In this issue of Blood, Ranganathan et al seek a holy grail: evidence that a drug with a novel mechanism of action may be effective for acute myeloid leukemia patients.1
Unfortunately, the overall remission rate for adult patients with acute myeloid leukemia (AML) is only approximately 40%, and has remained at that level for several decades despite great effort into developing new drugs. So, the prospect of a new approach is welcome news.
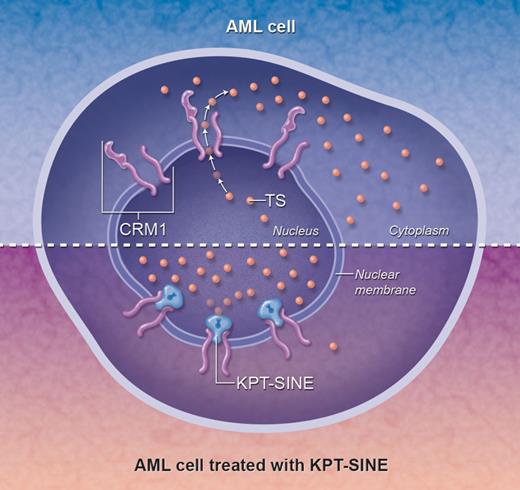
In order for proteins to function normally, they must be found within their proper subcellular compartment. In cancers, one way to disrupt protein function is to mis-localize it within the cell. For example, in AML the NPM mutation, found in almost 30% of all adult AML cases,2 results in a protein that fails to shuttle normally between the nucleus and cytoplasm, instead becoming localized inappropriately to the cytoplasm. Although more than 50 different NPM mutations have been identified in AML samples, they all result in unfolding of the C-terminus and the creation of a novel nuclear export motif recognized by CRM1 (XPO1, exportin), a protein found within the nuclear membrane that controls the export of various proteins (eg, NPM, p53, p21, topoisomerase II, and NF-κB/I-κB) into the cytoplasm (see top panel of figure). These consequences are so reliable that pathologists can use the cytoplasmic localization of NPM as a surrogate for molecular mutation testing.
The mechanism of action of KPT-SINE. The top half represents a typical AML cell, in which tumor suppressor (TS) proteins (small orange circles) escape through the nuclear membrane via the CRM1 nuclear exporter, resulting in a distribution of TS proteins throughout the cell. Arrows indicate the outward direction of flow of the TS proteins from the nucleus into the cytoplasm. In the bottom half, KPT-SINE irreversibly locks the transporter into a conformation that blocks the normal efflux of TS proteins from the nucleus, facilitating their accumulation within the nucleus and potentially allowing TS function. Professional illustration by Alice Y. Chen.
The mechanism of action of KPT-SINE. The top half represents a typical AML cell, in which tumor suppressor (TS) proteins (small orange circles) escape through the nuclear membrane via the CRM1 nuclear exporter, resulting in a distribution of TS proteins throughout the cell. Arrows indicate the outward direction of flow of the TS proteins from the nucleus into the cytoplasm. In the bottom half, KPT-SINE irreversibly locks the transporter into a conformation that blocks the normal efflux of TS proteins from the nucleus, facilitating their accumulation within the nucleus and potentially allowing TS function. Professional illustration by Alice Y. Chen.
The idea for a new drug, then, is simple: If proteins that are supposed to work in the nucleus are disrupted by merely sequestering them in the cytoplasm, an effective strategy might be to concentrate the protein in the nucleus, where it may function properly. To block the export of proteins out of the nucleus, researchers surmised the key residues required for the transport function of CRM1 by studying the known 3-dimensional structure of CRM1 with its cargo.3,4 With this information in hand, Karyopharm Therapeutics developed selective inhibitors of nuclear export (KPT-SINE), orally bioavailable small molecules that bind to the critical Cys528 site within CRM1 and irreversibly block the efflux of proteins into the cytoplasm, effectively concentrating them in the nucleus where they can potentially mediate apoptosis in response to chemotherapy (see bottom panel of figure).
Ranganathan and colleagues present the first in vitro data showing that KPT-SINE has efficacy against AML.1 KPT-SINE suppressed the proliferation of a variety of AML cell lines and samples of primary AML cells of several genotypes, inducing cell-cycle arrest in G1 and/or apoptosis as well as differentiation of several AML cell lines. As expected, KPT-SINE treatment resulted in nuclear accumulation of p53 and NPM, both within cell lines and primary blasts. Interestingly, the group observed a decrease in both FLT3 and KIT protein levels after drug treatment, with no effect on their respective mRNA levels, suggesting a posttranscriptional effect. To test the drug's effect in vivo, Ranganathan et al established mouse xenografts using the MV4-11 cell line, which carries the FLT3 internal tandem duplication (ITD) mutation. One week after the mice were inoculated with the leukemic cells, half of the cohort received a KPT-SINE with excellent bioavailability. Treated mice survived longer and had a lower leukemic cell burden than those who received vehicle alone, establishing preclinical efficacy for KPT-SINE in AML.
In abstracts presented at the American Society of Hematology's 2011 annual meeting, 2 groups demonstrated that KPT-SINE has efficacy in other hematopoietic malignancies beyond AML. Etchin and colleagues observed antileukemic activity, like Ranganathan, in AML as well as in T-cell acute lymphoblastic leukemia.5 Likewise, Kandarpa et al observed similar activity in primary plasma cells from patients with multiple myeloma.6
Ultimately of course, we await definitive studies in AML patients to determine the efficacy of the drug, but the path forward is clear for human studies. Given the disappointing clinical efficacy of FLT3 inhibitors,7 it will be exciting to see whether drugs that block traffic at the nuclear-cytoplasmic border, such as KPT-SINE, which decrease FLT3 protein levels, will be effective on their own or will have greatest usefulness in combination with other agents in the battle against our most aggressive leukemias.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal