Abstract
The genetic modification of T cells with a suicide gene grants a mechanism of control of adverse reactions, allowing safe infusion after partially incompatible hematopoietic stem cell transplantation (HSCT). In the TK007 clinical trial, 22 adults with hematologic malignancies experienced a rapid and sustained immune recovery after T cell–depleted HSCT and serial infusions of purified donor T cells expressing the HSV thymidine kinase suicide gene (TK+ cells). After a first wave of circulating TK+ cells, the majority of T cells supporting long-term immune reconstitution did not carry the suicide gene and displayed high numbers of naive lymphocytes, suggesting the thymus-dependent development of T cells, occurring only upon TK+-cell engraftment. Accordingly, after the infusions, we documented an increase in circulating TCR excision circles and CD31+ recent thymic emigrants and a substantial expansion of the active thymic tissue as shown by chest tomography scans. Interestingly, a peak in the serum level of IL-7 was observed after each infusion of TK+ cells, anticipating the appearance of newly generated T cells. The results of the present study show that the infusion of genetically modified donor T cells after HSCT can drive the recovery of thymic activity in adults, leading to immune reconstitution.
Introduction
Over the past 2 decades, clinical outcome after hematopoietic stem cell transplantation (HSCT) has seen a constant improvement and is therefore the treatment of choice for high-risk hematologic malignancies.1-4 Nevertheless, even if we overlook the unsolved issue of primary disease recurrence, 2 intertwined problems continue to account for most of the treatment-related mortality in transplanted patients: GVHD and opportunistic infections. Lethality related to these complications is particularly high in HLA-mismatched transplantation, explaining why alternative HSC sources such as umbilical cord blood and haploidentical family donors are only used in highly specialized centers.
The therapeutic options to treat or prevent GVHD have expanded considerably with the introduction into clinical practice of powerful tools such as infliximab, antithymocyte globulin, high-dose cyclophosphamide, and extracorporeal photoapheresis.5,6 Nevertheless, none of these agents has yet demonstrated a clearcut clinical benefit in severe steroid-resistant GVHD, and all carry as a drawback the risk of prolonged and profound immune suppression.
Furthermore, antimicrobial drugs are still insufficient to treat opportunistic pathogens and the incidence of microbial reactivations and diseases remains high until a functional and complete immune recovery is attained.7 To face this threat, in the past decades, several centers have successfully developed antigen-specific cellular therapies targeting the most lethal pathogens in transplanted patients.8-11 However, these targeted adoptive cell therapies require being ready in a short time, are associated with considerable costs, and ultimately grant protection against a limited array of pathogens. Another attractive strategy to improve immune reconstitution after HSCT is to promote the de novo development of new T cells, which is often compromised in adult patients by age-related involution of the thymus, which can be aggravated by the concomitant occurrence of GVHD.12 The infusion of ex vivo matured T-cell precursors and several soluble factors implicated in thymic recovery and T-cell maturation (eg, keratinocyte growth factor, growth hormone, and IL-7) are currently under evaluation in preclinical and phase 1 clinical studies.13-15
Given the issues encountered with currently available therapies, several groups of investigators have attempted cell-processing approaches that manipulate graft features to avoid GVHD while preserving pathogen-specific T cells. Examples are the photodynamic or pharmacologic elimination of allospecific T cells from the graft,16-18 the infusion of products enriched in T-regulatory cells,19 and the ex vivo anergization of T cells contained in the graft, which are reactive for patient-specific antigens.20,21
A different strategy have been pursued by us and others, with the development of “suicide gene therapy.”22,23 In this approach, patients receive purified stem cell grafts, followed by infusions of donor T cells that have been ex vivo manipulated to express an exogenous gene (the “suicide gene”) that is able to confer sensitivity to specific prodrugs, which activate the gene product and selectively eliminate genetically modified cells. The safety and efficacy of lymphocytes modified by this approach have been tested in several preclinical and clinical studies.24-26 In a recent multicentric phase 1/2 clinical trial (TK007), the infusion of suicide gene–modified T cells after HSCT from HLA-haploidentical family donors provided rapid and effective GVHD control in all patients who required activation of the suicide machinery.27 Most of the patients who received genetically modified T cells achieved rapid T-cell immune recovery, with the development of a polyclonal repertoire protective against pathogens, leading to a significant long-term reduction of infection-related mortality compared with the rates reported after haploidentical HSCT in the absence of T-cell add-backs.28 We observed that infusion of the purified, gene-modified cells (TK+ cells) was necessary to achieve T-cell recovery; however, such reconstitution was progressively enriched on T lymphocytes negative for the suicide gene (TK− cells).
In the present study, we provide new insights into the biologic events leading to T-cell immune reconstitution after TK+-cell infusions, which had the unexpected indirect effect of reversing thymic aging and promoting the de novo generation of T cells from the donor-derived precursors contained in the graft.
Methods
Patients, procedures, and biologic samples
Fifty-four adult patients with high-risk hematologic malignancies were enrolled in an open, nonrandomized, prospective phase 1-2 clinical trial of haploidentical HSCT and infusions of donor lymphocytes engineered to express the HSV-TK suicide gene (the TK007 trial). Details on the results of the trial were described previously.27 Briefly, after a myeloablative conditioning regimen, patients received CD34+ peripheral blood HSCs that were positively selected to achieve a final median CD34+ count of 11.6 × 106/kg (range, 4.6-16.8), with only 1.1 × 104/kg contaminant donor CD3+ cells (range, 0.26-10.0). For genetic modification, donor PBMCs were activated with muromonab anti-CD3 in the presence of 600 IU/mL of IL-2 (EuroCetus) and transduced with the replication-competent retrovirus–free SFCMM-3 retroviral vector to transfer HSV-TK and a truncated form of the low-affinity nerve growth factor receptor (ΔLNGFR) marker. After magnetic immune selection, gene-modified T cells were analyzed for expression of surface markers, vitality, in vitro sensitivity to activation of suicide gene machinery by ganciclovir (GCV), absence of adventitious agents, replication-competent virus, or independent cell growth. Cells with a vitality of more than 70%, a purity greater than 90%, and that met all of the above specifications were released for clinical use. Starting 28 days after HSCT and from the initial dose of 1 × 106 cells/kg (amended during the trial to 1 × 107 cells/kg), infusions of genetically modified T cells were repeated at monthly intervals in the absence of GVHD or immune reconstitution, defined as reaching an absolute CD3 cell count superior to 100/μL in 2 consecutive samples. None of the patients who achieved immune reconstitution after TK-cell infusions received systemic administration of IL-2. Peripheral blood samples of patients were collected at serial time points during treatment follow-up after written informed consent approved from the San Raffaele institutional ethical committee in accordance with the Declaration of Helsinki.
Flow cytometric analysis
Absolute quantification of circulating T and TK+ cells in TK007 patients was performed according to the International Society for Cell Therapy immunologic gating protocol.29 To assess the phenotype of circulating T cells and the frequency of CD31+ recent thymic emigrants (RTEs), patient and healthy donor PBMCs were stained with mAbs specific for CD3, CD4, CD8, CD45RA, CD62L, CD31, and LNGFR (all from BD Biosciences). Seven-color immunophenotypic analysis was performed using a FACSCanto II flow cytometer (BD Biosciences) and data were processed using FCS Express Version 3.00 software (De Novo Software).
qPCR for the HSV-TK suicide gene
The presence of the HSV-TK gene sequences was analyzed in genomic DNA extracted from PBMCs by quantitative PCR (qPCR) using the TK forward (GGACACGTTATTTACCCTGTTTCG) and TK reverse (GCCCAGGCAAACACGTTATAC) primers and the FAM-TTGCTGGCCCCCAAC-MGB fluorescent probe. Results were normalized on quantification of the telomerase reference gene (forward primer: GGCACACGTGGCTTTTCG; reverse primer: GGTGAACCTCGTAAGTTTATGCAA; probe: VIC-TCAGGACGTCGAGTGGACACGGTG-TAMRA), and relative expression was compared with a reference sample (100% of cells positive for HSV-TK) was obtained using the 2-ΔΔCT formula. Sensitivity of the assay allowed detection of up to 1% of HSV-TK+ cells.
Quantification of sjTRECs
Real-time qPCR for single-joint TCR excision circles (sjTRECs) was performed as described previously,30 using GAPDH as a control to standardize DNA content. Briefly, amplification reactions were performed in a final volume of 25 μL containing 50 ng of genomic DNA isolated from PBMCs, TaqMan universal PCR master mix (PerkinElmer/Applied Biosystems), and the appropriate primers and probes. The number of sjTRECs per 100 ng of DNA was determined on the basis of a standard curve developed in-house by cloning the sequence of α1 circles from human cord blood genomic DNA into a plasmid vector and diluting the plasmid into human DNA from a cell line devoid of TRECs (K562) with a lower limit of detection of 3 copies/100 ng of genomic DNA.
Thymic measurement from chest CT scans
Chest computed tomography (CT) scans performed for diagnostic purposes were analyzed retrospectively by 2 independent experienced radiologists who were blinded to the patients' clinical history. The radiologists scored a thymic index (TI) based on a semiquantitative grading scale ranging from 0 (no soft tissue and thymus replaced entirely by fat) to 5 (mass-like appearance of concern for hyperplasia or thymoma).31
Quantification of serum cytokines
The Bio-Plex Pro Human Cytokine 4-plex array (Bio-Rad) was used to analyze simultaneously the concentrations of IL-2, IL-7, IL-15, and IL-17 in patient sera. All samples were analyzed in duplicate. For each studied cytokine, a high-sensitivity standard curve was prepared by serial dilutions of recombinant proteins. Data were analyzed using Bio-Plex Manager Version 5.0 software (Bio-Rad).
Anti-CMV IFN-γ–release assay
T cell–specific response against CMV of selected patients transplanted from CMV-seropositive donors was assessed by IFN-γ ELISPOT assay, as described previously.27 Briefly, IFN-γ release from 105 PBMCs was assessed after a 24-hour incubation with 104 fibroblasts obtained from patient punch-skin biopsies infected with CMV strain AD169. IFN-γ spots were counted using a KS-ELISpot Reader (Zeiss).
Results
Infusion of gene-manipulated donor T cells after transplantation prompts the recovery of a transgene-negative T-cell repertoire
Between 2002 and 2008, 54 adult patients underwent HSCT from HLA-haploidentical family donors for high-risk hematologic malignancies in a multicenter, open, nonrandomized, prospective phase 1-2 clinical trial (TK007). The infused graft was extensively selected for CD34+ cells, and contaminating T cells were 1.1 × 104/kg. To improve immune reconstitution while controlling GVHD, patients with documented engraftment of donor HSCs were enrolled to receive TK cells. Excluding criteria for infusion included the presence of CMV reactivations requiring ganciclovir treatment, GVHD, or spontaneous T-cell reconstitution, which was never observed at the time of the scheduled TK-cell infusions. Twenty-eight patients received serial monthly infusions of donor lymphocytes genetically modified to express HSV-TK and ΔLNGFR starting from day 28 after transplantation. All infused cell products displayed a high purity of TK+ cells (average, 94.69% ± 1.92%, n = 43; Figure 1A).
Infusion of purified TK+ cells after T cell–depleted HSCT prompts the immune recovery of TK−-naive T cells. (A) Composition of 43 genetically modified T-cell products infused into 25 patients enrolled in the TK007 clinical trial. After magnetic selection, the large majority of infused cells expressed the surface marker ΔLNGFR (TK+ cells, shown in black), whereas only a minor fraction was negative for the transgene (TK− cells, shown in white). (B) Absolute counts of circulating T (circles), B (squares), and natural killer (NK; triangles) lymphocytes in TK007 patients. Shown is the average with SD. (C) Absolute counts of circulating T cells in TK007 patients before (baseline) and at different time points after the infusion of TK+-cell add-backs (at immune reconstitution, defined as the attainment of a CD3 cell count above 100 cells/μL; at 6 months after HSCT; and at 12 months after HSCT). Histogram bars are subdivided to represent the relative frequency of circulating TK+ (in black) and TK− (in white) T cells at each time point. Shown is the average with SD. (D) Correlation between the frequency of PBMCs expressing on their surface the ΔLNGFR marker (on the x axis) and that of PBMCs having integrated in their genome the HSV-TK transgene as assessed by specific qPCR (on the y axis) in 25 patients enrolled in the TK007 trial. (E) Absolute counts of circulating TK+ (left panels) and TK− (right panels) T cells at different time points after HSCT and TK+ cell add-backs subdivided according to the CD4 or CD8 subtype (in black and white, respectively; top panels) and to their naive, central memory or effector phenotype (in black, gray, and white, respectively; bottom panels). Shown is the average with SD of the results obtained from 10 TK007 patients.
Infusion of purified TK+ cells after T cell–depleted HSCT prompts the immune recovery of TK−-naive T cells. (A) Composition of 43 genetically modified T-cell products infused into 25 patients enrolled in the TK007 clinical trial. After magnetic selection, the large majority of infused cells expressed the surface marker ΔLNGFR (TK+ cells, shown in black), whereas only a minor fraction was negative for the transgene (TK− cells, shown in white). (B) Absolute counts of circulating T (circles), B (squares), and natural killer (NK; triangles) lymphocytes in TK007 patients. Shown is the average with SD. (C) Absolute counts of circulating T cells in TK007 patients before (baseline) and at different time points after the infusion of TK+-cell add-backs (at immune reconstitution, defined as the attainment of a CD3 cell count above 100 cells/μL; at 6 months after HSCT; and at 12 months after HSCT). Histogram bars are subdivided to represent the relative frequency of circulating TK+ (in black) and TK− (in white) T cells at each time point. Shown is the average with SD. (D) Correlation between the frequency of PBMCs expressing on their surface the ΔLNGFR marker (on the x axis) and that of PBMCs having integrated in their genome the HSV-TK transgene as assessed by specific qPCR (on the y axis) in 25 patients enrolled in the TK007 trial. (E) Absolute counts of circulating TK+ (left panels) and TK− (right panels) T cells at different time points after HSCT and TK+ cell add-backs subdivided according to the CD4 or CD8 subtype (in black and white, respectively; top panels) and to their naive, central memory or effector phenotype (in black, gray, and white, respectively; bottom panels). Shown is the average with SD of the results obtained from 10 TK007 patients.
Patients who did not receive TK+-cell add-backs failed to attain T-cell immune reconstitution (defined as an absolute count of circulating T cells greater than 100/μL) and experienced a dismal clinical outcome, mostly because of infectious complications. Conversely, 22 of 28 patients who received the purified TK+ cells recovered protective T-cell counts at a median time of 74 days after transplantation; these counts were maintained stably over time, with a concomitant improvement in clinical outcome mostly because of reduction of late transplantation-related mortality.27
These results demonstrate that the infused TK+ cells were necessary and sufficient in most treated patients to achieve a robust T-cell reconstitution. Moreover, posttransplantation immune recovery of TK007 patients demonstrated persistently high counts of circulating natural killer cells and, mostly because of the use of rituximab as part of the conditioning regimen, low B-cell counts that were eventually recovered at 1 year after transplantation (Figure 1B).
Unexpectedly, during the immunologic follow-up of treated patients, we detected in the peripheral blood consistent numbers of CD3+ cells that were negative for the surface marker ΔLNGFR (TK− cells) starting from the time of immune recovery and progressively growing in frequency and absolute counts to dominate the newly reconstituting T-cell repertoire (Figure 1C). Hematopoietic chimerism in the BM and peripheral blood was full donor for all of these patients. Donor-derived TK− cells could not be detected in patients who did not receive genetically modified T cells or in those who did not experience TK+-cell engraftment, precluding any further analysis on the features of their T-cell repertoire.
qPCR for the HSV-TK gene performed on the peripheral blood of treated patients confirmed the results obtained from the immunophenotype analysis, demonstrating a tight correlation with the expression of the surface marker (r2 = 0.597, P < .0001), excluding the loss or down-regulation of ΔLNGFR expression on TK+ cells (Figure 1D).
TK− cells were enriched in CD4 T cells compared with their TK+ counterparts or their respective infused cell products. Moreover, only in the TK− subset could we detect a recovery of T lymphocytes with a naive phenotype (CD62L+CD45RA+), which were barely detectable at the time of immune reconstitution (4.25 cells/μL or 3.22% of CD3+ cells) and increased over time to reach physiologic cell counts at 1 year after HSCT (250.70 cells/μL or 24.20% of CD3+ cells), which is of particular relevance in a patient population with a median age of 55 years (range, 17-64; Figure 1E). No significant enrichment in γδ T cells or CD4+CD25+CD127dimFoxP3+ T-regulatory cells were seen at any time point in TK007 patients compared with healthy controls (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This intriguing observation led us to investigate further the biologic origin of TK− cells and to hypothesize that they might have originated in the host from the donor progenitors through a thymic-dependent pathway.
Appearance of sjTRECs occurs even in elderly patients after TK+-cell add-backs
SjTRECs are by-products of the physiologic rearrangement of the α-chain of the TCR, and are maintained as episomal DNA in newly generated T lymphocytes when they egress from the thymus; therefore, their quantification represents a reliable assessment of thymic output.12
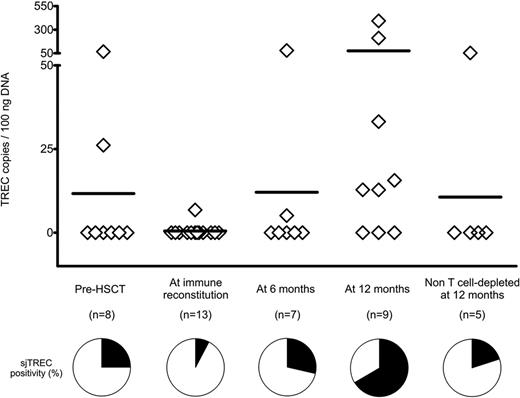
We measured longitudinally by qPCR the sjTREC counts in the peripheral blood of 13 patients treated with TK cells (Figure 2). As expected from the adult age of the patient cohort, sjTRECs at the time of transplantation were below the detection limit of the method in 6 of 8 patients (75%). At the time of immune reconstitution, sjTRECs were detectable in only 1 patient (7.7%). This results was consistent with the low numbers of circulating T cells at this early time point (14.67% of PBMCs), with a further dilution of the sjTREC-rich naive subset, a minor fraction of CD3+ cells (Figure 1D). Parallel to the increase in the numbers of naive T cells, sjTREC counts also increased over time, and at 1 year after HSCT, 6 of 9 patients (66.67%) had detectable circulating sjTRECs, demonstrating an important increase in thymic output compared with their pretransplantation levels and with an age-matched cohort of patients at 1 year after non–T cell–depleted haploidentical HSCT. Interestingly, 3 of 6 patients with detectable circulating sjTRECs were older than 50 years of age at the time of HSCT.
sjTRECs rise in the peripheral blood of TK007 patients after HSCT and suicide gene therapy. Absolute counts of sjTRECs per 100 ng of genomic DNA extracted from PBMCs of TK007 patients at different time points during their follow-up and, as control group, from patients who underwent non–T cell–depleted haploidentical HSCT. Pie charts below the graph represent the percentage of patients in whom circulating sjTREC counts were above (in black) or below (in white) the sensitivity of the detection method (3 TREC copies/100 ng of DNA).
sjTRECs rise in the peripheral blood of TK007 patients after HSCT and suicide gene therapy. Absolute counts of sjTRECs per 100 ng of genomic DNA extracted from PBMCs of TK007 patients at different time points during their follow-up and, as control group, from patients who underwent non–T cell–depleted haploidentical HSCT. Pie charts below the graph represent the percentage of patients in whom circulating sjTREC counts were above (in black) or below (in white) the sensitivity of the detection method (3 TREC copies/100 ng of DNA).
Naive TK− cells reconstituting after suicide gene therapy are RTEs
The surface Ig-like receptor CD31 (PECAM-1) is a cell adhesion and signaling receptor expressed on hematopoietic and endothelial cells. Among CD4+-naive T cells, positivity for CD31 can distinguish the sjTREC-rich RTEs from aged naive cells that did not encounter their cognate antigen and underwent homeostatic proliferation.32,33 The relative size of the CD31+ compartment among naive CD4 cells is an index of the contribution of thymic-dependent lymphopoiesis to the immune repertoire and decreases with age as thymic involution takes place.34,35
Because naive CD4+ cells were detectable at all time points after TK+ cell add-backs in treated patients (Figure 3A), we analyzed CD31 expression to quantify RTEs.
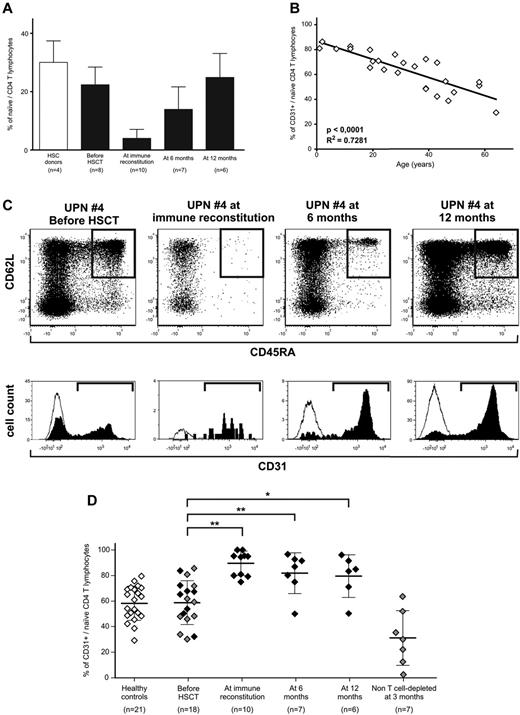
Naive T cells circulating after TK+-cell add-backs are CD31+ recent thymic emigrants. (A) Frequency of CD4-naive T cells among total CD3+ lymphocytes in healthy subjects (white bar) and in TK007 patients before treatment and at different time points during follow-up (black bars). Shown is average with SD. (B) Correlation between age of the subject (x-axis) and the frequency of CD31+ cells among the CD4-naive subset (y-axis) in 26 healthy subjects. (C) CD31 expression analysis in a representative patient from the TK007 clinical trial (UPN#4, age 57). In the top row are the dot plots of CD3+CD4+ΔLNGFR− T cells according to their expression of CD45RA (horizontal axis) and CD62L (vertical axis) at different time points during treatment. Gated in black are naive cells identified as CD45RA+ and CD62L+. Histograms below the dot plots represent CD31 positivity among naive CD4 cells (in black) and, as a negative control, among effectors (in white). (D) Frequency of CD31+ cells among naive CD4+ T lymphocytes in healthy subjects (white diamonds), in TK007 patients at different time points during follow-up (black diamonds), or in a control group of patients receiving haploidentical HSCT without T-cell depletion of the graft, followed by pharmacologic GVHD prophylaxis (gray diamonds). Shown is average with SD. *P < .05; **P < .001 in a paired-sample Student t test.
Naive T cells circulating after TK+-cell add-backs are CD31+ recent thymic emigrants. (A) Frequency of CD4-naive T cells among total CD3+ lymphocytes in healthy subjects (white bar) and in TK007 patients before treatment and at different time points during follow-up (black bars). Shown is average with SD. (B) Correlation between age of the subject (x-axis) and the frequency of CD31+ cells among the CD4-naive subset (y-axis) in 26 healthy subjects. (C) CD31 expression analysis in a representative patient from the TK007 clinical trial (UPN#4, age 57). In the top row are the dot plots of CD3+CD4+ΔLNGFR− T cells according to their expression of CD45RA (horizontal axis) and CD62L (vertical axis) at different time points during treatment. Gated in black are naive cells identified as CD45RA+ and CD62L+. Histograms below the dot plots represent CD31 positivity among naive CD4 cells (in black) and, as a negative control, among effectors (in white). (D) Frequency of CD31+ cells among naive CD4+ T lymphocytes in healthy subjects (white diamonds), in TK007 patients at different time points during follow-up (black diamonds), or in a control group of patients receiving haploidentical HSCT without T-cell depletion of the graft, followed by pharmacologic GVHD prophylaxis (gray diamonds). Shown is average with SD. *P < .05; **P < .001 in a paired-sample Student t test.
In a control cohort of 26 healthy subjects, CD31 expression in CD4-naive lymphocytes displayed a stringent correlation with age of the subject (r2 = 0.728, P < .0001; Figure 3B), with an average frequency of CD31+ RTEs of 58.28% ± 13.48% for adults.
The frequency of CD31+ RTEs measured in 18 adult patients before haploidentical HSCT for hematologic malignancies was comparable with that in age-matched healthy controls, demonstrating that no underlying disease or previous treatment had deregulated CD31 expression. Of these 18 patients, 8 were enrolled in the TK007 protocol and received a CD34-selected graft and gene-modified lymphocytes, whereas 10 underwent an unselected, non–T cell–depleted procedure followed by pharmacologic GVHD prophylaxis. As summarized in Figure 3D and exemplified for a representative patient in Figure 3C (UPN#4, age 57), we observed that in TK-cell–treated patients, almost all TK−-naive CD4 cells were positive for CD31 and were therefore bona fide RTEs (89.54% ± 9.55% at immune reconstitution, 81.84% ± 15.9% at 6 months after HSCT, and 79.55% ± 16.66% at 12 months after HSCT). Even at 1 year after transplantation, when naive T cells were fully recovered, more than 80% of these cells were thymic emigrants, indicating the continuous output of cellular elements with novel specificities. The control group of patients who underwent unselected HSCT, despite achieving comparable quantitative T-cell recovery with median CD3+-cell counts of 734 ± 474/μL at 90 days after HSCT, had very low frequencies of RTEs, suggesting that in this group of patients, peripheral expansion of donor T cells contained in the graft accounted for immune recovery.
Chest CT scans of patients treated with TK+ cells demonstrate regeneration of bioactive thymus
Irreversible, age-dependent fatty involution of thymic epithelia is the main cause of the low output of new T cells in adults. Chest CT scans can distinguish epithelial from adipose tissue, granting a noninvasive and reproducible way to measure the volume of the biologically active thymus.31,36 The volume of the CT-dense active thymus, expressed semiquantitatively as the TI, has been shown to decrease significantly over time in healthy subjects, reaching values below 1 (minimal soft tissue and barely recognizable) for subjects over the age of 50 years.
We evaluated retrospectively chest CT scans performed during follow-up of 28 patients enrolled in the TK007 study, 21 of whom received one or more infusions of purified TK+ cells.
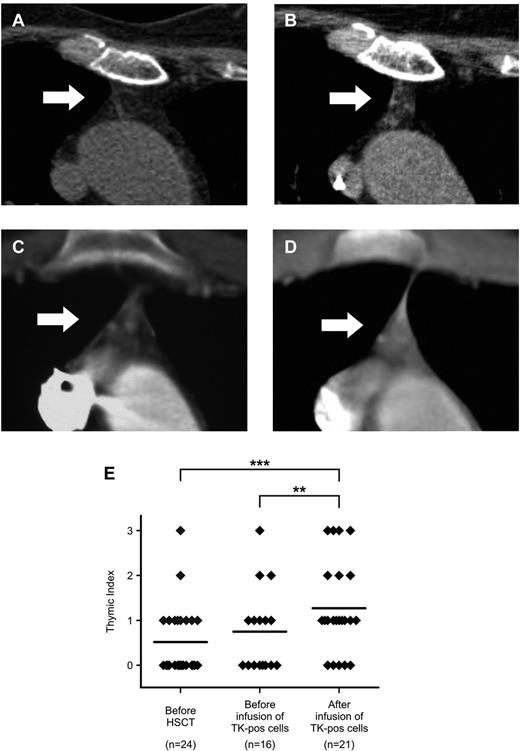
Consistent with their age, in most of our patients, no thymic soft tissue could be detected before HSCT (average TI, 0.48 ± 0.63). Infusion of the stem cell graft had a minimal effect on promoting thymic recovery, because in patients studied before TK+ cell infusions, no change in TI could be evidenced (mean TI, 0.75 ± 0.93; P = not significant). Conversely, even elderly patients treated with TK+ cells showed a consistent increase in the amount of soft tissue detectable by chest CT scan, with de novo appearance of well-defined tissue areas suggestive of bioactive epithelium (mean TI, 1.33 ± 1.01, P < .0001, vs before HSCT, P = .0039 vs before TK+-cell infusion; Figure 4A-E).
Chest CT scans document an increase in the volume of active thymic tissue after TK+-cell add-backs. (A-D) Detail on the thymic region (indicated by white arrows) from chest CT scans performed for diagnostic purposes in 2 representative TK007 patients (panels A-B: UPN# 25, age 64; panels C-D: UPN#15, age 17). Note the increase in density in the thymic region in the scans performed after TK+-cell add-backs (for panel B, TI = 2 and for panel D, TI = 3, performed at 50 and 135 days from last TK+-cell add-back, respectively) compared with their counterparts before treatment (panels A and C, both TI = 1). (E) Summary of results obtained from the retrospective analysis of 61 chest CT scans from TK007 patients, each scored a TI value by 2 independent expert radiologists (y-axis). **P = .0039; ***P < .0001 in a paired-sample Student t test.
Chest CT scans document an increase in the volume of active thymic tissue after TK+-cell add-backs. (A-D) Detail on the thymic region (indicated by white arrows) from chest CT scans performed for diagnostic purposes in 2 representative TK007 patients (panels A-B: UPN# 25, age 64; panels C-D: UPN#15, age 17). Note the increase in density in the thymic region in the scans performed after TK+-cell add-backs (for panel B, TI = 2 and for panel D, TI = 3, performed at 50 and 135 days from last TK+-cell add-back, respectively) compared with their counterparts before treatment (panels A and C, both TI = 1). (E) Summary of results obtained from the retrospective analysis of 61 chest CT scans from TK007 patients, each scored a TI value by 2 independent expert radiologists (y-axis). **P = .0039; ***P < .0001 in a paired-sample Student t test.
These results suggest the regeneration of the physiologic thymic tissue after the infusion of donor gene-modified T cells, which is paralleled by the emergence of RTEs and sjTRECs in the peripheral blood.
IL-7 serum levels peak after TK+-cell infusions and before T-cell reconstitution
Thymic activity and T-cell differentiation are tightly regulated by a group of concerted signals, including cell-to-cell interactions and soluble factors, among which IL-7 has been reported to play a major role.37 IL-7 is produced by stromal cells in the BM and thymus, where it is required for the development of mature T cells, and has a prominent role in the peripheral compartment, where it promotes the survival and proliferation of naive and memory T lymphocytes.38,39
We measured longitudinally the serum concentrations of IL-2, IL-7, IL-15, and IL-17 in selected TK007 patients who received TK+-cell add-backs. Neither IL-2 nor IL-17 could be detected in the peripheral blood of the patients studied and IL-15 was detectable only for the days immediately after HSCT conditioning, returning to basal levels by day 19 (data not shown).
A similar peak in serum concentration in the early posttransplantation days could be detected for IL-7, possibly induced by the lymphodepletion caused by the conditioning regimen (Figure 5). Strikingly, in most patients who experienced engraftment of the infused TK+ cells and T-cell recovery (7 of 8 patients studied), an additional sharp increase in the serum levels of IL-7 occurred early after the TK+-cell add-backs, reaching values not commonly observed in physiologic conditions (Figure 5B). This second peak in IL-7 serum concentration was often followed by a concomitant rise in peripheral T-cell counts, leading to immune reconstitution (Figure 5 dashed line). Interestingly, neither of the 2 patients who failed T-cell reconstitution after TK+-cell infusions showed an increase in IL-7 concentration (Figure 5D-E); this was also true for the only patient studied who received an unmanipulated T-cell add-back because of impending disease relapse (Figure 5A UPN#9, day 309).
TK+-cell add-backs prompt the systemic release of IL-7, leading to T-cell immune recovery. Line graphs indicate the serum concentration of IL-7 (solid lines) and the absolute T-cell counts (dashed lines) in 3 representative TK007 patients who experienced systemic engraftment of TK+ cells and subsequent immune reconstitution (3 of 12 studied patients are shown in the left panels) and in 2 of the 3 studied patients who received the suicide gene–modified cells but failed to attain T-cell reconstitution (right panels). Arrows indicate the time of HSCT (white), infusions of TK+ cells (black), or infusions of unmanipulated donor lymphocytes (gray).
TK+-cell add-backs prompt the systemic release of IL-7, leading to T-cell immune recovery. Line graphs indicate the serum concentration of IL-7 (solid lines) and the absolute T-cell counts (dashed lines) in 3 representative TK007 patients who experienced systemic engraftment of TK+ cells and subsequent immune reconstitution (3 of 12 studied patients are shown in the left panels) and in 2 of the 3 studied patients who received the suicide gene–modified cells but failed to attain T-cell reconstitution (right panels). Arrows indicate the time of HSCT (white), infusions of TK+ cells (black), or infusions of unmanipulated donor lymphocytes (gray).
Newly generated TK− cells ensure protective immunity after elimination of TK+ cells and control of GVHD
One of the major hurdles to the infusion of donor T cells in the context of HSCT from haploidentical donors is the extremely high risk of severe GVHD mediated by alloreactive T lymphocytes.40 This potentially lethal complication often requires prolonged immunosuppressive therapy, leading to loss of immune protection against pathogens and, ultimately, to an increase in infectious morbidity and mortality. In the TK007 clinical trial, 11 patients developed GVHD and all of them achieved complete resolution of all signs and symptoms by the activation of the suicide gene in TK+ cells through IV administration of GCV. As expected, this resulted in a consistent decrease in the counts of peripheral blood circulating TK+ cells, with only a minor effect on the numbers of TK− cells.27
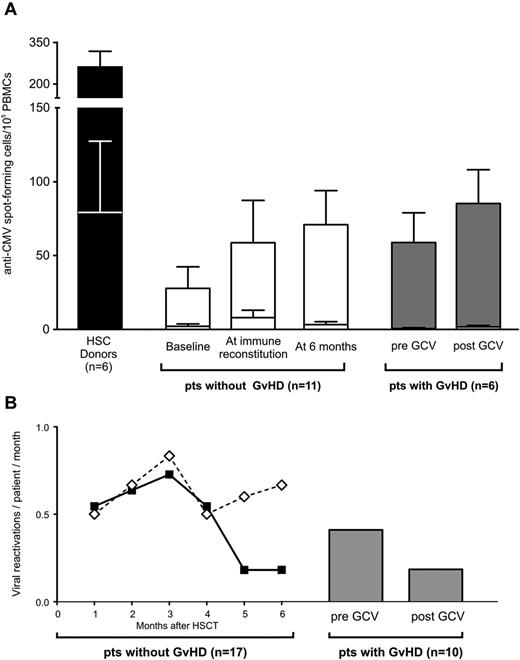
We tested the functional activity of T cells harvested from 6 TK007 patients who suffered GVHD against CMV, a life-threatening posttransplantation pathogen. In all of these patients, the frequency of T cells capable of releasing IFN-γ in response to CMV was not decreased after GCV treatment and elimination of TK+ cells, and was similar to that observed in patients who did not suffer GVHD (Figure 6A).
Once immune reconstitution is achieved, selective elimination of TK+ cells by activation of the suicide gene does not impair immunity against pathogens. (A) Histogram bars represent IFN-γ release in response to CMV-infected (top section of each bar) or uninfected (bottom section of each bar) fibroblasts by T cells harvested from HSC donors (in black), from TK007 patients who achieved T-cell immune reconstitution and did not experience GVHD (in white), and from TK007 patients who suffered GVHD assessed ex vivo before and after the administration of GCV and the resolution of GVHD (in gray). All studied patients were transplanted from CMV-seropositive donors. Shown is average with SEM. (B) Frequency of viral reactivations over time after HSCT in patients who received TK+ cells and achieved (solid line, black diamonds) or did not achieve (dashed line, white diamonds) T-cell recovery. Gray bars represent the frequency of viral reactivations in the 2 months preceding and after GVHD treatment in those TK007 patients in whom this complication occurred. Frequencies are expressed as number of viral reactivations per patient per month.
Once immune reconstitution is achieved, selective elimination of TK+ cells by activation of the suicide gene does not impair immunity against pathogens. (A) Histogram bars represent IFN-γ release in response to CMV-infected (top section of each bar) or uninfected (bottom section of each bar) fibroblasts by T cells harvested from HSC donors (in black), from TK007 patients who achieved T-cell immune reconstitution and did not experience GVHD (in white), and from TK007 patients who suffered GVHD assessed ex vivo before and after the administration of GCV and the resolution of GVHD (in gray). All studied patients were transplanted from CMV-seropositive donors. Shown is average with SEM. (B) Frequency of viral reactivations over time after HSCT in patients who received TK+ cells and achieved (solid line, black diamonds) or did not achieve (dashed line, white diamonds) T-cell recovery. Gray bars represent the frequency of viral reactivations in the 2 months preceding and after GVHD treatment in those TK007 patients in whom this complication occurred. Frequencies are expressed as number of viral reactivations per patient per month.
These ex vivo observations had a direct clinical counterpart: the frequency of viral reactivations did not increase in TK007 patients who experienced GVHD, remaining as low as in patients who achieved T-cell immune reconstitution in the absence of GVHD. Conversely, patients with no TK+-cell engraftment did not recover their immune repertoire against viruses and consequently experienced a much higher incidence of viral reactivations (Figure 6B).
These data demonstrate that the activation of the suicide gene machinery can abrogate GVHD without impairing physiologic immunity against pathogens, ultimately suggesting that once immune recovery is obtained after TK+-cell infusions, patients may rely on newly generated TK− cells for long-term protection against infections.
Discussion
Early recovery of a competent immune system to protect patients against opportunistic infections is a major open issue in the field of allogeneic HSCT. This is especially true in HLA-mismatched HSCT, a context in which donor T cells display an unfavorable balance between their antihost alloreactivity, the main determinant of GVHD, and their physiologic activity against pathogens.7 In the TK007 clinical trial, the infusion of donor T cells expressing the HSV-TK suicide gene after T cell–depleted HSCT promoted rapid immune reconstitution while allowing a safe mechanism of GVHD control in all patients facing this potentially lethal complication (11 of 11 or 100%).27 In the present study, we provide new insights into the immune recovery of TK007 patients, demonstrating that those genetically modified T cells promoted thymic renewal and the de novo development of T cells in adults. Other investigators have shown that T-cell infusions after HSCT can quicken immune reconstitution by accelerating the recovery of a polyclonal repertoire.41 The study context offered by TK007 is unique in allowing the in vivo tracking of infused, genetically marked donor T lymphocytes and their discrimination from other cells of donor origin infused with the graft that are negative for the transgene. This unique setting allowed us to show that the majority of T cells reconstituting after HSCT were not derived directly from peripheral expansion of the infused add-backs, but rather from the graft. Most naive lymphocytes expressed CD31, a hallmark of RTEs, which was confirmed by the reappearance of detectable sjTREC counts and bioactive thymic tissue in adult patients who were often older than 50 years of age. All of this evidence points to a thymic origin of the newly reconstituting T cells and helps to explain the clinical outcome of TK007 patients.27 In addition to efficient protection against infectious events, an unexpectedly low incidence of GVHD was recorded in these patients despite the absence of any pharmacologic immunosuppressive treatment. We can now speculate that this was because of the fact that the high numbers of T cells circulating in these patients had achieved central tolerance in the patient thymus, with negative selection of host-reactive cells.
The renewal of thymic activity was a direct consequence of the infusion of TK+ cells, as supported by several experimental results. First, in the few patients in whom TK+ cells were not infused for concomitant clinical reasons, we did not observe T-cell recovery, which is consistent with previous data on haploidentical T cell–depleted HSCT showing a time to T-cell reconstitution of up to 1 year.19,42 For this reason, we could not compare the quality of immune reconstitution obtained by TK-cell infusions with that observed after T cell–depleted haploidentical HSCT at similar time points. However, comparison with patients undergoing non–T cell–depleted HSCT showed that the infusion of mature donor T cells is not able by itself to enhance RTE generation in the thymus, as demonstrated by the low percentages of CD31+, CD4-naive T cells observed in this cohort of patients.
Another result supporting the direct role of TK+-cell add-backs in prompting thymic activity comes from the novel finding that these infusions are accompanied by a systemic release of IL-7, a relevant player in the early stages of T-cell maturation.37 The observation of the peak in the serum concentration of IL-7 elicited by TK+ cells is also intriguing because it apparently occurred exclusively in those patients in whom the add-backs led to immune reconstitution. Gene-expression profiling studies did not reveal IL-7 production by retrovirus-transduced T cells or by TK+ cells circulating in treated patients,43,44 so it is possible that this effect was achieved through the triggering of additional cellular mediators such as thymic epithelial cells, which are able to release IL-7 and propagate the process.38 IL-7 is not able to induce per se thymic renewal or thymopoiesis,15 so it should not be considered sufficient to attain the final effect of posttransplantation T-cell recovery.
Our finding that allogeneic T-cell infusions can prompt the renewal of the adult thymus are in apparent contradiction with historical data from animal models traditionally associating alloreactions with a detrimental effect on thymic output mainly because of the occurrence of thymic GVHD.45 Accordingly, GVHD is usually associated with low sjTREC counts and with a more profound posttransplantation immune deficiency.6,12 A possible explanation for the opposite effect mediated by the infusion of TK+ cells comes from the phenotype of the infused lymphocytes. To achieve efficient transduction by the retroviral vector, donor T cells underwent polyclonal stimulation with anti-CD3 Ab and IL-2, shifting their phenotype toward that of effector cells. Experimental transplantation studies have demonstrated that the risk of GVHD is correlated mainly with the presence of naive and central memory cells in the graft, whereas effectors pose a minor threat.46 This observation is also consistent with the overall low incidence of severe GVHD observed after infusions in TK007 patients despite the high numbers of haploidentical T cells that they received.27
Several studies on immune reconstitution after HSCT have consolidated the relevance of recovery of naive T lymphocytes, the polyclonal Vβ repertoire, and sjTREC counts as determinants of long-term clinical outcome.47,48 However, it must be taken into account that patients receiving HSCT face a 20-fold higher risk of infections after the procedure, which are a consistent cause of morbidity and mortality.49 TK007 patients displayed all of the features of a robust immune recovery, possibly explained by the continuous output of new T-cell specificities and, accordingly, no serious infectious event has been registered in any of the treated patients after day 166 (last death) from HSCT.
The process that we describe herein is long-lasting and self-maintaining: the frequency and absolute counts of naive T cells and CD31+ RTEs kept increasing over time. Consistent with this finding, serial CT scans obtained from patients after the add-backs showed stability of the active thymic tissue appearing after the infusions. The elucidation of the fine mechanisms underlying the long-term reversal of thymic aging would be a relevant step forward in the field, because most of the molecules currently in clinical use or under study to boost thymopoiesis and ameliorate posttransplantation immunity cease their stimulatory effect once discontinued.14
Finally, in the present study, activation of the suicide gene and elimination of TK+ cells in GVHD did not come at the price of losing immune competence against pathogens, allowing us to separate the detrimental GVHD reaction from the desired graft-versus-infection effect. Nevertheless, the direct contribution of TK+ cells to the outcome of HSCT should be emphasized. In fact, the process of thymic renewal that they promote is lengthy and unlikely to protect against pathogens in the early months after transplantation. Therefore, infusion of the polyclonal effector TK+ cells may have a major role as a first line of defense, bridging the recovery of the new T-cell repertoire matured in the thymus. Moreover, it is unlikely that the newly reconstituted T lymphocytes, possibly tolerized to host antigens during their development, may display antitumor activity. Conversely, the emergence of mutant variants of the original leukemia with de novo loss of the patient-specific HLA after serial TK+-cell infusions in 2 TK007 patients is a strong indirect evidence of the robust antileukemic potential of TK+ cells.27,50
The results of the present study suggest that thymopoiesis is reactivated after the infusion of gene-modified lymphocytes, prompting the generation of a protective T-cell compartment that is able to reduce the incidence of infectious events after HSCT. Not only was a fully competent immune system reestablished, but also the morbidity and mortality related to GVHD was reduced dramatically in patients treated with TK+ cells. The combined effects of reduction of infections and control of GVHD, for which our novel findings provide a biologic explanation, ultimately result in a considerable gain in terms of clinical outcome.27 A multicenter, randomized phase 3 clinical trial (the TK008 study) to assess the efficacy of TK+ cells in the context of haploidentical HSCT for leukemia started in 2010 in Italy and is currently expanding to multiple centers throughout Europe and the United States.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Italian Ministry of Health, the Italian Ministry of University and Research, the Associazione Italiana Ricerca sul Cancro, and the Cariplo Foundation.
Authorship
Contribution: L.V. designed the study and performed the experiments; G.O., M.N., I.B., and S.F. performed the experiments; A.B., C.T., A.A., C. Bordignon, and C. Bonini provided scientific counseling; C.S., D.G., and A.D.M. analyzed the CT scans; R.G., M.T.L.S., J.P., M.D.F., and F.C. supervised the clinical care of the patients; and L.V., G.O., and C. Bonini wrote the manuscript.
Conflict-of-interest disclosure: S.F., M.D.F., C.T., and C. Bordignon are employees of MolMed SpA. C. Bonini has a research contract with MolMed SpA. The remaining authors declare no competing financial interests.
Correspondence: Dr Chiara Bonini, Experimental Hematology Unit, San Raffaele Scientific Institute, via Olgettina 60, Milano, Italy; e-mail: bonini.chiara@hsr.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal