Abstract
Previous studies have shown that fibroblast growth factor (FGF) signaling promotes hematopoietic stem and progenitor cell (HSPC) expansion in vitro. However, it is unknown whether FGF promotes HSPC expansion in vivo. Here we examined FGF receptor 1 (FGFR1) expression and investigated its in vivo function in HSPCs. Conditional knockout (CKO) of Fgfr1 did not affect phenotypical number of HSPCs and homeostatic hematopoiesis, but led to a reduced engraftment only in the secondary transplantation. When treated with 5-fluorouracil (5FU), the Fgfr1 CKO mice showed defects in both proliferation and subsequent mobilization of HSPCs. We identified megakaryocytes (Mks) as a major resource for FGF production, and further discovered a novel mechanism by which Mks underwent FGF-FGFR signaling dependent expansion to accelerate rapid FGF production under stress. Within HSPCs, we observed an up-regulation of nuclear factor κB and CXCR4, a receptor for the chemoattractant SDF-1, in response to bone marrow damage only in control but not in Fgfr1 CKO model, accounting for the corresponding defects in proliferation and migration of HSPCs. This study provides the first in vivo evidence that FGF signaling facilitates postinjury recovery of the mouse hematopoietic system by promoting proliferation and facilitating mobilization of HSPCs.
Introduction
Fibroblast growth factors (FGFs) are a large group of secreted molecules that regulate cell migration, proliferation, and differentiation in both embryonic and adult development.1,2 FGFs mediate their cellular responses by binding to and activating a family of 4 receptor tyrosine kinases designated as the FGF-receptors FGFR1 through FGFR4, which display different ligand-binding characteristics and biologic functions.3 FGF signaling is important for hematopoietic developmental regulation,4,5 and FGFR1 was shown to be preferentially expressed in adult hematopoietic stem and progenitor cells (HSPCs).6 Although FGF ligands support HSPC expansion in vitro,7,8 the role of FGF signaling via FGFR1 in vivo has not been elucidated.
Treatment with chemotherapeutic drugs, such as cyclophosphamide and 5-fluorouracil (5FU),9,10 induces a multistep bone marrow (BM) stress response: (1) actively cycling cells are eliminated, including cycling HSPCs9,10 ; (2) surviving quiescent long-term hematopoietic stem cells (LT-HSCs) are subsequently activated to expand; (3) some expanded HSCs give rise to short-term HSCs (ST-HSCs) for further proliferation; and (4) some HSPCs egress from BM to the blood circulation and extramedullary sites, such as spleen (ie, mobilization), to further proliferate and differentiate.11-13 In homeostatic hematopoiesis, HSPCs are primarily localized within BM where they associate with niches that regulate their activity.14-18 Although a small percentage of HSPCs routinely circulate from BM to peripheral blood (PB) and home back to BM,19,20 the number of HSPCs that migrate from BM can be markedly increased by certain stimuli during mobilization.21-25 These stimuli include tissue damaging chemotherapeutic drugs as previously mentioned and various cell signaling molecules, such as stromal derived factor-1 (SDF-1)26 and AMD3100, a small molecule that interferes with the interaction between SDF-1 and its receptor CXCR4.27
In this report, we used 3 conditional knockout (CKO) mouse models: Mx1-Cre, Scl-Cre-ERT (hereafter referred to as Scl-Cre), and Tek-Cre (or Tie2-Cre) to study the role of FGFR1 in HSPCs. Although each model has its own advantage and disadvantage, the results from testing FGFR1 in all 3 models support a critical role of FGFR1 signaling in promoting proliferation and facilitating mobilization of HSPCs as an essential process of hematopoietic recovery in response to BM damage.
Methods
Animals and treatment protocol
Fgfr1fx/fx mice28 were mated with Mx1-Cre,29 Scl-Cre,30 or Tek-Cre31 lines to generate Mx1-Cre:Fgfr1fx/fx, Scl-Cre:Fgfr1fx/fx, or Tek-Cre:Fgfr1fx/fx Fgfr1 CKO lines, respectively. All mice were backcrossed with C57Bl/6 to achieve the C57Bl/6 background. Genotyping was performed on tail biopsies using a polymerase chain reaction (PCR)–based method developed by Transnetyx (Cordova). To induce gene deletion, polyinosinic:polycytidylic acid (pIpC; GE Healthcare) was injected intraperitoneally every other day at a dose of 250 μg per injection to Mx1-Cre:Fgfr1fx/fx mice for a total of 7 injections, or tamoxifen (TMX; Sigma-Aldrich) was injected intraperitoneally every day at a dose of 2 mg per injection to Scl-Cre:Fgfr1fx/fx mice for 5 days. Mice received 5FU or AMD3100 treatment only after 2 to 3 weeks following completion of induction for gene-deletion. FVB/N and FVB/N Fgf2 knockout mice were as described.32 The adult mice were defined as beyond 2 months old. All mice used in this study were housed in the animal facility at the Stowers Institute for Medical Research (SIMR) and handled according to SIMR and National Institutes of Health (NIH) guidelines. Mice were treated with reagents as follows: injected once via tail vein with 5FU (Sigma-Aldrich) at 150 μg/g body weight (BW),33 injected once subcutaneously with AMD3100 (Sigma-Aldrich) at 5 μg/g BW.27 PB, BM, and/or spleen tissue was harvested at various time points after 5FU treatment, and 60 minutes after AMD3100 treatment. All procedures were approved by the Institutional Animal Care and Use Committee of SIMR.
Flow cytometry analysis of hematopoietic cells
Hematopoietic cells were harvested from spleen, PB, and BM of the femurs and tibias. The flow analysis for HSCs was previously described.34,35 Megakaryocytes (Mks) were identified by their large size (forward scatter high, FSChi) combined with staining with a monoclonal antibody to CD41 (eBioscience). For detection of FGFR1 and FGF2 expression in Mks, total BM cells were incubated with rat anti CD41-PE (eBioscience) and with rabbit anti-FGFR1 (Abcam) antibodies followed by incubation with 2nd 488 anti–rabbit (Jackson ImmunoResearch Laboratories) or cells were stained with rat anti–CD41-PE and permeabilized using BD Perm/Fix kit (BD Biosciences) according to the manufacturer's protocol, and then incubated with biotinylated anti-FGF1 (Peprotech) antibody followed by incubation with streptavidin-APC (Biolegend). Gating on FSChi CD41+ cell population that enriches Mks, we measured the mean fluorescence intensity (MFI) as the levels of FGFR1 or FGF1. Cell viability was tested by annexin V (Invitrogen) and 7-AAD (Invitrogen). Cell sorting and analysis were done on the Cyan ADP (Dako), MoFlo (Dako), and/or Influx (BD Biosciences). Data analysis was performed using FlowJo Version 7.6.4 software.
Transplantation assays
Transplantation experiments were performed with BM, spleen or PB cells from donor mice (CD45.2) that had received either none or with 5FU 12 days prior. Two × 105 competitor/rescue whole Ptprc BM cells (CD45.1) were transplanted into each lethally irradiated (10 Gy) Ptprc (CD45.1) recipient with indicated numbers of donor cells. Repopulation was measured every 4 weeks after transplantation by collection of PB, red blood cell lysis, and staining of CD45.1 (recipient) versus CD45.2 (donor) engraftment.
Quantitative real-time RT-PCR and RNA-sequencing analysis
Total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's protocol. Real-time reverse transcription (RT)–PCR reactions were performed in triplicate using the Quantitect SYBR Green RT-PCR kit (QIAGEN) on an iQ5 RT-PCR detection system (Bio-Rad Laboratories) or ABI 7900 (Applied Biosystems) according to the manufacturer's instructions. The RNA-sequencing library was prepared using illumina TruSeq RNA sample prep kit (no. FC-122-1001). A total of 10 fmol library fragments were loaded to cBot to generate clusters, followed by sequencing on an Illumina HiSeq 2000. Gene expression was quantitated using Cufflinks 1.0.3. For details please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Genotyping
DNA was purified from targeted cells using QIAamp DNA blood kit (QIAGEN) and PCR was performed using primers, Fgfr1 a, Fgfr1 c for recombined allele, and Fgfr1 b, Fgfr1 c for unrecombined allele. Sequences for primers Fgfr1 a, b, and c are 5′gtattgctggcccactgttc3′, 5′ctggtatcctgtgcctatc3′ and 5′caatctgatcccaagaccac3′, respectively.
Cell migration assays
Cell migration was studied using 6.5 mm, 5 μm pore size transwell inserts in 24-well cluster plates (Corning-Costar). Five-thousand lineage−Sca-1+c-Kit+ (LSK) cells were flow sorted into 0.1 mL Dulbecco modified Eagle medium (DMEM) supplemented with 5% horse serum in the upper chamber. Chemotaxis toward 100 ng/mL murine CXCL12/SDF-1α (R&D Systems) in 0.6 mL DMEM + 5% HS in the lower chamber was allowed to continue for 4 hours at 37°C and 5% CO2. Cells that had migrated to the lower chamber were visualized and enumerated using a Leica DM IL (Leica Microsystems). Where indicated, 100 ng/mL FGF1or FGF2 (R&D Systems) and the following chemical inhibitors were included in the media of both the upper and lower chambers: SU5402 at 25μM (Calbiochem), LY294002 at 50μM (BIOMOL Research Laboratories), and PD98059 at 50μM (Sigma-Aldrich).
Cell culture
Flk2−LSK cells, LSK cells, or total BM cells were sorted into 96-well U-bottom tissue culture plates at 500 Flk2−LSK cells, 600 LSK cells or 1 to 2 × 105 BM cells/well with 180μL media/well. Cells were incubated at 37°C, 5% O2, 5% CO2 (balance N2). Defined HSC expansion media was based on previous reports,36 which included StemSpan serum-free expansion medium (SFEM; StemCell Technologies) supplemented with 10 mg/mL heparin (Sigma-Aldrich), 10 ng/mL recombinant mouse stem cell factor (Biovision), and 20 ng/mL Tpo (Cell Sciences). Where indicated, the chemical inhibitor SU5402 (Calbiochem) or nuclear factor (NF)κB inhibitor (EMD Biosciences) was added to the culture media at 1 or 5μM. FGF1, FGF2, FGF4, and FGF10 (R&D Systems) were added at 50 ng/mL to the culture media, respectively, for 2 weeks.
Immunohistochemistry and immunostaining
Tissues collected for immunohistochemistry were fixed in unbuffered zinc formalin (Richard-Allan Scientific) for 24 hours at room temperature. Femurs and tibias were decalcified in Immunocal (Decal Chemical) for 24 hours at room temperature. Tissues were embedded in paraffin and 3μM sections were obtained. Tissues were stained with a rabbit polyclonal antibody to von Willibrand factor (VWF; AbCam) at a dilution of 1:300. Secondary staining was done with the EnVision+ System-HRP Labeled anti–rabbit polymer (Dako). For single-cell staining, cells were sorted onto poly-lysine coated slides, fixed with methanol and stained for NFκB P65 (Cell Signaling Technology) using 1:200 dilution. Images were taken on an Axiomager Z1 (Zeiss) with AxioVision 4.7.2.0.
Enzyme-linked immunosorbent assays
For the FGF1, FGF2, and SDF-1 detection, a monoclonal antibody to FGF1 (PeproTech), FGF2 (R&D Systems), or SDF-1 monoclonal antibody 79018 (R&D Systems) was used for capture, and biotinylated antibodies FGF1 (PeproTech), FGF2 (R&D Systems), or biotinylated anti–SDF-1 (R&D Systems) were used for detection using streptavidin-horseradish peroxidase. Plates were read with an enzyme-linked immunosorbent assay (ELISA) reader and analyzed according to the standard curve and Bradford assay results. The standard curve was made using recombinant mouse FGF1, FGF2 (PeproTech), or hSDF-1 (R&D Systems).
Colony forming unit assays
In vitro colony forming unit (CFU) assays detected a mixture of myeloid progenitors including: erythroid (BFU-E), granulocyte-macrophage (CFU-G, CFU-M, and CFU-GM), and multipotential granulocyte, erythroid, macrophage, and Mk (CFU-GEMM). The assay was performed using 2 × 105 PB cells per well of a 12-well tissue culture plate (Becton Dickinson) and 0.9 mL MethoCult GF M3434 Media (StemCell Technologies) following the manufacturer's instructions. Colonies were evaluated and counted on day 12 of culture using a Leica DM IL microscope (Leica Microsystems).
CFU-Mk assays
Mk progenitor assay was performed on BM cells using MegaCult-C media (StemCell Technologies) supplemented with 50 ng/mL rmTpo, 20 ng/mL rmIL-6, and 10 ng/mL rmIL-3. CFU-Mks were stained for acetylcholinesterase activity and scored after 7 days incubation according to the manufacturer's protocol.
Statistical analysis
Data were expressed as mean ± SD. Pairwise comparisons were performed using the Student t test.
Results
FGFR1-mediated signaling is dispensable for homeostatic hematopoiesis but important for BM recovery under stress
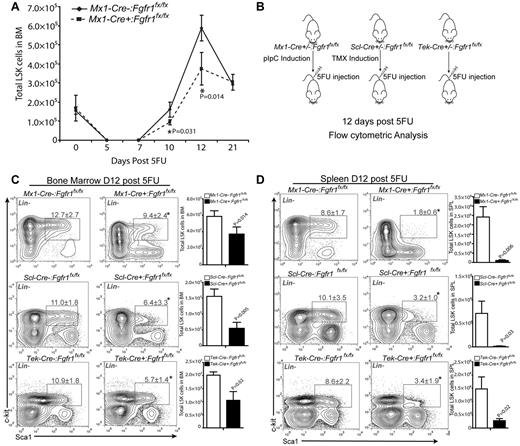
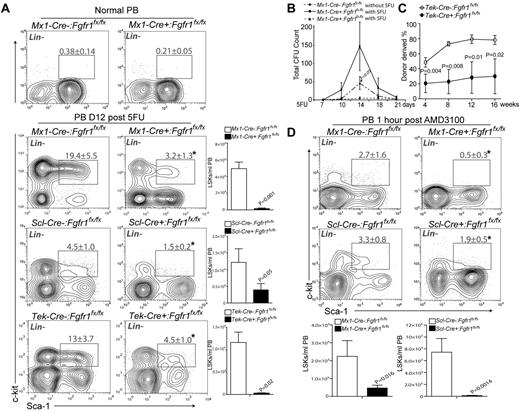
To investigate the function of FGF signaling in HSPCs, we induced Mx1-Cre:Fgfr1fx/fx control and CKO mice for gene inactivation. After a recovery period of 3 weeks, Mx1-Cre+:Fgfr1fx/fx mice exhibited normal hematopoiesis compared with Mx1-Cre−:Fgfr1fx/fx controls, as indicated by similar numbers of HSPCs (LSK cells) in BM, as well as normal levels of mature myeloid and lymphoid lineages in PB, spleen, and thymus (supplemental Figure 1). Because pIpC used for induction of Mx1-Cre can cause HSPC proliferation independent of FGF signaling and Scl-Cre has lower recombination efficiency, we used the Tek-Cre induced Fgfr1 CKO model to conduct the repopulation assay. We demonstrated that Fgfr1 CKO did not significantly affect HSPC numbers and function in primary transplantation but did influence HSPC repopulation after secondary transplantation (supplemental Figures 2-3). These results indicate that loss of FGF signaling via FGFR1 does not impact homeostatic hematopoiesis but can compromise HSC function when multiple rounds of expansion are required.
Although not required for homeostasis, FGF signaling was previously reported to be involved in neonatal motor cortex injury recovery.37 We asked whether FGF signaling is involved in BM stress response. As a cytotoxic agent, 5FU initiated BM stress response by killing actively cycling cells, including cycling HSPCs,9,10 causing the number of LSK cells in BM to decline within the first week after 5FU treatment (Figure 1A). Extensive cell death led to activation of surviving HSCs, followed by proliferation of LT-HSCs, ST-HSCs, and multipotent progenitor cells (MPPs; all contained in LSK cells) from day 7 through day 12 after 5FU.38 We observed that Mx1-Cre−:Fgfr1fx/fx and Mx1-Cre+:Fgfr1fx/fx mice showed similar nadirs of LSKs between days 5 and 7 after 5FU (Figure 1A). Whereas LSKs in BM of Mx1-Cre−:Fgfr1fx/fx mice expanded dramatically on days 10 and 12 after 5FU, LSK expansion in Mx1-Cre+:Fgfr1fx/fx mice was significantly impaired (Figure 1A). Because the Mx-1 promoter-driving Cre expression is in hematopoietic cells as well as in stromal cells,29 we also used a TMX-induced HSPC specific Scl-Cre mouse line,30 and a hematopoietic and endothelial specific Tek-Cre line to determine whether FGFR1 is required for HSPC recovery after 5FU treatment or whether it acts indirectly on HSPCs through nonhematopoietic stromal components. For this purpose, we compared the pIpC-induced Mx1-Cre, TMX-induced Scl-Cre, and Tek-Cre–mediated Fgfr1 CKO models. After Cre induction, mice were treated with 5FU (Figure 1B). Twelve days after 5FU, when the largest difference between controls and Fgfr1 CKO models was observed (Figure 1A), all 3 Fgfr1 CKO models had decreased frequency and absolute number of LSK cells in BM compared with littermate controls (37%, 66.5%, and 57.2% decrease, respectively in Mx1-Cre, Scl-Cre, Tek-Cre; Figure 1C). In response to 5FU-induced BM damage, surviving LSK cells undergo mobilization and significant expansion as seen in spleen of control mice; however, we observed substantially fewer LSK cells in the spleens of Fgfr1 CKO mice (95.6%, 98%, and 90.45% decrease, respectively in Mx1-Cre, Scl-Cre, and Tek-Cre; Figure 1D). We also noticed the extent of HSPC increase in the Cre− control group was greater with pIpC induction (5.8 × 105 in BM, 2.4 × 106 in spleen) than with TMX induction (1.7 × 105 in BM, 7.1 × 105 in spleen), and greater than in the noninduced Tek-Cre model (2.2 × 105 in BM, 1.4 × 106 in spleen; Figure 1C-D). This discrepancy can be explained, at least in part, by the added effect of interferon (IFN) induced by pIpC.39 These results indicate that FGFR1 promotes HSPC proliferation and potential migration during BM recovery in response to BM damage.
FGFR1 inactivation impairs HSPC recovery after BM damage. (A) Comparison of LSK numbers in BM of Mx1:Control and Mx1:CKO mice at the indicated times after 5FU (n = 2-4). (B) Illustration of Cre induction, 5FU-induced BM damage, and analyses of Fgfr1 control (Cre−:Fgfr1fx/fx) and CKO (Cre+:Fgfr1fx/fx) mice. Polyinosinic:polycytidylic acid (pIpC). Tamoxifen (TMX). (C) Flow cytometric analyses of the LSK population and comparison of absolute numbers of LSK cells in BM from Mx1:Control, Mx1:CKO (n = 4), Scl:Control, Scl:CKO (n = 6), and Tek:Control, Tek:CKO (n = 6) mice 12 days after 5FU. (D) Flow cytometric analyses of the LSK population and comparison of absolute numbers of LSK cells in spleen from Mx1:Control, Mx1:CKO (n = 4), Scl:Control, Scl:CKO (n = 5), and Tek:Control, Tek:CKO (n = 6) mice 12 days after 5FU (*P < .05). Data pooled from at least 2 independent experiments.
FGFR1 inactivation impairs HSPC recovery after BM damage. (A) Comparison of LSK numbers in BM of Mx1:Control and Mx1:CKO mice at the indicated times after 5FU (n = 2-4). (B) Illustration of Cre induction, 5FU-induced BM damage, and analyses of Fgfr1 control (Cre−:Fgfr1fx/fx) and CKO (Cre+:Fgfr1fx/fx) mice. Polyinosinic:polycytidylic acid (pIpC). Tamoxifen (TMX). (C) Flow cytometric analyses of the LSK population and comparison of absolute numbers of LSK cells in BM from Mx1:Control, Mx1:CKO (n = 4), Scl:Control, Scl:CKO (n = 6), and Tek:Control, Tek:CKO (n = 6) mice 12 days after 5FU. (D) Flow cytometric analyses of the LSK population and comparison of absolute numbers of LSK cells in spleen from Mx1:Control, Mx1:CKO (n = 4), Scl:Control, Scl:CKO (n = 5), and Tek:Control, Tek:CKO (n = 6) mice 12 days after 5FU (*P < .05). Data pooled from at least 2 independent experiments.
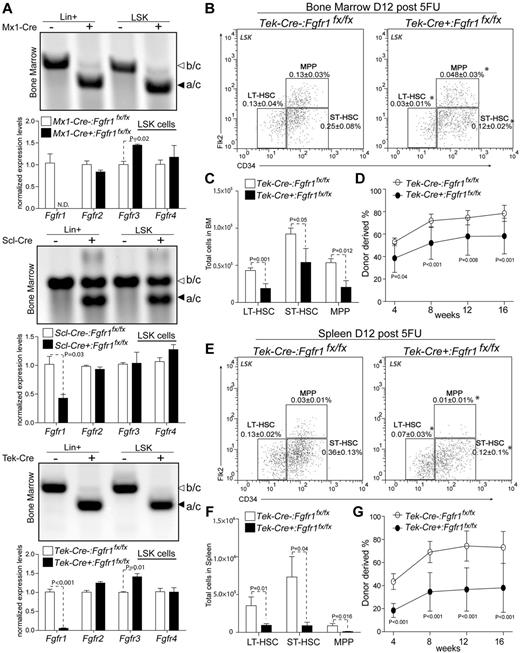
Given the potential side effect from pIpC in Mx1-Cre model, we further verified our finding by performing whole BM transplantations from Scl-Cre−:Fgfr1fx/fx and Scl-Cre+:Fgfr1fx/fx donors into lethally irradiated recipients (supplemental Figure 4). We found that the BM cells from Scl-Cre+:Fgfr1fx/fx exhibited lower short-term engraftment at 4 weeks but recovered at 12 and 16 weeks compared with control donors. To exclude the potential cytotoxicity caused by Cre-ERT, we tested the Scl-Cre+:Fgfr1+/+ control mice after TMX induction and 5FU treatment, and this resulted in normal HSPC recovery (supplemental Figure 5). Because the HSPCs that escaped Cre-induced Fgfr1 excision could potentially contribute to long-term repopulation, we measured recombination efficiency and found that both Mx1-Cre and Tek-Cre models resulted in complete excision in both the LSK and lineage-positive (mature cells) populations; the Scl-Cre model, however, revealed incomplete recombination (∼ 50%) in both populations (Figure 2A). The gene inactivation efficiencies in LSK populations were confirmed by qRT-PCR (Figure 2A).The mRNA levels of Fgfr1 in both Mx1-Cre+:Fgfr1fx/fx and Tek-Cre+:Fgfr1fx/fx were largely reduced; however, Scl-Cre+:Fgfr1fx/fx showed only a 50% reduction compared with littermate controls. In addition, we noticed a 44% and 41% increase of Fgfr3, respectively, in Mx1-Cre+:Fgfr1fx/fx and Tek-Cre+:Fgfr1fx/fx mice compared with littermate controls. This suggested a potential compensation from FGFR3 when FGFR1 was inactivated. However, Fgfr1 CKO still adversely affected after BM damage recovery of HSPCs (Figure 1), which indicated an influence from FGFR3 compensation for BM recovery is not sufficient.
Comparison of gene deletion efficiency in Fgfr1 CKO models and testing functions of HSPCs in BM and spleen after BM damage. (A) Purified DNA and mRNA from sorted lineage positive (Lin+) and LSK populations in BM of Fgfr1 control and Fgfr1 CKO animals at 12 days after 5FU. PCR to detect recombined (primer a/c 300 bp) and unrecombined allay (primer b/c 400 bp). qRT-PCR detection of gene expression analysis of Fgfr1, 2, 3, 4 in LSK cells. ND = not detected. (B-E) Flow cytometric analyses of HSPCs (LT-HSC, ST-HSC, MPP) in LSK population in BM (B) and spleen (E) of Tek:Control, Tek:CKO (n = 4-6) mice 12 days after 5FU. Frequencies of total TNC shown in plots. (C-F) Comparison of absolute numbers of HSPCs (LT-HSC, ST-HSC, MPP) in BM (C) and spleen (F) from Tek:Control, Tek:CKO (n = 4-6) mice 12 days after 5FU. (D-G) Two × 105 BM cells (D) or 2.5 × 105 spleen cells (G) from Tek:Control, Tek:CKO mice (CD45.2) transplanted 12 days after 5FU with 2 × 105 rescue BM cells (CD45.1) into lethally irradiated Ptprc recipients. PB analysis for total engrafted donor cells at 4, 8, 12 and 16 weeks posttransplantation (n = 10 per group). Error bars indicate SD (*P < .05).
Comparison of gene deletion efficiency in Fgfr1 CKO models and testing functions of HSPCs in BM and spleen after BM damage. (A) Purified DNA and mRNA from sorted lineage positive (Lin+) and LSK populations in BM of Fgfr1 control and Fgfr1 CKO animals at 12 days after 5FU. PCR to detect recombined (primer a/c 300 bp) and unrecombined allay (primer b/c 400 bp). qRT-PCR detection of gene expression analysis of Fgfr1, 2, 3, 4 in LSK cells. ND = not detected. (B-E) Flow cytometric analyses of HSPCs (LT-HSC, ST-HSC, MPP) in LSK population in BM (B) and spleen (E) of Tek:Control, Tek:CKO (n = 4-6) mice 12 days after 5FU. Frequencies of total TNC shown in plots. (C-F) Comparison of absolute numbers of HSPCs (LT-HSC, ST-HSC, MPP) in BM (C) and spleen (F) from Tek:Control, Tek:CKO (n = 4-6) mice 12 days after 5FU. (D-G) Two × 105 BM cells (D) or 2.5 × 105 spleen cells (G) from Tek:Control, Tek:CKO mice (CD45.2) transplanted 12 days after 5FU with 2 × 105 rescue BM cells (CD45.1) into lethally irradiated Ptprc recipients. PB analysis for total engrafted donor cells at 4, 8, 12 and 16 weeks posttransplantation (n = 10 per group). Error bars indicate SD (*P < .05).
To prove the decrease of functional HSPCs in Fgfr1 CKO mice after BM damage, we conducted a competitive repopulation assay using the Tek-Cre CKO model that has high recombination efficiency (Figure 2A) and no side effect from pIpC. First, we observed, in Fgfr1 CKO mice at 12 days after 5FU treatment, that the frequency and absolute number of HSPCs decreased in BM (56.1% for LT-HSCs, 41.5% for ST-HSCs, and 61.5% for MPPs; Figure 2B-C) and in spleen (73.3% for LT-HSCs, 87.8% for ST-HSCs, and 87.6% for MPPs; Figure 2E-F). Next, we transplanted equal number of BM or spleen cells isolated from Tek-Cre−:Fgfr1fx/fx and Tek-Cre+:Fgfr1fx/fx mice at 12 days after 5FU with rescue cells into lethally irradiated recipients. We observed that BM cells and spleen cells from Tek-Cre+:Fgfr1fx/fx mice resulted in lower engraftment than those from Tek-Cre−:Fgfr1fx/fx (Figure 2D-G; 26% decrease in BM cells and 48% decrease in spleen cells, respectively, at 16 weeks posttransplantation). These data demonstrate that the numbers of functional HSPCs in BM and spleen of Fgfr1 CKO mice are indeed reduced after BM damage, supporting the functional requirement of FGFR1 for HSPCs during BM recovery.
FGF-FGFR1 signaling is activated by 5FU treatment
Because we observed that FGFR1 is dispensable for homeostatic hematopoiesis but important for BM recovery under stress, we examined whether FGF-FGFR1 signaling is activated under stress. First, we performed an RNA-sequencing analysis to examine the expression of Fgfrs in HSPCs. Among the 4 Fgfrs, we detected Fgfr1 and Fgfr3 expressions in LT-HSCs, ST-HSCs, and MPPs, with Fgfr1 expressing higher than Fgfr3 (Figure 3A). We then compared expressions of Fgfrs under stressed conditions. BM CD34−LSK (enriched for LT-HSCs) cells were harvested from untreated and 5FU-treated wild-type (WT; C57Bl/6) mice, and expressions of Fgfrs were measured by qRT-PCR. At day 5 after 5FU, we observed that only Fgfr1 had a significant increase (2-fold), supporting the role of FGFR1-mediated signaling in promoting HSPC proliferation after stress (Figure 3B). Next, we compared expression levels of FGF ligands specific for FGFR1 (FGF1, 2, 3, 4, 5, and 10) in BM of C57/B6 mice under normal and 5FU-induced stress conditions.1 Five days after 5FU, the mRNA levels of Fgf1, Fgf2, Fgf5, and Fgf10 showed significant increase, with Fgf1 displaying the highest increase (12, 2.6, 2.3, and 4.7-fold, respectively; Figure 3C). In addition, the protein levels of FGF1 and FGF2 in BM increased 1.7-fold and 1.5-fold, respectively after 5FU, as measured by ELISAs (Figure 3D). These data suggest that FGF1, FGF2, FGF5, and FGF10 are probably the signals promoting HSPC proliferation in response to stress, which is consistent with published studies using FGF1 and FGF2 to expand HSPCs in vitro.7,8
FGF signal is activated post BM damage. (A) RNA-seq analysis of HSPCs for Fgfrs. Expression level shown by FPKM (fragments per kilobase of exon per million fragments mapped). (B) Gene expression analysis of Fgfr1, 2, 3, 4 using qRT-PCR on BM CD34−LSK cells from C57Bl/6 WT mice 5 days after 5FU (n = 3). (C) Gene expression analysis of Fgf1, 2, 3, 4, and 10 using qRT-PCR on BM cells from C57Bl/6 WT mice on the days indicated after 5FU (n = 3). (D) FGF1 and FGF2 protein levels as determined by ELISA of BM supernatants of C57Bl/6 WT mice after 5FU at 5 and 10 days, respectively (n = 3).
FGF signal is activated post BM damage. (A) RNA-seq analysis of HSPCs for Fgfrs. Expression level shown by FPKM (fragments per kilobase of exon per million fragments mapped). (B) Gene expression analysis of Fgfr1, 2, 3, 4 using qRT-PCR on BM CD34−LSK cells from C57Bl/6 WT mice 5 days after 5FU (n = 3). (C) Gene expression analysis of Fgf1, 2, 3, 4, and 10 using qRT-PCR on BM cells from C57Bl/6 WT mice on the days indicated after 5FU (n = 3). (D) FGF1 and FGF2 protein levels as determined by ELISA of BM supernatants of C57Bl/6 WT mice after 5FU at 5 and 10 days, respectively (n = 3).
FGF signaling drives HSPC expansion in vitro
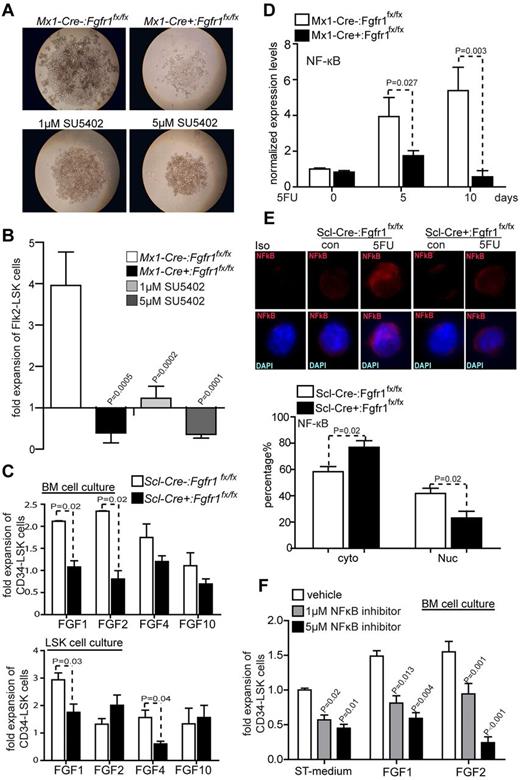
To further test whether FGF signaling via FGFR1 plays a role in HSPC expansion, we conducted an in vitro culture experiment using a previously reported method, in which the functional HSCs are maintained.35,36 BM Flk2−LSKs (enriched with both LT and ST-HSCs) from Mx1-Cre−:Fgfr1fx/fx and Mx1-Cre+:Fgfr1fx/fx mice were sorted and used for in vitro culture. After 2 weeks culture, we found that BM Flk2−LSKs from Mx1-Cre−:Fgfr1fx/fx mice expanded much more robustly (by 10.1-fold) than those from Mx1-Cre+:Fgfr1fx/fx mice (Figure 4A-B). Furthermore, the FGFR inhibitor SU5402 blocked expansion of WT Flk2−LSKs in vitro 3.2-fold at 1μM and 11-fold at 5μM (Figure 4B). These in vitro data confirm the importance of FGFR1 signaling in facilitating the expansion of HSPCs.
FGF signals facilitate HSPC expansion through FGFR1. (A) BM Flk2−LSK cells from Mx1:Control or Mx1:CKO cultured with or without SU5402 14 days. Representative data from 3 independent experiments. (B) Comparison of fold increase of Flk2−LSK cells from Mx1:Control, Mx1:CKO and Mx1:Control plus SU5402 at 1 or 5μM after 14 days culture (n = 6). (C) Comparison of fold increase of CD34−LSK cells from BM cells and sorted LSK cells from Scl:Control and Scl:CKO mice, respectively, plus FGF1, 2, 4, or 10 with standard medium after 14 days culture (n = 3). (D) Gene expression analysis of NFκB using qRT-PCR on BM Flk2−LSK cells from Mx1:Control, Mx1:CKO mice on the days indicated after 5FU (n = 3). (E) Representative immunostaining and quantification of subcellular location of NFκB in CD34−LSK cells sorted from Scl:Control and Scl:CKO mice. Data pooled from 2 independent experiments. (F) Comparison of fold increase of CD34−LSK cells from BM cells in C57Bl/6 WT mice plus FGF1, FGF2, or with standard medium plus NFκB inhibitor at 1 or 5μM, respectively, as indicated after 14 days culture (n = 4).
FGF signals facilitate HSPC expansion through FGFR1. (A) BM Flk2−LSK cells from Mx1:Control or Mx1:CKO cultured with or without SU5402 14 days. Representative data from 3 independent experiments. (B) Comparison of fold increase of Flk2−LSK cells from Mx1:Control, Mx1:CKO and Mx1:Control plus SU5402 at 1 or 5μM after 14 days culture (n = 6). (C) Comparison of fold increase of CD34−LSK cells from BM cells and sorted LSK cells from Scl:Control and Scl:CKO mice, respectively, plus FGF1, 2, 4, or 10 with standard medium after 14 days culture (n = 3). (D) Gene expression analysis of NFκB using qRT-PCR on BM Flk2−LSK cells from Mx1:Control, Mx1:CKO mice on the days indicated after 5FU (n = 3). (E) Representative immunostaining and quantification of subcellular location of NFκB in CD34−LSK cells sorted from Scl:Control and Scl:CKO mice. Data pooled from 2 independent experiments. (F) Comparison of fold increase of CD34−LSK cells from BM cells in C57Bl/6 WT mice plus FGF1, FGF2, or with standard medium plus NFκB inhibitor at 1 or 5μM, respectively, as indicated after 14 days culture (n = 4).
To distinguish direct and indirect influence of FGFs in this regard, we performed in vitro experiments to culture mixed BM cells or sorted LSK cells. Total BM cells harvested from Scl-Cre−:Fgfr1fx/fx and Scl-Cre+:Fgfr1fx/fx mice were cocultured with different FGF ligands for 2 weeks. BM cells cultured with FGF1 or FGF2 expanded CD34−LSK cells 2 times greater than without FGF1 or FGF2 (Figure 4C). This result is consistent with a previous report.8 FGF4 and FGF10 did not expand HSPCs. When we conducted this culture with sorted LSK cells, we surprisingly found that only FGF1 expanded HSPCs 3 times greater than without FGF1, but not with FGF2, and also that FGF4 instead of FGF10 slightly expanded HSPCs (Figure 4C). This result suggests that FGF1 more directly regulates HSPCs, whereas FGF2 may indirectly influence HSPCs by regulating other types of BM cells.
Mechanistically, we noted by measuring with qRT-PCR that the FGFR1 downstream target NFκB, a known HSC survival factor, showed an up-regulation in Flk2−LSK cells from Mx1-Cre−:Fgfr1fx/fx mice but not from Mx1-Cre+:Fgfr1fx/fx mice on day 5 (3.9-fold) and day 10 (5.4-fold) after 5FU treatment (Figure 4D). As the NFκB activity is associated with its nuclear translocalization (the p65 form), we compared NFκB p65 in CD34−LSK cells isolated from Scl-Cre−:Fgfr1fx/fx and Scl-Cre+:Fgfr1fx/fx mice using immunostaining. We found that NFκB was more abundant in the nucleus of CD34−LSK cells from Scl-Cre−:Fgfr1fx/fx mice (42%) than from Scl-Cre+:Fgfr1fx/fx mice (23%) at 12 days after 5FU (Figure 4E). We further examined whether NFκB pathway is functionally required for HSPC expansion in vitro. We found that in mixed BM cell culture, NFκB inhibitor (at 1μM and 5μM, respectively) reduced the expansion of CD34−LSKs in vitro without FGF (43% and 54%), with FGF1 (45% and 60%), or with FGF2 (36% and 84%; Figure 4F). Taken together, these data further support the conclusion that FGF1 and FGF2 signaling, mediated mainly by FGFR1 and potentially other receptors, such as FGFR3, are important for HSPC expansion, at least phenotypically, in response to BM damage.
FGFR1 inactivation reduces the number of megakaryocytes and the associated up-regulation of FGF induced by 5FU treatment
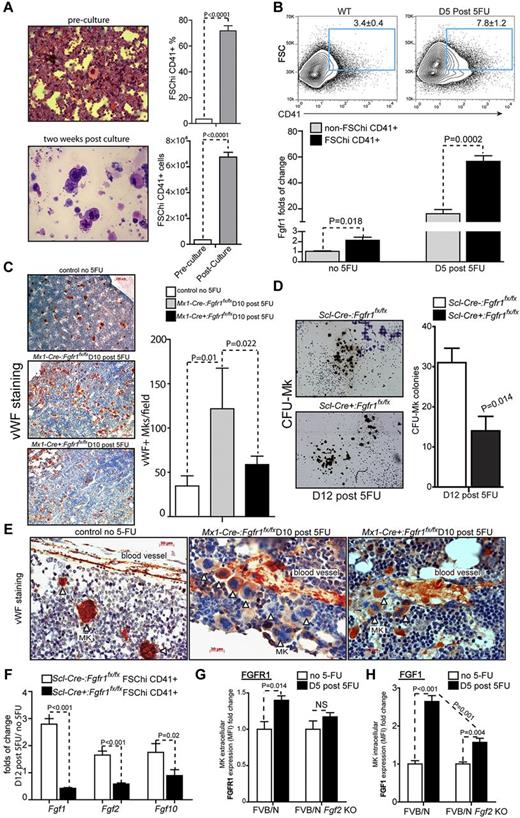
As observed in our previous report, HSPCs were surrounded by Mks35 at 2 weeks post culture as shown by cytospin and hematoxylin and eosin (H&E) staining (Figure 5A). We further measured the frequency and number of Mks post culture by analyzing FSChiCD41+ cells.40 The frequency of Mks initially was 3.4% but increased to 71.8% post culture, and the absolute number increased 20.6-fold (Figure 5A), suggesting that Mks may play a role in supporting HSPC expansion. Consistent with this observation, Mks have been reported to migrate to the endosteum in response to irradiation-induced BM damage to facilitate recovery and expansion of the osteoblastic niche, which in turn promotes HSC expansion.41 Next, we examined Mks response to 5FU-induced BM damage in C57Bl/6 BM in vivo. The frequency of Mks (FSChiCD41+) increased from 3.4% in BM in steady-state (no 5FU) to 7.8% in BM at 5 days after 5FU (Figure 5B). We also found that Mks (FSChiCD41+) in steady-state had relatively higher Fgfr1 levels (2.1-fold) than non-Mks (non-FSChiCD41+ cells). Strikingly, on day 5 after 5FU, Fgfr1 levels increased much more significantly in Mks (56.5-fold) than in non-Mks cells (16-fold; Figure 5B).
FGFR1 inactivation impairs megakaryocyte proliferation and FGF production induced by BM damage. (A) Cytospin followed by H&E staining and comparison of the frequency and absolute number of FSChi CD41-positive population in BM cells pre and post–2-week culture (n = 3). (B) Flow cytometric analyses of BM FSChiCD41+ cells and gene expression analysis of Fgfr1 using qRT-PCR on BM FSChiCD41+ and non-FSChiCD41+ cells from C57Bl/6 WT mice on the days indicated after 5FU (n = 4). (C) Mks stained using VWF (red) and hematoxylin (blue) to label nuclei. Quantification of the numbers of VWF+ Mks per field of view from BM and representative BM sections of Fgfr1 Mx1:Control, Mx1:CKO mice at 10 days after 5FU compared with day 0 (n = 3). (D) Quantification of CFU-Mks and representative CFU-Mk (brown colonies) from Fgfr1 Scl:Control, Scl:CKO BM at 12 days after 5FU. Representative data from 2 independent experiments. (E) Mks attached to blood vessel stained by VWF at 10 days after 5FU. (F) Gene expression analysis of Fgf1, 2 and 10 using qRT-PCR on BM Mks (FSChiCD41+ cells) from Fgfr1 Scl:Control and Scl:CKO mice on the days indicated after 5FU. (G-H) Expression analysis of FGFR1(G) and FGF1(H) using mean fluorescence intensity (MFI) on BM Mks (FSChiCD41+ cells) from FVB/N WT and FVB/N Fgf2 KO mice (n = 4).
FGFR1 inactivation impairs megakaryocyte proliferation and FGF production induced by BM damage. (A) Cytospin followed by H&E staining and comparison of the frequency and absolute number of FSChi CD41-positive population in BM cells pre and post–2-week culture (n = 3). (B) Flow cytometric analyses of BM FSChiCD41+ cells and gene expression analysis of Fgfr1 using qRT-PCR on BM FSChiCD41+ and non-FSChiCD41+ cells from C57Bl/6 WT mice on the days indicated after 5FU (n = 4). (C) Mks stained using VWF (red) and hematoxylin (blue) to label nuclei. Quantification of the numbers of VWF+ Mks per field of view from BM and representative BM sections of Fgfr1 Mx1:Control, Mx1:CKO mice at 10 days after 5FU compared with day 0 (n = 3). (D) Quantification of CFU-Mks and representative CFU-Mk (brown colonies) from Fgfr1 Scl:Control, Scl:CKO BM at 12 days after 5FU. Representative data from 2 independent experiments. (E) Mks attached to blood vessel stained by VWF at 10 days after 5FU. (F) Gene expression analysis of Fgf1, 2 and 10 using qRT-PCR on BM Mks (FSChiCD41+ cells) from Fgfr1 Scl:Control and Scl:CKO mice on the days indicated after 5FU. (G-H) Expression analysis of FGFR1(G) and FGF1(H) using mean fluorescence intensity (MFI) on BM Mks (FSChiCD41+ cells) from FVB/N WT and FVB/N Fgf2 KO mice (n = 4).
Because our results indicate that Mk expansion after 5FU is correlated with FGFR1 up-regulation, we therefore analyzed Mks stained by VWF in Mx1-Cre−:Fgfr1fx/fx and Mx1-Cre+:Fgfr1fx/fx BM 10 days after 5FU treatment. Quantification of VWF+ Mks showed that 5FU induced a significant increase (4.3-fold) in the number of Mks in BM compared with control mice (Figure 5C). Intriguingly, the number of Mks in BM from Mx1-Cre+:Fgfr1fx/fx mice was 51.7% less than from Mx1-Cre−:Fgfr1fx/fx mice (Figure 5C). In addition, using functional CFU-Mk assays, we found significantly fewer CFU-Mk colonies (54.8% less) in Scl-Cre+:Fgfr1fx/fx BM 12 days after 5FU than in Scl-Cre−:Fgfr1fx/fx BM (Figure 5D). In addition, we noticed that Mks were attracted to the perivascular area after 5FU treatment, and this aggregation was much reduced in Fgfr1 CKO mice (Figure 5E). This is consistent with a previous report that FGF signaling is involved in Mk recovery after myelosuppression, in which FGF4-enhanced Mk progenitor localization to the vascular niche, survival, and maturation.42
These observations raised the possibility that Mks are a major resource of FGF production and that Mks may themselves respond to FGF signals. To test whether Mks secrete FGFs that might support HSPC expansion under 5FU-induced stress, we measured FGFs in sorted Mks (FSChiCD41+) from Scl-Cre−:Fgfr1fx/fx and Scl-Cre+:Fgfr1fx/fx BM. Fgf1, Fgf2, and Fgf10 expressions were increased (2.8, 1.7, and 1.75-fold, respectively) after 5FU treatment in sorted Mks (FSChiCD41+) from Scl-Cre−:Fgfr1fx/fx mice but not from Scl-Cre+:Fgfr1fx/fx mice (Figure 5F). Furthermore, Fgf1 and Fgf2 mRNA levels declined in Scl-Cre+:Fgfr1fx/fx mice (58% and 42%, respectively) compared with the untreated group (Figure 5F). We further examined FGF1 and FGFR1 levels in the Fgf2 KO mouse model. Whereas protein levels of FGFR1 and FGF1 in MKs (FSChiCD41+) after 5FU treatment increased 40% and 1.64-fold, respectively, in the control mice, there was no significant increase of FGFR1 and a much lower increase (65.2% less) of FGF1 in MKs (FSChiCD41+) in Fgf2 KO mice (Figure 5G-H).
Taken together, these data further confirm that Mks serve as a major resource of FGFs, including FGF1 and FGF2 to support HSPC recovery during BM damage.
FGFR1 inactivation impairs HSPC mobilization induced by BM damage
The reduction of HSPCs in spleen after 5FU treatment (Figure 1D) suggested that FGFR1 may also affect the mobilization of HSPCs. To test this possibility, we monitored numbers of HSPCs in PB by flow cytometry. Under homeostasis, the number of circulating HSPCs is extremely low (Figure 6A). However, 12 days after 5FU, HSPC numbers in PB were substantially increased in control mice. In contrast, the numbers of HSPCs in PB derived from all 3 mouse models, Mx1-Cre+:Fgfr1fx/fx, Scl-Cre+:Fgfr1fx/fx, and Tek-Cre+:Fgfr1fx/fx, were significantly lower (96.9%, 67.5%, and 97.3%, respectively; Figure 6A). Again, we noticed that the absolute numbers of LSK cells were higher in Mx1-Cre−:Fgfr1fx/fx mice (4.9 × 104 LSKs/mL) than in Scl-Cre−:Fgfr1fx/fx (1.2 × 104 LSKs/mL) or in Tek-Cre−:Fgfr1fx/fx (1.16 × 104 LSKs/mL; Figure 6A), which is probably because of the previously mentioned pIpC side effect. Furthermore, we tracked hematopoietic progenitor cell (HPC) mobilization in response to 5FU by measuring changes in the total CFUs. We administered 5FU to Mx1-Cre−:Fgfr1fx/fx and Mx1-Cre+:Fgfr1fx/fx mice and performed CFU assays on PB at multiple intervals after treatment. Mx1-Cre−:Fgfr1fx/fx mice displayed a 3.2-fold greater number of CFUs than did Mx1-Cre+:Fgfr1fx/fx mice at 14 days after 5FU (Figure 6B). This is consistent with the report that mobilized HPCs peaked at 14 days after 5FU.43 According to the kinetics of 5FU-induced mobilization, the time window between day 10 and day 18 is important for HPC mobilization. Next, we performed a repopulation assay to test the functional HSPCs in the Fgfr1 CKO mice. We transplanted equal numbers of mononuclear cells from PB at 12 days after 5FU from Tek-Cre−:Fgfr1fx/fx and Tek-Cre+:Fgfr1fx/fx mice with rescue cells into lethally irradiated recipients. As shown in Figure 6C at 16 weeks after transplantation, PB cells from Tek-Cre+:Fgfr1fx/fx mice resulted in much lower engraftment (62% less) than those from Tek-Cre−:Fgfr1fx/fx mice.
FGFR1 inactivation impairs HSPC mobilization induced by BM damage. (A). Flow cytometric analyses of the LSK population in PB and/or comparison of absolute numbers of LSKs/mL in PB between Mx1:Control, Mx1:CKO at homeostasis and Mx1:Control, Mx1:CKO (n = 4), Scl:Control, Scl:CKO (n = 3), Tek:Control, Tek:CKO (n = 4) mice 12 days after 5FU. (B) Comparison of total CFUs from PB of Mx1:Control, Mx1:CKO, mice at 14 days after 5FU (n = 4). (C) PB mononuclear cells 6 × 105) from Tek:Control, Tek:CKO mice (CD45.2) transplanted 12 days after 5FU together with 2 × 105 rescue BM cells (CD45.1) into lethally irradiated Ptprc recipients PB analysis for total engrafted donor cells at 4, 8, 12, and 16 weeks posttransplantation (n = 5). (D) Flow cytometric analyses of the LSK population in PB and comparison of absolute numbers of LSKs/mL PB between of Mx1:Control, Mx1:CKO, Scl:Control, and Scl:CKO mice at 1 hour after AMD3100 treatment (n = 3).
FGFR1 inactivation impairs HSPC mobilization induced by BM damage. (A). Flow cytometric analyses of the LSK population in PB and/or comparison of absolute numbers of LSKs/mL in PB between Mx1:Control, Mx1:CKO at homeostasis and Mx1:Control, Mx1:CKO (n = 4), Scl:Control, Scl:CKO (n = 3), Tek:Control, Tek:CKO (n = 4) mice 12 days after 5FU. (B) Comparison of total CFUs from PB of Mx1:Control, Mx1:CKO, mice at 14 days after 5FU (n = 4). (C) PB mononuclear cells 6 × 105) from Tek:Control, Tek:CKO mice (CD45.2) transplanted 12 days after 5FU together with 2 × 105 rescue BM cells (CD45.1) into lethally irradiated Ptprc recipients PB analysis for total engrafted donor cells at 4, 8, 12, and 16 weeks posttransplantation (n = 5). (D) Flow cytometric analyses of the LSK population in PB and comparison of absolute numbers of LSKs/mL PB between of Mx1:Control, Mx1:CKO, Scl:Control, and Scl:CKO mice at 1 hour after AMD3100 treatment (n = 3).
The observed reduction in PB HSPCs in Fgfr1 CKO mice could be because of impaired HSPC migration and/or to impaired HSPC proliferation before BM egress to the bloodstream. To address this issue, we tested whether FGFR1 is required for HSPC mobilization in response to another clinically used mobilizing reagent, an SDF-1 inhibitor AMD3100. AMD3100 acts directly on HSPC-stromal cell interactions and results in rapid HSPC mobilization into the bloodstream.44 One hour after AMD3100 injection, we observed increased numbers of circulating HSPCs in Mx1-Cre−:Fgfr1 fx/fx and Scl-Cre−:Fgfr1fx/fx mice, indicating AMD3100-induced HSPC mobilization is independent of prior expansion. HSPC mobilization in Fgfr1 CKO mice was severely impaired (79.0% and 98.9% fewer absolute LSK cells in Mx1-Cre+:Fgfr1fx/fx and Scl-Cre+:Fgfr1fx/fx mice, respectively; Figure 6D). These data show that FGFR1-mediated signaling also promotes HSPC migration, and the rapid mobilization within 1 hour after induction excluded the possibility that HSPC expansion was involved in AMD3100-induced mobilization.
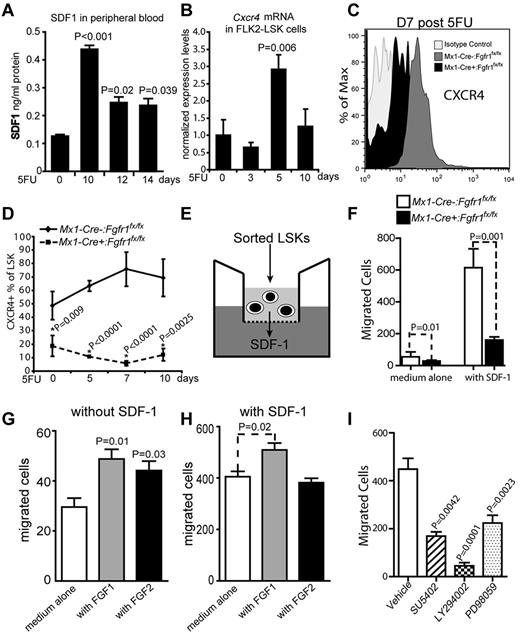
SDF-1 ligand is distributed in gradient in vivo and attracts HSPC migration through its cell surface receptor CXCR4 on HSPCs.23 Recently it was reported that SDF-1 is increased in PB after AMD3100 treatment, and functional CXCR4 on HSPCs is needed for AMD3100-induced migration.44 Therefore, we measured the protein level of SDF-1 in PB after 5FU treatment using ELISAs to compare PB supernatants collected from C57Bl/6 mice at homeostasis and multiple intervals after 5FU. Interestingly, SDF-1 protein level increased 3.5, 2.0, and 1.9-fold at day 10, 12, and 14 after 5FU, respectively (Figure 7A), correlating with the mobilization trend of HSPCs after 5FU (Figure 6B). The increase of SDF-1 level in PB led us to analyze the expression of its receptor, CXCR4, in HSPCs at multiple intervals after 5FU. We found that Cxcr4 mRNA was up-regulated and peaked at day 5, when HSPCs were expanding within BM and preparing to migrate,10,45 and then regressed at day 10, when HSPCs were possibly already mobilizing (Figure 7B). Furthermore, we used flow cytometry to compare surface protein levels of CXCR4 on HSPCs from Fgfr1 CKO and control mice after 5FU. The percentage of CXCR4+LSK cells was 2.6-fold higher in Mx1-Cre−:Fgfr1fx/fx mice than in Mx1-Cre+:Fgfr1fx/fx mice at homeostasis, and this difference increased to 12.7-fold at day 7 and 5.8-fold at day 10 after 5FU (Figure 7C-D).
FGFR1 inactivation disrupts SDF-1-CXCR4 pathway for HSPC migration. (A) SDF-1 protein level as determined by ELISA of PB supernatants of C57Bl/6 WT mice on days indicated after 5FU (n = 4). (B) Gene expression analysis of Cxcr4 using qRT-PCR on BM Flk2−LSK cells from C57Bl/6 WT mice on the days indicated after 5FU (n = 2). (C-D) Timeline of CXCR4 surface expression as a percentage of LSK cells in BM from Mx1:Control and Mx1:CKO mice after 5FU (n = 3). (E) Illustration of transwell migration assay. (F) Comparison of the chemotactic ability of Mx1:Control, and Mx1:CKO LSKs (n = 4). (G-H) Comparison of the chemotactic ability of LSKs from C57Bl/6 WT mice plus FGF1, FGF2, or with standard medium (G) without the presence of SDF-1 (n = 3) and (H) with the presence of SDF-1 (n = 3). (I) Comparison of the chemotactic ability of LSKs from C57Bl/6 WT mice in the presence of the following inhibitors: SU5402, LY294002, and PD98059 (n = 6). All results repeated at least 2 times.
FGFR1 inactivation disrupts SDF-1-CXCR4 pathway for HSPC migration. (A) SDF-1 protein level as determined by ELISA of PB supernatants of C57Bl/6 WT mice on days indicated after 5FU (n = 4). (B) Gene expression analysis of Cxcr4 using qRT-PCR on BM Flk2−LSK cells from C57Bl/6 WT mice on the days indicated after 5FU (n = 2). (C-D) Timeline of CXCR4 surface expression as a percentage of LSK cells in BM from Mx1:Control and Mx1:CKO mice after 5FU (n = 3). (E) Illustration of transwell migration assay. (F) Comparison of the chemotactic ability of Mx1:Control, and Mx1:CKO LSKs (n = 4). (G-H) Comparison of the chemotactic ability of LSKs from C57Bl/6 WT mice plus FGF1, FGF2, or with standard medium (G) without the presence of SDF-1 (n = 3) and (H) with the presence of SDF-1 (n = 3). (I) Comparison of the chemotactic ability of LSKs from C57Bl/6 WT mice in the presence of the following inhibitors: SU5402, LY294002, and PD98059 (n = 6). All results repeated at least 2 times.
These results led us to predict that HSPCs from Fgfr1 CKO mice would be defective in their ability to migrate through a microporous membrane in response to the chemotactic signal SDF-1 (Figure 7E).34 Indeed, in the SDF-1–induced migration assay, HSPCs isolated from Mx1-Cre+:Fgfr1fx/fx BM showed 2.7-fold fewer migrated cells than from Mx1-Cre−:Fgfr1fx/fx BM (Figure 7F). To further investigate whether FGF signaling directly facilitates HSPC mobilization, we examined the effects of FGF1 and FGF2 on HSPC migration. We found that adding FGF1or FGF2 led to 65% and 49% increase, respectively, in migrated LSK cells in the medium without SDF-1 (Figure 7G). And FGF1 instead of FGF2 increased migrated LSK cells 26% in the medium containing SDF-1 (Figure 7H). These data indicate that FGF, especially FGF1, may directly facilitate HSPC mobilization. We also studied the effects of different FGFR1 downstream pathways on LSK cell migration from BM of Mx1-Cre−:Fgfr1fx/fx mice. We found that an FGFR inhibitor (SU5402) reduced the number of LSK cells able to migrate by 2.9-fold; a PI3K inhibitor (LY294002) reduced the number 18.5-fold; and a MEK inhibitor (PD98059) reduced the number 2.1-fold (Figure 7I). These inhibitors did not affect the viability of HSPCs (supplemental Figure 6). As PI3K and MEK are key molecules in the 2 major downstream pathways of the FGF/FGFR signaling complex, these data support our results from FGFR1 inactivation and SU5402-treated LSK cells, and also indicate that FGFR1 signaling is involved in SDF-1–induced chemotaxis. Overall, these results indicate that FGFR1 signaling facilitates mobilization of HSPCs in response to stress.
Discussion
In this study, we used 3 mouse models to inactivate FGFR1. Mx1-Cre has a very high recombination efficiency but is not hematopoietic specific and also has a side effect from pIpC that affected our interpretation of the observed result. Scl-Cre is hematopoietic specific; however, incomplete gene deletion resulted in a misleading conclusion regarding a ST versus LT requirement of FGFR1 for HSC engraftment. Tek-Cre, mediating a constitutive gene deletion in both hematopoietic and endothelial tissues, has very high gene deletion efficiency. The observation that Tek-Cre dependent FGFR1 inactivation did not affect homeostatic hematopoiesis ruled out the possibility of not just intrinsic (HSPC) but also extrinsic (endothelial cells) influences from loss of FGFR1. Transplantation of BM derived from Fgfr1 CKO mice into wild type recipient mice further tested FGFR1 function when deleted from the hematopoietic system. The experimental results obtained from these models are consistent and complementary in both phenotype and functionality revealed by FGFR1 inactivation. Our results demonstrate that FGFR1-mediated signaling is important for HSPC proliferation and mobilization in vivo in response to BM damage but is not essential under homeostasis. However, we cannot rule out a potential compensation from FGFR3 in homeostasis.
The increase of expression of FGFR1 in HSPCs and their ligands FGF1 and FGF2 in BM after 5FU treatment indicate that FGF pathway activation is part of the hematopoietic stress response. We have shown that FGF signaling, mediated by FGF1 or FGF2 via by FGFR1, is required for both in vitro HSPC expansion and in vivo HSPC proliferation. We further showed that FGF1 may directly and that FGF2 may indirectly regulate HSPC expansion. This observation is consistent with the role of FGF signaling in regulating multiple cellular components and processes to promote survival and growth during cytotoxic stress.7,8,46
Regarding the potential source of FGFs in response to BM damage, we observed that Mks after 5FU had increased cell numbers and were enriched in the perivascular region. Because Mks also express CXCR4,42 the aggregation of Mks is possibly because of the increased SDF-1 level in PB after 5FU treatment (Figure 7A) as suggested by a previous report.42 Our data indicate that Mks support HSPC recovery by secretion of FGF factors, including FGF1 and FGF2; however FGF production by Mks and expansion of Mks postinjury are severely affected when FGF-FGFR signaling is blocked. Further study is required to investigate the role of Mks in facilitating HSPC proliferation and BM recovery.
Furthermore, the failure of Fgfr1 CKO mice to up-regulate NFκB in HSPCs, which is functionally required for HSPC expansion in vitro, partially explains the mechanism of FGFR1 on HSPC expansion, consistent with a previous report that MAPK stimulates NFκB transcription.47 In addition, AKT has been shown to regulate NFκB activity by controlling its subcellular localization, specifically its nuclear accumulation.48 NFκB inhibition may have nonspecific effects beyond FGFR signaling; the observation that failure to up-regulate NFkΒ in response to injury in Fgfr1 mutant HSPCs still suggests that regulation of NFκB by FGF signaling may play a role in supporting HSPC survival during expansion and mobilization.
In addition to affecting HSPC proliferation, FGFR1 inactivation directly affects HSPC migration from BM to bloodstream as evidenced by the mobilzation defect induced by interruption of SDF-1–CXCR4 interactions via AMD3100.49 Mechanistically, we found that SDF-1 was increased in PB, and that FGFR1 signaling was required for up-regulation of CXCR4 within HSPCs in response to BM damage, accounting for the diminished responsiveness of FGFR1-null HSPCs to stress-induced changes in SDF-1 gradients.50 In vitro migration assays (Figure 7I) confirmed that the downstream components of FGF signaling, AKT and MAPK, are involved in FGFR1-facilitated HSPC migration.
In summary, we provide the first in vivo evidence showing that FGFR1-mediated signaling is important for HSPC proliferation and mobilization in response to severe BM damage, though blockage of this pathway does not affect homeostatic hematopoiesis, thus opening a new avenue for improving HSPC mobilization before harvesting in conjunction with AMD3100, and for promoting blood recovery after BM damage.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Joachim Goethert for Scl-Cre mice; Brandy Lewis and Debra Dukes for technical support; Teri Johnson and Nannette Marsh for assistance with immunohistochemistry; Andrew Box and Craig Semerad for assistance with flow cytometry; Leanne Wiedemann, Joan Conaway, Jay Vivian, Patrick Fields, Mike Werle, and members of the Li laboratory for scientific discussion; and Karen Tannen for editing the paper.
This work was funded by the Stowers Institute for Medical Research. J.M.P. is a Fellow of the Leukemia & Lymphoma Society. A.V. is a recipient of an overseas associateship from Department of Biotechnology, Ministry of S&T, Government of India.
Authorship
Contribution: M.Z. and J.T.R. primarily conducted the experiments; T.I. and T.L. conducted ELISA assay for FGF protein measurement; J.M.P. assisted with in vitro HSPC culture; A.V. provided the RNA seq data; J.S.H. assisted with cell sorting; M.J.H. for genotyping; C.-X.D. provided the Fgfr1 line; X.C.H. helped with training and trouble shooting and performed quantitative RT-PCR; and L.L. directed the overall project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Linheng Li, Stowers Institute for Medical Research, 1000 E 50th St, Kansas City, MO 64110; e-mail: lil@stowers.org.
References
Author notes
M.Z. and J.T.R contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal