Abstract
Loss of the fibroblastic reticular cell (FRC) network in lymphoid tissues during HIV-1 infection has been shown to impair the survival of naive T cells and limit immune reconstitution after antiretroviral therapy. What causes this FRC loss is unknown. Because FRC loss correlates with loss of both naive CD4 and CD8 T-cell subsets and decreased lymphotoxin-β, a key factor for maintenance of FRC network, we hypothesized that loss of naive T cells is responsible for loss of the FRC network. To test this hypothesis, we assessed the consequences of antibody-mediated depletion of CD4 and CD8 T cells in rhesus macaques and sooty mangabeys. We found that only CD4 T-cell depletion resulted in FRC loss in both species and that this loss was caused by decreased lymphotoxin-β mainly produced by the CD4 T cells. We further found the same dependence of the FRC network on CD4 T cells in HIV-1–infected patients before and after antiretroviral therapy and in other immunodeficiency conditions, such as CD4 depletion in cancer patients induced by chemotherapy and irradiation. CD4 T cells thus play a central role in the maintenance of lymphoid tissue structure necessary for their own homeostasis and reconstitution.
Introduction
Highly active antiretroviral therapy (HAART) has had a great impact on decreasing morbidity and mortality in HIV infection1 by suppressing HIV replication and restoring CD4 T-cell populations to levels where the immune system can better control opportunistic infections and cancers associated with AIDS. However, controlling viral replication has not necessarily led to full reconstitution of the immune system. More than one-fourth of the patients after years of HAART still have CD4 T-cell counts not significantly increased from pretreatment levels and/ or below the critical threshold of 200 cells/mm2 ; and even in patients with significant increases in peripheral CD4 T-cell counts, few reach the levels in uninfected populations after long-term HAART.2-10
Limited immune reconstitution is most prevalent in patients starting HAART in the chronic stage of disease (CD4 < 350 cells/μL) and in older age patients, and this failure in reconstitution strongly correlates with significantly higher morbidity and mortality.4-6,9,11-13 Further, the magnitude of CD4+ T-cell reconstitution in peripheral blood does not necessarily reflect the real magnitude of immune reconstitution in lymphoid tissues (LTs) where these cells mostly reside. Compared with the pace and extent of restoration of peripheral blood CD4 T cells, the normalization of LT CD4 T cell is significantly slower and less significant.14-20
There are important functional immunologic abnormalities that accompany this limited restoration of T cells. These include persistently poor vaccine responses,21,22 increased frequency of reactivation of latent herpes simplex infection and human papilloma virus infections,23-25 and other less well-characterized persistent defects in immune function that probably contribute to the increasing incidence of non-AIDS–related clinical events, such as cardiovascular disease, liver disease, and non-AIDS–related cancer,26-29 and increased susceptibility to bacterial infections.30 These enduring and pervasive defects in immune surveillance despite the great benefits conferred by suppression of viral replication point to the importance of understanding the mechanisms that limit immune reconstitution after HAART to devise strategies to improve outcomes.
HIV and SIV infections' greatest impact on immune reconstitution is depletion-naive T-cell populations, and this is also the case in immunodeficiencies caused by chemotherapy and irradiation treatment of cancer or patients receiving allogeneic hematopoietic stem cell transplantation.20,31-38 In these conditions, the loss of naive T cells is greater than in other T-cell populations, and the restoration of naive T cells is slower and to lower levels than other T-cell subsets with HAART or cessation of cancer treatments.20,31-33,36,37
Although the mechanisms underlying depletion and impaired immune reconstitution particularly of naive T cells have yet to be fully defined, we do know that damage to LT structure plays an important role. Because naive T cells within secondary LTs rely for their survival on interacting with the fibroblastic reticular cell (FRC) network in the T-cell zone to supply factors, such as IL-7 and self-antigen–major histocompatibility complex signals,39-43 LT damage resulting from the losses of the FRC network and collagen deposition in HIV-1 and pathogenic SIV infection of rhesus macaques (Macaca mulatta) leads to loss of production of IL-7. This limits access of naive T cells to the source of IL-7, thereby impairing the survival of naive T cells.44,45 The damaged LT structure also recovers very slowly and incompletely when HAART is initiated during the chronic stage of infection, which perpetuates the elevated level of apoptosis in naive T cells to thereby limit the reconstitution of naive T cells.45 Thus, LT structure, and particularly the FRC network, is critical for the life or death particularly of naive T cells, and a critical factor in depletion and immune reconstitution.
The causes of the loss of FRC networks during HIV-1 infection are unknown, but there are good reasons to suspect that lymphotoxin-β contributes to FRC depletion. First, lymphotoxin-β, together with lymphotoxin-α, can trigger the lymphotoxin-β receptor-signaling pathway to maintain the stromal FRC network in T-cell zone and follicular dendritic cell network in B-cell follicles.44,46-51 Second, during SIV infection in rhesus macaques, there is a coordinate loss of lymphotoxin-β associated with T cells and depletion of the FRC network.44
These findings led to a hypothetical model in which loss of T cell–derived lymphotoxin-β during HIV-1 infection would lead to loss of FRC network, which in turn would deplete primarily naive T cells. Then the interdependencies between T cells and the FRC network for survival factors create a vicious cycle perpetuating loss of both networks and T cells.44
In this model, the source(s) of lymphotoxin-β associated with T-cell loss were not identified, but, from in vitro experiments, there were reasons to suspect that naive CD4 T cells might be a major source.46,52 If this were the case, the vicious cycle model would be “closed” because the loss of naive CD4 T cells through collagen-denied access to IL-7 would be the cause of the loss of lymphotoxin-β and the FRC network that compounds and perpetuates the loss of primarily naive CD4 T cells.
However, naive CD8 T cells are also depleted, so that they also could be responsible for the loss of lymphotoxin-β. In the studies we now describe, we therefore examined the effects of anti-CD4 and anti-CD8 antibody depletion of CD4 and CD8 T cells in nonhuman primate models to understand how depletion of each subset affected the FRC network. We first show that naive CD4 T cells are indeed the principal source of lymphotoxin-β for maintenance of the FRC network because antibody-mediated depletion of CD4 T cells, but not CD8 T cells, in rhesus macaques reproduced the depletion of the FRC network in SIV-infected rhesus macaques and HIV-1 infection. Furthermore, we found that, in the nonpathogenic model of SIV infection of sooty mangabeys (Cercocebus atys),53,54 the maintenance of CD4 T cells in sooty mangabeys correlated with maintenance of the FRC network and that CD4 T-cell depletion by antibody in uninfected sooty mangabeys, also significantly depleted the FRC and follicular dendritic cell networks, reproducing the pathogenic effect in SIV-infected rhesus macaques.
In HIV-1 infection, we also found that the loss of CD4 T cells in LTs correlates with the depletion of FRC network before HAART and with the extent of restoration after HAART. Lastly, we extend our conclusions to the more general case of CD4 T-cell depletion induced by chemotherapy and irradiation in cancer patients. We thus provide in vivo evidence to show that the decreased production of lymphotoxin-β resulting from depletion of CD4 T cells leads to loss of FRC networks in various immunodeficiency conditions. It is thus critical to minimize CD4 T-cell loss and preserve LT structure to improve immune reconstitution.
Methods
Ethics statement
This human study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board of the University of Minnesota. All patients provided written informed consent for the collection of samples and subsequent analysis.
LN biopsy specimens
Inguinal LN biopsies from HIV negative persons and HIV-1–infected persons at different clinical stages (7 at acute/early stage, 18 at presymptomatic stage, and 8 at AIDS stage)45 were obtained for this University of Minnesota Institutional Review Board–approved study. Viral load measurements were obtained the same day as biopsies. Each LN biopsy was immediately placed in fixative (4% neutral buffered paraformaldehyde or Streck tissue fixative) and paraffin embedded. LN biopsies from cancer patients are provided by the University of Minnesota Biologic Materials Procurement Network (BioNet).
Animals, SIV infection, and LN biopsy specimens
Adult rhesus macaques and sooty mangabeys used in these studies were housed in accordance with the regulations of the American Association of Accreditation of Laboratory Animal Care and the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal studies were approved by the University of Pennsylvania and Emory University Institutional Animal Care and Use Committees. For the chronically infected rhesus macaque and sooty mangabey study, LTs were obtained in longitudinal studies from 5 rhesus macaques that were inoculated intravenously with 10 000 TCID50 of SIVmac239 (generous gift from R. Desrosiers, the New England Primate Research Center, Southborough, MA), additional LTs from 5 rhesus macaques obtained in previously described cross-sectional studies.44,55 Infection of 5 sooty mangabeys by intravenous inoculation with 1 mL of plasma from an experimentally SIVsmm-infected sooty mangabey sampled at day 11 after infection with a viral load of 1 × 107 copies/mL of plasma. In addition, 4 naturally SIV-infected and 3 uninfected sooty mangabeys were included in the cross-sectional analysis. Blood collection was performed by venipuncture. Each LN biopsy was immediately placed in fixative (4% neutral buffered paraformaldehyde or Streck tissue fixative) and paraffin embedded. For the CD4 and CD8 T cell–depletion study, the animals and LTs isolation were previously described in detail.56 Briefly, depletion of CD4 and CD8 lymphocytes was performed using 10 mg/kg intravenous anti-CD4 mAb (OKT4A) on day −10 and 5 mg/kg on days −7, −3, and 0, whereas for CD8 lymphocyte depletion animals were treated with 4 mg/kg intravenous anti-CD8 mAb (OKT8F) on days −2, −1, and 0, a protocol that has been shown to deplete CD4 and CD8 lymphocytes in vivo in both rhesus macaques and sooty mangabeys.56 Blood and LN collection was performed at baseline and at different time points after the last antibody administration. Each LN biopsy was immediately placed into 70% ethanol immediately upon collection, then 24 hours later transferred to 4% PFA or Streck fixative and paraffin embedded.
Immunofluorescence staining, immunohistochemistry staining, and quantitative image analysis
All staining procedures were performed as previously described using 5- to 10-μm tissue sections mounted on glass slides.45,55 Tissues were deparaffinized in 60°C incubator for 2 hours and rehydrated through graded ethanols and rehydrated in deionized water. Heat-induced epitope retrieval was performed using a high-pressure cooker (125°C) in either DIVA Decloaker or EDTA Decloaker (Biocare Medical), followed by cooling to room temperature. Tissue sections were washed with PBS with 0.5% Tween 20 (Sigma-Aldrich) and then blocked with Fc receptor blocker (Innovex) for 30 minutes and SNIPER Blocking Reagent (Biocare Medical) for 30 minutes at room temperature. Primary antibodies were diluted in TNB (0.1M Tris-HCl, pH 7.5, 0.15M NaCl, 0.05% Tween 20 with Dupont blocking buffer) and incubated overnight at 4°C. After the primary antibody incubation, sections were washed with PBS with 0.5% Tween 20 for 3 times and then incubated with fluorochrome-conjugated secondary antibodies (AlexaFluor-488, -586, and -647–conjugated antibodies) in TNB for 2 hours at room temperature. Finally, sections were washed with PBS, and nuclei were counterstained blue with 4,6-diamidino-2-phenylindole and mounted using Aqua Poly/Mount (Polysciences Inc). Immunofluorescent micrographs were taken at room temperature using an Olympus FV1000 Fluoview confocal microscope with the following objectives: ×20 (0.75 NA), ×40 (0.75 NA), and ×60 (1.42 NA).
Immunohistochemistry staining
After overnight incubation of primary antibody at 4°C, tissues were then washed with PBS with 0.5% Tween 20 for 3 times. Endogenous peroxidase inactivated with 3% (volume/volume) H2O2 in PBS for 10 minutes. Tissues were then washed with PBS with 0.5% Tween 20 for 3 times. Primary antibody was detected with Mach-3 (Biocare Medical) and DAB kits (Vector). Stained sections were examined by light microscopy at ambient temperatures. Light micrographs were taken at room temperature using an Olympus BX60 upright microscope with the following objectives: ×10 (0.3 NA), ×20 (0.5 NA), and ×40 (0.75 NA); images were acquired using a Spot color mosaic camera (model 11.2) and Spot acquisition software (Version 4.5.9; Diagnostic Instruments).
Isotype-matched negative control antibodies in all instances yielded negative staining results (see Table 1, which lists the primary antibodies and antigen retrieval methodologies).
List of primary antibodies and antigen retrieval methodologies
| Antibody . | Clone/manufacturer and catalog no. . | Antigen-retrieval pretreatment . | Antibody dilution . | Species . |
|---|---|---|---|---|
| Desmin | D33/Lab Vision; no. MS-376-S1 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/200 | Mouse |
| Desmin | Polyclonal/Lab Vision; no. RB-9014-P1 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/200 | Rabbit |
| CD35 | Ber-MAC-DRC/Dako North America; no. M0846 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Mouse |
| CD21 | 1F8/Dako North America; no. M0784 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Mouse |
| IL-7 | 7417/R&D Systems; no. MAB207 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C Proteinase K treatment for 15 minutes | 1/100 | Mouse |
| Lymphotoxin-β | 135105/R&D Systems; no. MAB1684 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Mouse |
| CD4 | Polyclonal/R&D Systems; no. AF-379-NA | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Goat |
| CD4 | 1F6/Novacastra; no. NCL-CD4-1F6 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Mouse |
| CD8 | SP16/Neomarkers; no. RM-9116-s | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Rabbit |
| Lymphotoxin-β | Polyclonal/Santa Cruz Biotechnology; no. sc-23561 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Goat |
| In Situ Cell Death Detection Kit | Roche Applied Science; no. 1 684 817 | |||
| IgG isotype controls | Dako North America, Jackson ImmunoResearch Laboratories | Diva Decloaker; high pressure cooker for 30 seconds at 125°C Protease K (10 μg/mL) | 1/100 | Mouse, rabbit, goat |
| Antibody . | Clone/manufacturer and catalog no. . | Antigen-retrieval pretreatment . | Antibody dilution . | Species . |
|---|---|---|---|---|
| Desmin | D33/Lab Vision; no. MS-376-S1 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/200 | Mouse |
| Desmin | Polyclonal/Lab Vision; no. RB-9014-P1 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/200 | Rabbit |
| CD35 | Ber-MAC-DRC/Dako North America; no. M0846 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Mouse |
| CD21 | 1F8/Dako North America; no. M0784 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Mouse |
| IL-7 | 7417/R&D Systems; no. MAB207 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C Proteinase K treatment for 15 minutes | 1/100 | Mouse |
| Lymphotoxin-β | 135105/R&D Systems; no. MAB1684 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Mouse |
| CD4 | Polyclonal/R&D Systems; no. AF-379-NA | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Goat |
| CD4 | 1F6/Novacastra; no. NCL-CD4-1F6 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Mouse |
| CD8 | SP16/Neomarkers; no. RM-9116-s | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Rabbit |
| Lymphotoxin-β | Polyclonal/Santa Cruz Biotechnology; no. sc-23561 | Diva Decloaker; high pressure cooker for 30 seconds at 125°C | 1/100 | Goat |
| In Situ Cell Death Detection Kit | Roche Applied Science; no. 1 684 817 | |||
| IgG isotype controls | Dako North America, Jackson ImmunoResearch Laboratories | Diva Decloaker; high pressure cooker for 30 seconds at 125°C Protease K (10 μg/mL) | 1/100 | Mouse, rabbit, goat |
Quantitative image analysis was performed using 10 to 20 randomly acquired, high-powered images (original magnification ×200 or ×400) by either manually counting the cells in each image or by determining the percentage of LT area occupied by positive fluorescence signal using an automated action program in Adobe Photoshop CS with tools from Reindeer Graphics.
Statistical analysis
Data analysis using a Student t test, 1-way analysis of variance with a Bonferroni correction, and linear regression analysis was performed using Prism Version 5.01 (GraphPad Software).
Results
CD45RA+ naive CD4 T cells are the major producers of lymphotoxin-β
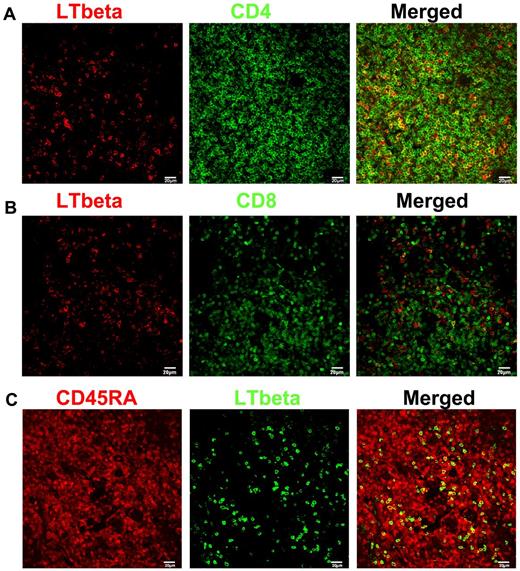
We had previously shown that lymphotoxin-β was predominantly expressed in T cells in LT and now asked in which T-cell subset lymphotoxin-β might be produced by colocalizing lymphotoxin-β with cell markers. We found that approximately 60% to 70% of the lymphotoxin-β colocalized with CD4 T cells, and mainly in the CD45RA+ naive subset (Figure 1). This conclusion is consistent with previous findings on lymphotoxin-α, suggesting that naive CD4 T cells are the key cell subset for the maintenance of the FRC network.46,52 However, given the fact that a minor subset of memory CD4 T cells (∼ 1%) are also expressing CD45RA, this minor memory population might also be a potential minor source of lymphotoxin-β.57
CD4+ T cells are the major producers of lymphotoxin-β (LTbeta). (A-B) Immunofluorescent staining of LTbeta (red), CD4 or CD8 (green) in LNs from rhesus macaques (RMs), showing that LTbeta is mainly expressed by CD4+ T cells. (C) Immunofluorescent staining of CD45RA (red), LTbeta (green) in LNs from RMs, showing that LTbeta is mainly expressed by naive T cells.
CD4+ T cells are the major producers of lymphotoxin-β (LTbeta). (A-B) Immunofluorescent staining of LTbeta (red), CD4 or CD8 (green) in LNs from rhesus macaques (RMs), showing that LTbeta is mainly expressed by CD4+ T cells. (C) Immunofluorescent staining of CD45RA (red), LTbeta (green) in LNs from RMs, showing that LTbeta is mainly expressed by naive T cells.
Sparing of FRC and follicular dendritic cell networks in SIV-infected sooty mangabeys and loss of both networks with CD4 depletion
Because the depletion of CD4 T cells, particularly naive CD4 T cells within LTs, strongly correlates with the progression of HIV infection,20,58 we tested one prediction of the hypothesis that CD4 T cells play a critical role in maintenance of the FRC network by examining the correlation between depletion of CD4 T cells and the FRC network in pathogenic SIV infection of rhesus macaque and comparing this result with nonpathogenic SIV infection of sooty mangabey where CD4 T cells are not significantly depleted in LNs.39,59 Because destruction of the follicular dendritic cell network in the B-cell follicles parallels CD4 T-cell depletion in HIV and pathogenic SIV infections, we also examined and compared the impact of SIV infection on the follicular dendritic cell network when CD4 T cells are depleted or preserved.60
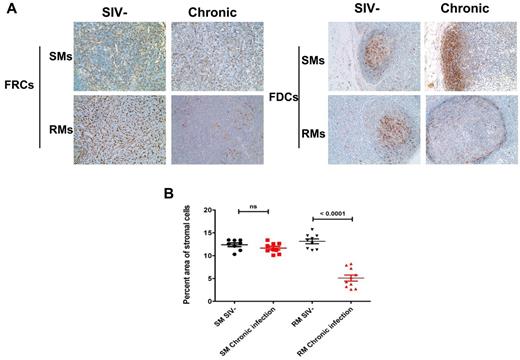
In support of the hypothesis that CD4 T cells are critical for the maintenance of the FRC network, we found that the FRC network was destroyed in pathogenic SIV infection of rhesus macaques. This correlated with the depletion of CD4 T cells within LTs. Interestingly, there was a parallel depletion of the follicular dendritic cell network in the B-cell follicles after CD4 depletion because of SIV infection, suggesting that the follicular dendritic cell network also relies at least partially on CD4 T cells for its maintenance. By contrast, the FRC and follicular dendritic cell networks were maintained in the nonpathogenic animal model of SIV-infected sooty mangabeys in which CD4 T-cell counts are preserved despite the similar level of viral replication to SIV-infected rhesus macaques (Figure 2).45,55
Maintenance of FRC and follicular dendritic cell (FDC) networks within LTs in chronically SIV infected sooty mangabeys (SMs) but not in RMs. (A) Immunohistochemical staining of the FDC network with antibodies to desmin (left panel) and the FDC network with antibodies to CD35 (right panel) in LNs from uninfected and chronically infected SMs and RMs, showing the intact FRC and FDC network in SMs during chronic infection compared with RMs. Original magnification ×200. (B) Quantitative image analysis of the percent area of the stromal cell networks, showing the preservation of both FDC and FRC networks in SMs but not in RMs. Bars represent the mean ± SEM.
Maintenance of FRC and follicular dendritic cell (FDC) networks within LTs in chronically SIV infected sooty mangabeys (SMs) but not in RMs. (A) Immunohistochemical staining of the FDC network with antibodies to desmin (left panel) and the FDC network with antibodies to CD35 (right panel) in LNs from uninfected and chronically infected SMs and RMs, showing the intact FRC and FDC network in SMs during chronic infection compared with RMs. Original magnification ×200. (B) Quantitative image analysis of the percent area of the stromal cell networks, showing the preservation of both FDC and FRC networks in SMs but not in RMs. Bars represent the mean ± SEM.
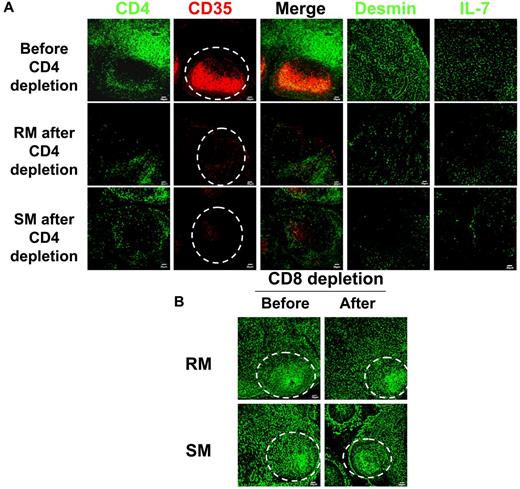
To further test the hypothesis that CD4 T cells are necessary for the maintenance of the FRC and follicular dendritic cell networks, we depleted CD4 T cells in uninfected sooty mangabeys and rhesus macaques with anti-CD4 antibodies, with the prediction that this would result in the loss of both networks. We had previously reported that the majority of CD4 T cells in both peripheral blood and LTs could be depleted by repeated treatment of animals with CD4-depleting antibody, assessed by flow cytometric analysis,56 and we now show by immunofluorescence analysis of tissue sections from these animals that (1) the CD4 T cells in the T-cell zone and B-cell follicles are both severely depleted (Figure 3A); and that (2) depletion of CD4 T cells leads to the loss of both follicular dendritic cell and FRC networks and loss of IL-7 production in rhesus macaques and sooty mangabeys (Figure 3A). In contrast to CD4 depletion, the depletion of CD8 T cells with CD8-depleting antibody did not significantly affect follicular dendritic cell and FRC networks (Figure 3B), further supporting the hypothesis that the CD4 T-cell population is playing the principal role in the preservation of the FRC and follicular dendritic cell networks.
Depletion of CD4 T cells leads to depletion of both FDC and FRC networks in both RMs and SMs. (A) Immunofluorescent staining of CD4, CD35, desmin, and IL-7 in LNs from RMs and SMs before and after CD4+ T-cell depletion, showing that CD4 T-cell depletion leads to depletion of FRC and FDC networks and loss of IL-7 production in both SMs and RMs. Dotted circles represent the position of B-cell follicles. (B) Immunofluorescent staining of desmin, a marker for both FRCs and FDCs in LNs from RMs and SMs before and after CD8 T-cell depletion, showing the preservation of FDC and FRC networks after CD8+ T-cell depletion in both RMs and SMs. Dotted circles represent the position of B-cell follicles.
Depletion of CD4 T cells leads to depletion of both FDC and FRC networks in both RMs and SMs. (A) Immunofluorescent staining of CD4, CD35, desmin, and IL-7 in LNs from RMs and SMs before and after CD4+ T-cell depletion, showing that CD4 T-cell depletion leads to depletion of FRC and FDC networks and loss of IL-7 production in both SMs and RMs. Dotted circles represent the position of B-cell follicles. (B) Immunofluorescent staining of desmin, a marker for both FRCs and FDCs in LNs from RMs and SMs before and after CD8 T-cell depletion, showing the preservation of FDC and FRC networks after CD8+ T-cell depletion in both RMs and SMs. Dotted circles represent the position of B-cell follicles.
Consequences of loss of the FRC network for CD4 T-cell restoration and depletion of CD8 T cells
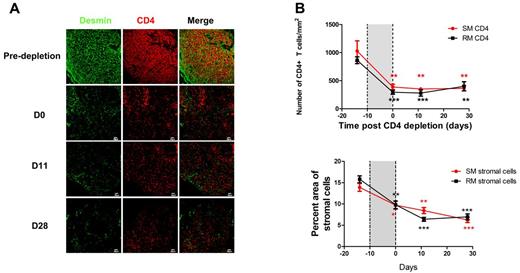
The FRC network is critical for the maintenance of homeostasis of T-cell populations by providing critical survival factors, such as IL-7, so that the concomitant loss of the network and IL-7 (Figure 3A) should slow the restoration of CD4 T-cell populations after termination of CD4-depleting antibody treatment. We indeed found that there was no significant increase of CD4 T cells within LTs 28 days after the last administration of the CD4-depleting antibody (Figure 4A), and there was also little restoration of the FRC network in sooty mangabeys and rhesus macaques (Figure 4A-B). Because IL-7 is a survival factor, particularly critical for both naive CD4+ and CD8+ T cells,40-42 the loss of the FRC network and IL-7 would also be expected to be associated with depletion of CD8 T cells through increased apoptosis. We indeed found that CD8+ T cells are also significantly depleted within LTs in both CD4 T cell–depleted rhesus macaques and sooty mangabeys coincident with increased apoptosis (Figure 5), which was sustained to account for continued lower CD8+ T-cell counts 30 days after the end of the CD4 T cell–depleting treatments (Figure 5B).
Slow restoration of CD4+ T cells correlates with slow restoration of FRC and FDC networks. (A) Immunofluorescent staining of CD4 and desmin in LNs from RMs before and at different time points after stopping CD4-depleting antibody treatment, showing that the slow restoration of CD4 T cells after stopping antibody treatment correlates with slow restoration of FRC and FDC networks. (B) Quantitative image analysis of the number of CD4 T cells in LNs and the percent area of stromal cells, showing the slow restoration of both CD4 T cells and stromal cells. Bars represent the mean ± SEM; and the dotted lines, the timing of anti-CD4 antibody administrations. All the comparisons are made between labeled time point and predepletion. Star color matches line color. *P < .05. **P < .01. ***P < .001.
Slow restoration of CD4+ T cells correlates with slow restoration of FRC and FDC networks. (A) Immunofluorescent staining of CD4 and desmin in LNs from RMs before and at different time points after stopping CD4-depleting antibody treatment, showing that the slow restoration of CD4 T cells after stopping antibody treatment correlates with slow restoration of FRC and FDC networks. (B) Quantitative image analysis of the number of CD4 T cells in LNs and the percent area of stromal cells, showing the slow restoration of both CD4 T cells and stromal cells. Bars represent the mean ± SEM; and the dotted lines, the timing of anti-CD4 antibody administrations. All the comparisons are made between labeled time point and predepletion. Star color matches line color. *P < .05. **P < .01. ***P < .001.
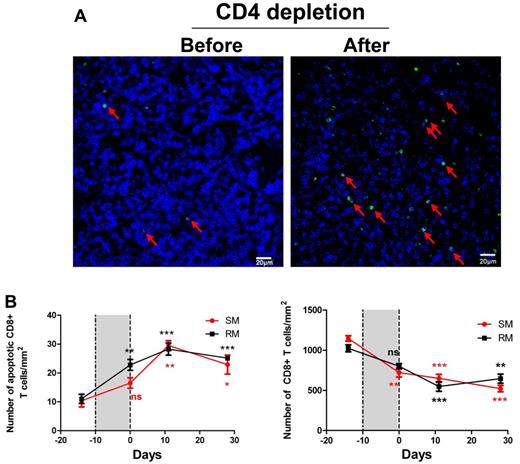
Depletion of both FDC and FRC networks leads to depletion of CD8+ T cells in both RMs and SMs. (A) Immunofluorescent staining of CD8 (blue) and TUNEL (green) in LNs before and after CD4 T-cell depletion, showing that apoptosis level in CD8 population within LTs is elevated after CD4 depletion, which correlates with the depletion of blue staining CD8+ T cells. (B) Quantitative image analysis of the number of apoptotic CD8+ T cells (left panel) and the number of CD8 T cells (right panel) in RMs and SMs before and after receiving CD4-depleting antibody, showing that CD4 depletion leads to increased apoptosis in CD8+ T-cell populations, therefore depleting CD8 T cells. Bars represent the mean ± SEM; and the dotted lines, the timing of anti-CD4 antibody. All the comparisons are made between labeled time point and predepletion. Star color matches line color. ns indicates not significant. *P < .05. **P < .01. ***P < .001.
Depletion of both FDC and FRC networks leads to depletion of CD8+ T cells in both RMs and SMs. (A) Immunofluorescent staining of CD8 (blue) and TUNEL (green) in LNs before and after CD4 T-cell depletion, showing that apoptosis level in CD8 population within LTs is elevated after CD4 depletion, which correlates with the depletion of blue staining CD8+ T cells. (B) Quantitative image analysis of the number of apoptotic CD8+ T cells (left panel) and the number of CD8 T cells (right panel) in RMs and SMs before and after receiving CD4-depleting antibody, showing that CD4 depletion leads to increased apoptosis in CD8+ T-cell populations, therefore depleting CD8 T cells. Bars represent the mean ± SEM; and the dotted lines, the timing of anti-CD4 antibody. All the comparisons are made between labeled time point and predepletion. Star color matches line color. ns indicates not significant. *P < .05. **P < .01. ***P < .001.
Depletion of CD4 T cells and FRCs and follicular dendritic cells in HIV-1 infection and other immunodeficiency conditions
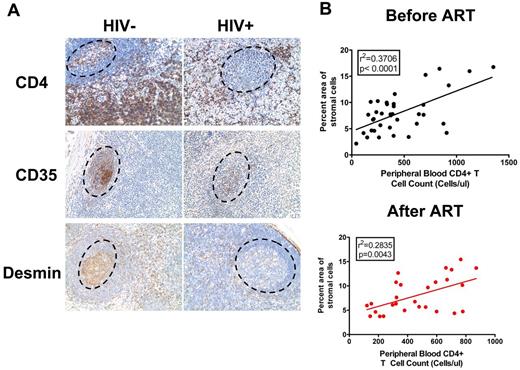
In support of the hypothesized CD4 depletion-mediated mechanism of depletion of FRC and follicular dendritic cell networks, we found that the number of CD4 T cells significantly correlated with the sizes of the FRC and follicular dendritic cell networks in HIV-1 infection before the initiation of antiretroviral therapy (ART) and that the slow restoration of CD4 T cells after initiation of ART correlated with the slow restoration of FRC and follicular dendritic cell networks (Figure 6).
Depletion of CD4 T cells correlates with depletion of FRC and FDC networks in HIV-1 infection. (A) Immunohistochemical staining of CD4, CD35, and desmin in LNs from uninfected and chronically HIV-1–infected subjects, showing the depletion of CD4 T cells in B-cell follicles and T-cell zone correlates with depletion of FDCs and FRCs in B-cell follicles and T-cell zone. Dotted circles represent the position of B-cell follicles. Original magnification ×200. (B) Correlation between the percent area of stromal cells and peripheral blood CD4 T-cell counts (cells/μL) before (top panel) and after (bottom panel) the initiation of ART.
Depletion of CD4 T cells correlates with depletion of FRC and FDC networks in HIV-1 infection. (A) Immunohistochemical staining of CD4, CD35, and desmin in LNs from uninfected and chronically HIV-1–infected subjects, showing the depletion of CD4 T cells in B-cell follicles and T-cell zone correlates with depletion of FDCs and FRCs in B-cell follicles and T-cell zone. Dotted circles represent the position of B-cell follicles. Original magnification ×200. (B) Correlation between the percent area of stromal cells and peripheral blood CD4 T-cell counts (cells/μL) before (top panel) and after (bottom panel) the initiation of ART.
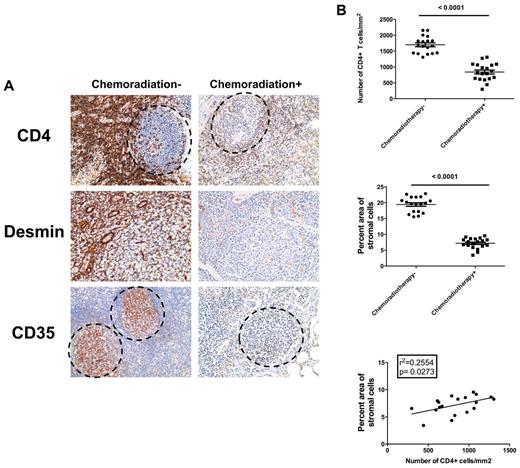
Severe depletion of CD4 T cells and other lymphocytes and slow and incomplete immune reconstitution, particularly of naive T cells, are significant issues in chemotherapy and irradiation for cancer treatment.20,31-38 Because we had found that these same major impacts on T-cell homeostasis in HIV infection are related to the “health” of the supportive FRC network, we tested the hypothesis that chemotherapy and irradiation lead to loss of FRC network to thereby deplete T cells and restrict immune reconstitution after the cessation of the therapy. To test this hypothesis, we examined effects of chemoradiotherapy on depletion of FRC and follicular dendritic cell networks and in cancer patients receiving chemoradiotherapy compared with age-, sex-, and cancer type–matched patients without chemoradiotherapy (Table 2). We found that chemotherapy and irradiation depleted CD4 T cells and diminished FRC and follicular dendritic cell networks in treated cancer patients compared with patients who had not been treated (Figure 7). These findings again strongly support the model that loss of particularly CD4 T cells leads to LT structure damage, including loss of FRC and follicular dendritic cell networks, which in turn impairs the survival of lymphocytes and restricts their restoration.
Demographic characteristics and clinical information of subjects
| Patient ID . | Disease (grade) . | Chemotherapy drugs history (drug doses) . | Radiation therapy dose . | Time since chemotherapy and radiation, wks . | Race . | Sex . | Age, y . |
|---|---|---|---|---|---|---|---|
| Breast carcinoma-chemotherapy group | |||||||
| 4915 | Breast carcinoma (T2, N1/2, M0) | 4 cycles of adriamycin (105 mg) + Cytoxan (1050 mg) | NA | 18 | White | Female | 70 |
| 8871 | Breast carcinoma (grade 1) | 4 cycles of adriamycin (103 mg) + Cytoxan (1000 mg), followed by 4 cycles of Taxol (300 mg) and Neulasta (6 mg) | NA | 25 | White | Female | 49 |
| 4700 | Breast carcinoma (T3, N1, M0) | 4 cycles of adriamycin + Cytoxan (performed off site; dosing information not available) | NA | 14 | Unknown | Female | 57 |
| 9942 | Breast carcinoma (T4, N1c, M0) | 4 cycles of idamycin + Cytoxan (performed off site; dosing information not available) | NA | 14 | White | Female | 59 |
| 9576 | Breast carcinoma (stage not available) | 4 cycles of FU (280 mg) + epirubicin (140 mg) + Cytoxan (920 mg) + Herceptin (140 mg) followed by weekly Herceptin (140 mg) + Taxol (150 mg) for 12 wks | NA | 30 | White | Female | 38 |
| 9493 | Breast carcinoma (T2, N1, M0) | 3 cycles of Taxol dose 2 (300 mg) + Neulasta (6 mg), followed by adriamyacin (102 mg) + Cytoxan (1020 mg) + Neulasta (6 mg) | NA | 25 | White | Female | 51 |
| 1594 | Breast carcinoma (T3, N3c, M0) | 4 cycles of adriamyacin and Cytoxan + Neulasta (6 mg), followed one Taxol (175 mg/m2) | NA | 16 | Unknown | Female | 36 |
| 7204 | Breast carcinoma (T2, N0, M0) | 4 cycles of Taxotere (130 mg) and Cytoxan (1044 mg) + Neulasta (6 mg) | NA | 22 | White | Female | 68 |
| 4060 | Breast carcinoma (T2, N1) | Femar 2.5 mg/d for 13 wks, followed by 1 cycle of Taxotere (130 mg), Cytoxan (1000 mg), and 1 cycle of Nuelasta (6 mg) | NA | 23 | White | Female | 64 |
| 3438 | Breast carcinoma (T3, N1, grade 2) | 4 cycles of adriamycin, Cytoxan (dosing information not available), followed by 12 doses of weekly Paclitaxel (dosing information not available), followed by 1 cycle of Neulasta (6 mg), followed by weekly Taxol (122 mg) for 12 wks | NA | 26 | White | Female | 42 |
| Breast carcinoma-control group (no chemoradiotherapy before LN biopsy) | |||||||
| 4914 | Breast carcinoma (T2.5, N0, M0) | NA | NA | NA | White | Female | 32 |
| 3873 | Breast carcinoma (T2, N0, M0) | NA | NA | NA | White | Female | 40 |
| 9492 | Breast carcinoma (pT1c, N0, M0) | NA | NA | NA | White | Female | 47 |
| 5333 | Breast carcinoma (T1c, N0, Mx) | NA | NA | NA | White | Female | 76 |
| 4197 | Breast carcinoma (T2, N0, M0) | NA | NA | NA | Black | Female | 51 |
| 4934 | Breast carcinoma (T2, N0, M0) | NA | NA | NA | White | Female | 39 |
| 2021 | Breast carcinoma (grade 1) | NA | NA | NA | White | Female | 62 |
| 1979 | Breast carcinoma (T1c, N0, M0) | NA | NA | NA | White | Female | 57 |
| 1626 | Breast carcinoma (pT1c, N0(i-)(sn), MX) | NA | NA | NA | White | Female | 42 |
| 4131 | Breast carcinoma pTis, N0, MX G2) | NA | NA | NA | White | Female | 44 |
| Esophageal carcinoma-chemoradiotherapy group | |||||||
| 5491 | Esophageal carcinoma (stage not available) | Treatment performed off site; information not available | NA | 11 | White | Male | 46 |
| 3445 | Esophageal adenocarcinoma (stage not available) | Cisplatin and infusional 5-FU (performed off site; dosing information not available) | NA | Unknown | Unknown | Male | 66 |
| 9921 | Adenocarcinoma of the gastroesophageal junction (T3, N0, M0) | Epirubicin (50 mg/m2), oxaliplatin (130 mg/m2) and Xeloda (625 mg/m2), followed by 1 cycle Neulasta (6 mg) | NA | 23 | White | Male | 75 |
| 2670 | Distal esophageal adenocarcinoma (T3, N0, M0) | 2 cycles of concurrent chemotherapy (performed off site; drug information not available) | Area of tumor involvement and regional LNs received a dose of 4500 cGy in 25 fractions using a four-field technique with 10 MV photons | 11 | White | Male | 56 |
| 2409 | Adenocarcinoma of the gastroesophageal junction (T3, N1-2) | 4 cycles of cisplatin (75 mg/m2), 5-FU (2300 mg/d) | 25 fractions 1.8 Gy to gastroesophageal junction, regional LNs and margin; 50.4 Gy total dose to primary site | 27 | White | Male | 40 |
| 7852 | Esophageal carcinoma (stage not available) | 3 cycles carboplatin, taxol, and 5-FU, followed by concurrent cisplatin and 5-FU (performed off site; dosing information not available) | Dosing information not available | Unknown | White | Male | 51 |
| 5595 | Esophageal carcinoma (stage not available) | Treatment performed off site; information not available | Dosing information not available | 22 | White | Male | 59 |
| 2341 | Esophageal adenocarcinoma (T3, N1, MX) | 2 cycles of Cisplatin (75 mg/m2), 5-FU (100 mg/m2) | Dosing information not available | 26 | White | Male | 78 |
| 7932 | Esophageal adenocarcinoma (T3, N1, M0) | 3 cycles of Carboplatin (658 mg), Taxol (125 mg) | Radiation 25 fractions with dose of 45 Gy | 31 | White | Male | 54 |
| Esophageal carcinoma-control group (no chemoradiotherapy before LN biopsy) | |||||||
| 6669 | Moderately differentiated adenocarcinoma (T1, N0) | NA | NA | NA | White | Male | 55 |
| 4818 | Esophageal leiomyoma (T3, N0) | NA | NA | NA | White | Male | 48 |
| 4917 | Adenocarcinoma, moderately to poorly differentiated (T2, N0) | NA | NA | NA | White | Male | 75 |
| 4307 | Adenocarcinoma, poorly differentiated, with papillaryand clear cell features (T2, N0) | NA | NA | NA | White | Female | 78 |
| 5924 | Adenocarcinoma, poorly differentiated (stage not available) | NA | NA | NA | White | Female | 44 |
| 3159 | Moderately differentiated adenocarcinoma (T1, N0) | NA | NA | NA | White | Male | 57 |
| 6067 | Moderately to poorly differentiated adenocarcinoma (stage not available) | NA | NA | NA | Not available | Female | 67 |
| 4602 | Multifocal high-grade dysplasia (stage not available) | NA | NA | NA | White | Male | 51 |
| 6669 | Moderately differentiated adenocarcinoma (T1, N0) | NA | NA | NA | White | Male | 55 |
| Patient ID . | Disease (grade) . | Chemotherapy drugs history (drug doses) . | Radiation therapy dose . | Time since chemotherapy and radiation, wks . | Race . | Sex . | Age, y . |
|---|---|---|---|---|---|---|---|
| Breast carcinoma-chemotherapy group | |||||||
| 4915 | Breast carcinoma (T2, N1/2, M0) | 4 cycles of adriamycin (105 mg) + Cytoxan (1050 mg) | NA | 18 | White | Female | 70 |
| 8871 | Breast carcinoma (grade 1) | 4 cycles of adriamycin (103 mg) + Cytoxan (1000 mg), followed by 4 cycles of Taxol (300 mg) and Neulasta (6 mg) | NA | 25 | White | Female | 49 |
| 4700 | Breast carcinoma (T3, N1, M0) | 4 cycles of adriamycin + Cytoxan (performed off site; dosing information not available) | NA | 14 | Unknown | Female | 57 |
| 9942 | Breast carcinoma (T4, N1c, M0) | 4 cycles of idamycin + Cytoxan (performed off site; dosing information not available) | NA | 14 | White | Female | 59 |
| 9576 | Breast carcinoma (stage not available) | 4 cycles of FU (280 mg) + epirubicin (140 mg) + Cytoxan (920 mg) + Herceptin (140 mg) followed by weekly Herceptin (140 mg) + Taxol (150 mg) for 12 wks | NA | 30 | White | Female | 38 |
| 9493 | Breast carcinoma (T2, N1, M0) | 3 cycles of Taxol dose 2 (300 mg) + Neulasta (6 mg), followed by adriamyacin (102 mg) + Cytoxan (1020 mg) + Neulasta (6 mg) | NA | 25 | White | Female | 51 |
| 1594 | Breast carcinoma (T3, N3c, M0) | 4 cycles of adriamyacin and Cytoxan + Neulasta (6 mg), followed one Taxol (175 mg/m2) | NA | 16 | Unknown | Female | 36 |
| 7204 | Breast carcinoma (T2, N0, M0) | 4 cycles of Taxotere (130 mg) and Cytoxan (1044 mg) + Neulasta (6 mg) | NA | 22 | White | Female | 68 |
| 4060 | Breast carcinoma (T2, N1) | Femar 2.5 mg/d for 13 wks, followed by 1 cycle of Taxotere (130 mg), Cytoxan (1000 mg), and 1 cycle of Nuelasta (6 mg) | NA | 23 | White | Female | 64 |
| 3438 | Breast carcinoma (T3, N1, grade 2) | 4 cycles of adriamycin, Cytoxan (dosing information not available), followed by 12 doses of weekly Paclitaxel (dosing information not available), followed by 1 cycle of Neulasta (6 mg), followed by weekly Taxol (122 mg) for 12 wks | NA | 26 | White | Female | 42 |
| Breast carcinoma-control group (no chemoradiotherapy before LN biopsy) | |||||||
| 4914 | Breast carcinoma (T2.5, N0, M0) | NA | NA | NA | White | Female | 32 |
| 3873 | Breast carcinoma (T2, N0, M0) | NA | NA | NA | White | Female | 40 |
| 9492 | Breast carcinoma (pT1c, N0, M0) | NA | NA | NA | White | Female | 47 |
| 5333 | Breast carcinoma (T1c, N0, Mx) | NA | NA | NA | White | Female | 76 |
| 4197 | Breast carcinoma (T2, N0, M0) | NA | NA | NA | Black | Female | 51 |
| 4934 | Breast carcinoma (T2, N0, M0) | NA | NA | NA | White | Female | 39 |
| 2021 | Breast carcinoma (grade 1) | NA | NA | NA | White | Female | 62 |
| 1979 | Breast carcinoma (T1c, N0, M0) | NA | NA | NA | White | Female | 57 |
| 1626 | Breast carcinoma (pT1c, N0(i-)(sn), MX) | NA | NA | NA | White | Female | 42 |
| 4131 | Breast carcinoma pTis, N0, MX G2) | NA | NA | NA | White | Female | 44 |
| Esophageal carcinoma-chemoradiotherapy group | |||||||
| 5491 | Esophageal carcinoma (stage not available) | Treatment performed off site; information not available | NA | 11 | White | Male | 46 |
| 3445 | Esophageal adenocarcinoma (stage not available) | Cisplatin and infusional 5-FU (performed off site; dosing information not available) | NA | Unknown | Unknown | Male | 66 |
| 9921 | Adenocarcinoma of the gastroesophageal junction (T3, N0, M0) | Epirubicin (50 mg/m2), oxaliplatin (130 mg/m2) and Xeloda (625 mg/m2), followed by 1 cycle Neulasta (6 mg) | NA | 23 | White | Male | 75 |
| 2670 | Distal esophageal adenocarcinoma (T3, N0, M0) | 2 cycles of concurrent chemotherapy (performed off site; drug information not available) | Area of tumor involvement and regional LNs received a dose of 4500 cGy in 25 fractions using a four-field technique with 10 MV photons | 11 | White | Male | 56 |
| 2409 | Adenocarcinoma of the gastroesophageal junction (T3, N1-2) | 4 cycles of cisplatin (75 mg/m2), 5-FU (2300 mg/d) | 25 fractions 1.8 Gy to gastroesophageal junction, regional LNs and margin; 50.4 Gy total dose to primary site | 27 | White | Male | 40 |
| 7852 | Esophageal carcinoma (stage not available) | 3 cycles carboplatin, taxol, and 5-FU, followed by concurrent cisplatin and 5-FU (performed off site; dosing information not available) | Dosing information not available | Unknown | White | Male | 51 |
| 5595 | Esophageal carcinoma (stage not available) | Treatment performed off site; information not available | Dosing information not available | 22 | White | Male | 59 |
| 2341 | Esophageal adenocarcinoma (T3, N1, MX) | 2 cycles of Cisplatin (75 mg/m2), 5-FU (100 mg/m2) | Dosing information not available | 26 | White | Male | 78 |
| 7932 | Esophageal adenocarcinoma (T3, N1, M0) | 3 cycles of Carboplatin (658 mg), Taxol (125 mg) | Radiation 25 fractions with dose of 45 Gy | 31 | White | Male | 54 |
| Esophageal carcinoma-control group (no chemoradiotherapy before LN biopsy) | |||||||
| 6669 | Moderately differentiated adenocarcinoma (T1, N0) | NA | NA | NA | White | Male | 55 |
| 4818 | Esophageal leiomyoma (T3, N0) | NA | NA | NA | White | Male | 48 |
| 4917 | Adenocarcinoma, moderately to poorly differentiated (T2, N0) | NA | NA | NA | White | Male | 75 |
| 4307 | Adenocarcinoma, poorly differentiated, with papillaryand clear cell features (T2, N0) | NA | NA | NA | White | Female | 78 |
| 5924 | Adenocarcinoma, poorly differentiated (stage not available) | NA | NA | NA | White | Female | 44 |
| 3159 | Moderately differentiated adenocarcinoma (T1, N0) | NA | NA | NA | White | Male | 57 |
| 6067 | Moderately to poorly differentiated adenocarcinoma (stage not available) | NA | NA | NA | Not available | Female | 67 |
| 4602 | Multifocal high-grade dysplasia (stage not available) | NA | NA | NA | White | Male | 51 |
| 6669 | Moderately differentiated adenocarcinoma (T1, N0) | NA | NA | NA | White | Male | 55 |
If the patients also had radiation therapy, the LN biopsy is included in the radiation field.
NA indicates not applicable; and 5-FU, 5-fluorouracil.
Depletion of CD4+ T cells by chemoradiotherapy leads to depletion of FRC and FDC networks in cancer patients. (A) Immunohistochemical staining of CD4, CD35, and desmin in LNs from chemoradiotherapy-treated patients and untreated patients, showing the depletion of CD4 T cells in B-cell follicles and T-cell zone correlates with depletion of FDCs and FRCs. Dotted circles represent the position of B-cell follicles. Original magnification ×200. (B) Quantitative image analysis of the number of CD4 T cells (top panel) and amount of stromal cells (middle panel) within LNs of chemoradiotherapy-treated patients and untreated patients, showing that both CD4 T cells and stromal cells are depleted during chemoradiotherapy. The depletion of CD4 T cells significantly correlates with the depletion of stromal cells within chemoradiotherapy-treated patients.
Depletion of CD4+ T cells by chemoradiotherapy leads to depletion of FRC and FDC networks in cancer patients. (A) Immunohistochemical staining of CD4, CD35, and desmin in LNs from chemoradiotherapy-treated patients and untreated patients, showing the depletion of CD4 T cells in B-cell follicles and T-cell zone correlates with depletion of FDCs and FRCs. Dotted circles represent the position of B-cell follicles. Original magnification ×200. (B) Quantitative image analysis of the number of CD4 T cells (top panel) and amount of stromal cells (middle panel) within LNs of chemoradiotherapy-treated patients and untreated patients, showing that both CD4 T cells and stromal cells are depleted during chemoradiotherapy. The depletion of CD4 T cells significantly correlates with the depletion of stromal cells within chemoradiotherapy-treated patients.
Discussion
The depletion of naive CD4 T has been well documented during HIV and SIV infections.20,38,58,61-63 This depletion strongly correlates with the depletion of memory CD4 T-cell population and disease progression58,64,65 before initiation of HAART. Furthermore, the continuous deficiency of naive T cells also correlates with increasing incidence of non-AIDS–related clinical events, such as cardiovascular disease, liver disease, and non-AIDS–related cancer,26-29,64 and increased susceptibility to bacterial infections.30 These observations suggest that naive T cells play a critical role in the pathogenesis of HIV infection. In support of this view, it has been recently reported in a nonhuman primate model that naive T-cell depletion markedly impaired SIV-specific CD4+ T-cell responses, SIV env-specific antibody responses, and SIV-specific CD8+ T-cell responses but did not affect the homeostasis of memory T-cell populations.66 This suggests that the depletion of naive T-cell population may have a broader negative impact on the other arms of immune functions during HIV infection, independent of the impact of loss of memory CD4 populations.
Therefore, given the importance of naive T cells in immune defense and immuocompetency, it is critical to understand the mechanisms that lead to naive T-cell depletion during HIV infection. We had previously shown that there is a vicious cycle mechanism of naive CD4 T-cell depletion in SIV infection of rhesus macaque in which (1) collagen deposition impedes access of naive T cells to IL-7 on the FRC network and loss of IL-7 production by loss of FRC network itself, leading to the depletion of naive T cells through increased apoptosis; and (2) depletion of naive T cells as the source of lymphotoxin-β on which the FRC network depends for survival, leads to loss of the FRC network, thereby amplifying and perpetuating the cycle of depletion of both naive T cells and stromal cells.44,45 Because both CD4 and CD8 T cells were depleted in HIV and SIV infection, we could not unequivocally identify the source(s) of lymphotoxin-β and other FRC survival factors. Therefore, in the studies we report here, we compared FRC network in pathogenic and nonpathogenic models of SIV infection in which CD4 T cells are depleted or preserved, analyzed changes in the FRC network in uninfected animals depleted of CD4 and CD8 T cells with antibodies, and examined the general relationships between CD4 T cells and the FRC network in HIV-1 infection and cancer patients.
We show that (1) preservation of CD4 T cells in SIV-infected sooty mangabeys correlates with the maintenance of FRC network; (2) depletion of CD4 T cells, but not CD8 T cells, in uninfected sooty mangabeys and rhesus macaques by antibody recapitulates the LT damage occurring in pathogenic SIV infection in rhesus macaques; and (3) CD4 T-cell depletion in HIV-1 infection or chemotherapy and irradiation in cancer patients is associated with the loss of the FRC network. Taken together, these findings support the general concept that naive CD4 T-cell populations are the source for lymphotoxin-β and other FRC survival factors that are critical for the maintenance of FRC network, and the depletion of naive CD4 T cells thereby leads to the loss of FRC network during immunodeficiency conditions.
The link established here between CD4 T-cell depletion and damage to the LT niche clearly argues that therapeutic approaches for maintaining and restoring functional LT structure should be beneficial in improving immune reconstitution. Because the CD4 T-cell depletion and damage to the FRC network operate cumulatively during HIV-1 infection, the most straightforward way to do this would be through earlier initiation of HAART to limit the depletion of both CD4 T cells and FRC (and follicular dendritic cell) networks. Furthermore, these findings strongly suggest that activation of lymphotoxin-β receptor signaling pathway might be helpful for the restoration of FRC (and follicular dendritic cell) networks during HAART. With availability of such reagents as the soluble form of lymphotoxin-α1β2 or with a specific anti–lymphotoxin-β receptor agonistic monoclonal antibody,67 it will be worthwhile to determine whether treatment of SIV-infected rhesus macaques with these reagents improves the restoration of LTs and immune reconstitution. Lastly, devising treatment regimens that minimize CD4 T-cell loss and that preserve/restore LT structure may also help to improve immune reconstitution for other immune cell subsets, including naive CD8 T-cell and B-cell populations. Taken together, our findings have identified a novel mechanism by which HIV-1 infection depletes FRC and follicular dendritic cell networks, and pointed potential therapeutic approaches to restore the functional stromal cell networks needed for immune reconstitution.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. O'Neill and T. Leonard for help in preparing the manuscript and figures; Ann Sequin and Katelyn Hanneman for their help in organizing patient information; Dr Stephen Schmechel and Sarah Bowell from University of Minnesota Biologic Materials Procurement Network (BioNet) for their help in preparing patients' tissues; Dr Katherine A. Staskus for her critical and thoughtful suggestions and discussion; and all of the donor participants in this study.
This study used BioNet histology and digital imaging core facilities, which are supported by the National Institutes of Health (grants P30-CA77598, P50-CA101955, and KL2-RR033182), and the University of Minnesota Academic Health Center. This work was supported in part by the University of Minnesota (Doctoral Dissertation Fellowship; M.Z.), the National Institutes of Health (grants R37 AI028246, R01 AI048484, and R01 AI056997; A.T.H.), Yerkes National Primate Research Center (grants P51 00165, R21 AI054234, and R01 HL075766; G.S.), and the National Cancer Institute, National Institutes of Health (contract HHSN261200800001E).
National Institutes of Health
Authorship
Contribution: M.Z. and A.T.H. conceived and designed the tissue analysis experiments and wrote the manuscript; M.Z. performed the tissue analyses and contributed reagents, materials, and analysis tools; M.P. contributed to the design and supervision of the CD4+ lymphocyte depletion experiments; J.C.E. conducted the experiments of CD4+ lymphocyte depletion; G.J.B., J.G.C., and T.W.S. provided lymphoid tissue samples; T.W.S. provided CD4 count data from HIV-1–infected patients; G.S. designed and supervised the experiments of CD4+ lymphocyte depletion in primates; and all authors contributed to manuscript revision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ashley T. Haase, Department of Microbiology, University of Minnesota, MMC 196, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: haase001@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal