kit, which is highly expressed by FGF-2–stimulated HSPCs. Activated c-Kit expands the pool of HSPCs and increases LTR-HSC capacity by decreasing intracellular ROS levels and activating stem cell–maintaining factors such as STAT5.
Scheme of the proposed model. At steady-state conditions, HSPCs reside in proximity to “niche” supportive Nestin+ MSCs. FGF-2 is expressed locally in low levels, allowing low-key steady-state maintenance of HSPCs. CXCL12 is expressed at high levels, keeping HSPCs in a quiescent state. After FGF-2 treatment, a dual increase in the Nestin+ MSC and the HSPC pool size occurs. Stromal CXCL12 levels are reduced via mir-31 up-regulation, allowing HSPCs to exit from their quiescent state and begin cycling. Stromal SCF levels are up-regulated, activating c-Kit, which is highly expressed by FGF-2–stimulated HSPCs. Activated c-Kit expands the pool of HSPCs and increases LTR-HSC capacity by decreasing intracellular ROS levels and activating stem cell–maintaining factors such as STAT5.
Transplantation of human CD34+ HSPCs is the traditional therapy for the treatment of different hematologic malignancies and immunodeficiency conditions. However, there are still some unresolved clinical limitations to this approach, such as the suboptimal CD34+ cell numbers that can be collected for autologous transplantation from elderly or extensively chemotherapy-treated donors. Cord blood is also a potential source for transplantable CD34+ HSPC, but its clinical use is also limited by the low number of collected cells. Therefore, expansion of HSPCs prior to or after transplantation is of paramount importance. A promising study showed that the expansion of murine HSCs could be achieved by long-term PTH treatment10 via support of the modified BM microenvironment. Our present data indicate that FGF-2 is required for PTH-induced expansion. In addition, our results suggest that FGF-2 is required for HSPC recovery after myeloablative treatments. Similar to long-term PTH treatment, we show herein for the first time that short-term in vivo FGF-2 treatment can enhance HSPC numbers significantly and accelerate bone growth. Although downstream to PTH, which preferentially expands LTR-HSCs, FGF-2 differs in its ability to expand heterogeneous progenitor populations that range from the most primitive LTR-HSCs to more committed progenitors. This is particularly significant because, unlike murine HSCs, human HSCs have much slower cycling and self-renewal rates.42 This characteristic is crucial when rapid replenishment of hematopoiesis is required pursuant to clinical transplantations, especially in patients with suboptimal numbers of transplanted HSPCs. Therefore, human BM failure may also result from insufficient numbers of committed progenitors, not just from the absence of LTR-HSCs.43 The ability of FGF-2 to expand committed progenitors capable of quickly restoring hematopoiesis may increase the chances of successful engraftment and repopulation.
Another important question we addressed in the present study was the active involvement of the stromal microenvironment in HSPC expansion. Because it is known that stromal cells present in the vascular niche, such as MSCs or endothelial cells, provide HSPCs with a supportive microenvironment,13,20,24,44 we investigated whether these cells respond to FGF-2 stimulation. Indeed, Nestin+ MSC proliferation was triggered by FGF-2 stimulation. In addition, it was shown that AKT-mTOR–activated endothelial cells support HSPC expansion in an FGF-2–dependent manner.45 FGF-2 modulated Nestin+ MSCs by generating higher levels of SCF. In addition, FGF-2 down-regulated CXCL12 levels in Nestin+ MSCs. CXCL12 KO in adult mice results in HSPC hypercycling and exhaustion of the LTR-HSC pool.38 CXCL12 KO mice had reduced SCF levels,which may have contributed to the observed loss of LTR-HSCs. Unfortunately, ROS levels were not examined in CXCL12 KO mice to complete this scenario. Maintenance of low ROS levels is crucial for the expansion of the pool of LTR-HSCs under cycling conditions. Our recently published investigations show that ROS up-regulation in HSPCs is part of a general mechanism mediating G-CSF– and AMD3100-induced mobilization.46,47 Therefore, mobilized HSPCs may have reduced LTR-HSC potential that could be restored and increased on ROS inhibition or FGF-2 treatment after harvest or transplantation.
A recent study has shown that stromal KO of the miRNA master regulator Dicer induced myelodysplasia and secondary leukemia.48 The role of mir-31 has been studied mainly in cancer models, where it was shown to affect tumor cell migration and metastasis in a cell-type– and tissue-dependent manner.49 Intriguingly, mir-31 is expressed in cancer-associated fibroblasts composing the tumor microenvironment.49 We propose that in BM supportive stromal cells, the FGF-2/mir-31 pathway acts to fine-tune CXCL12 levels during steady-state conditions and to drastically down-regulate CXCL12 during conditions that require expansion of the HSPC pool.
In conclusion, the results of the present study provide mechanistic insights into the physiology of stress-induced HSPC expansion. We show how the reciprocal rapport between HSPCs and the stromal microenvironment culminates in HSPC cycling without deleterious loss of the HSC pool. It is our contention that during steady-state conditions, FGF-2 signaling is activated locally and in a low-key manner to support and maintain both MSCs and HSCs. In stress situations, the FGF signaling cascade undergoes robust amplification, resulting in an increased MSC pool size, followed by restoration and expansion of the HSPC pool.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the Lapidot laboratory, Prof Linheng Li, and Meng Zhao for fruitful scientific discussions, and Hagar Oppenheim from the Hornstein laboratory for technical assistance and advice about performing miRNA in situ studies.
This study was supported in part by the Israeli Science Foundation (544/09), the European Union (Advance Cell–based Therapies for the Treatment of Primary Immunodeficiency HEALTH-F5-2010-261387), and The Edith Arnoff Stein Professorial Chair in Stem Cell Research (T.L.). The work of T.B. was generously supported by the Department of Molecular Biology (director Axel Ullrich) at the Max Planck Institute of Biochemistry.
Authorship
Contribution: T.I. designed and performed the experiments, analyzed the data, and wrote the manuscript; A.L., B.G., S.G.-C., A.K., A.S., Y.O., O.K., J.C., and E.S. performed the experiments and analyzed the data; D.J.C. provided FGF-2 KO mice and advice on experimental design; G.N.E. provided Nestin-GFP mice and advice on experimental design; T.B. synthesized the STAT5 inhibitor and provided advice on experimental design; W.P. provided advice on primary in vitro experimental design; E.H. provided reagents and designed the miRNA experiments; and T.L. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tsvee Lapidot, Department of Immunology, Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: tsvee.lapidot@weizmann.ac.il.



![Figure 3. FGF signaling regulates HSPC quiescence versus cycling states and HSPC intracellular ROS levels. C57BL/6 mice were treated with either PBS or FGF-2 (n > 7). (A) Percentage of G0 quiescent (Ki67−/7-amino-actinomycin D-low [7AADlow]) SKL cells determined by flow cytometry. (B) Representative flow cytometric analysis of cell-cycle status of SKL cells. Numbers indicate the mean percentage ± SEM of G0 quiescent (Ki67−/7AADlow) SKL cells. (C) Percentage of G0 quiescent (Ki67−) SLAM cells determined by flow cytometry. (D) Representative flow cytometric analysis of Ki67 expression inside SLAM cells. Numbers indicate mean percentage ± SEM of G0 quiescent (Ki67−) SLAM cells. (E) Percentage of ROSlow-expressing SKL cells as determined by flow cytometry. (f) Representative flow cytometric analysis of ROS expression inside SKL cells. SKL were further divided into subpopulations of ROSlow (ROS-L)–, ROSintermediate (ROS-I)–, and ROShigh (ROS-H)–expressing cells. Lin− progenitors were enriched from C57BL/6 mice total BM cells and cultured for 5 days with a proliferation-inducing cytokine mixture (erythropoietin, SCF, GM-CSF, and IL-3) supplemented with FGF-2 or FGFR inhibitor (SU5402; n = 4 experimental repeats). (G) Frequency of cultured SKL cells as measured by flow cytometry. (H) Percentage of ROSlow-expressing cultured SKL cells as determined by flow cytometry. (I) Representative flow cytometric analysis of ROS expression inside cultured SKL cells. (J) B6.SJL recipient mice were sublethally irradiated (n = 15) and transplanted with C57BL/6 3 × 104 Lin− cultured cells from DMSO-, FGF-2-, or SU5402-treated cultures (n = 5). Mice were killed and levels of engraftment were determined by examining the percentage of CD45.2 donor–derived chimerism. *P < .05; **P < .01. Data shown are means ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/9/10.1182_blood-2011-11-394692/4/m_zh89991293720003.jpeg?Expires=1765909161&Signature=KslCa6U5kRSBUlIzRPZUkvqieEJqpFfEXF~d0pBitRN06pkt9DaVWNzidqQzlXMVPd~qS7xfOXSSw~9F-AAA~hpbIkX-N8qDZXllvR9si-SCV3m1jdBX7W7TtmQX7MAmW5geQXD3r507QPtarcKnAmMk7i2FtFpqshlRj09EFhfeyPNlrAcMbiaIQ-VBFWJoeIDa~oWEbqv7csm7uNJSeBpSuZVLOvJJb6WqoSU-~9BV3O5DVXx970y4aNezzmQ4RBnoknbhcxlJ8F1rEorwfAVAGtop~xLpVa034QuF2UCuE6x8q0r99L3bRcB5eHH7jNRoq0r6pp~379-crtKZ0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal