In this issue of Blood, Tai and colleagues identify Bruton tyrosine kinase (BTK) inhibition as a new and effective strategy for the treatment of myeloma and the associated bone disease.1
Despite many recent advances in our understanding of the pathogenesis of multiple myeloma and the discovery of powerful agents such as thalidomide and bortezomib, myeloma still remains a fatal malignancy. The reciprocal relationship that develops between myeloma cells (the “seed”) and cells of the host microenvironment (the “soil”) results in a vicious cycle that exacerbates both tumor growth and the development of the osteolytic bone disease (see figure).2,3 Thus, an optimal drug would not only have direct antimyeloma effects but would also act on the host microenvironment to disrupt this relationship resulting in indirect antitumor effects and/or prevention of the osteolytic bone disease.
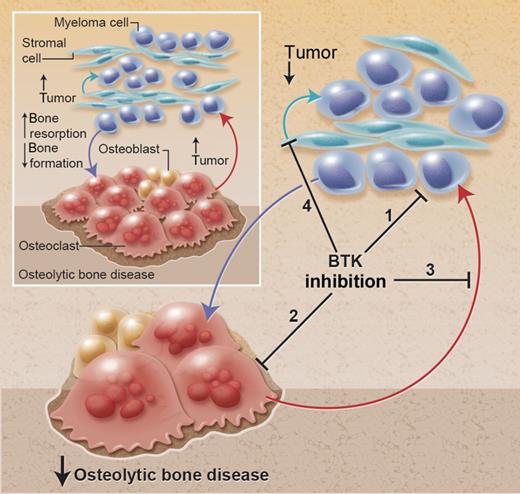
Effect of BTK inhibition in the bone microenvironment in multiple myeloma. Myeloma cells promote the development of the associated bone disease, and the bone disease promotes tumor growth and survival, resulting in a vicious cycle of increased tumor burden and increased osteolytic bone disease. BTK inhibition has multiple effects to (1) directly inhibit tumor growth, (2) directly inhibit osteoclastic bone resorption, (3) inhibit the release of osteoclast-derived tumor growth factors, and (4) prevent adhesion to bone marrow stromal cells (BMSCs) and release of BMSC-derived growth factors. The culmination of these effects is to reduce tumor burden and osteolytic bone disease. Professional illustration by Alice Y. Chen.
Effect of BTK inhibition in the bone microenvironment in multiple myeloma. Myeloma cells promote the development of the associated bone disease, and the bone disease promotes tumor growth and survival, resulting in a vicious cycle of increased tumor burden and increased osteolytic bone disease. BTK inhibition has multiple effects to (1) directly inhibit tumor growth, (2) directly inhibit osteoclastic bone resorption, (3) inhibit the release of osteoclast-derived tumor growth factors, and (4) prevent adhesion to bone marrow stromal cells (BMSCs) and release of BMSC-derived growth factors. The culmination of these effects is to reduce tumor burden and osteolytic bone disease. Professional illustration by Alice Y. Chen.
BTK is a nonreceptor tyrosine kinase that is expressed in many hematopoietic lineages and plays a critical role in B-cell maturation. Targeting BTK in chronic lymphocytic leukemia (CLL) has been shown to have potent antitumor effects,4,5 leading to the clinical investigation of an oral, irreversible, selective BTK inhibitor, PCI-32765 (Ibrutinib).6 Tai and colleagues demonstrate strong expression of BTK in malignant plasma cells from patients with myeloma. This observation combined with the recently discovered role for BTK in the promotion of osteoclastic bone resorption,7,8 led them to hypothesize that BTK inhibition may disrupt multiple components of the vicious cycle and, as such, represent an ideal target in multiple myeloma. To investigate this they used the BTK inhibitor PCI-32765 in a series of elegant in vitro studies designed to reflect the cellular interactions within the bone marrow microenvironment and finally in an in vivo model of myeloma.
The knockdown of BTK expression or treatment of myeloma cells directly with PCI-32765 resulted in a modest reduction in cell viability and induction of apoptosis, an expected result consistent with other hematopoietic malignancies. Further enthusiasm for the direct potential of BTK inhibition was generated by the ability of PCI-32765 to suppress the clonogenicity of myeloma stem-like cells, a subpopulation of cells postulated to contribute to chemo-resistance and disease relapse. In addition to these promising effects of PCI-32765 on the seed, Tai and colleagues also examined the effect of BTK inhibition on the soil. PCI-32765 reduced osteoclast formation and bone resorption, supporting the direct role for BTK in osteoclast bone resorption previously identified in genetically modified mice.7,8 The release of tumor growth factors, either from osteoclasts, bone marrow stromal cells (BMSCs), or resorbed bone, is an important component of the vicious cycle. PCI-32765 was found to reduce cytokine and chemokine secretion from both osteoclasts and BMSCs, with inhibition of a number of key myeloma growth factors, including a proliferation-inducing ligand, activin A, macrophage inflammatory protein-1α, transforming growth factor-β, and stromal cell–derived factor. Working concomittently with the release of tumor growth factors to perpetuate the vicious cycle is the release of so-called “osteoclast activating factors” from myeloma cells,2 and it would have been of interest to determine whether BTK inhibition had similar effects to inhibit secretion of these factors. Further evidence for BTK inhibition to target multiple cellular interactions within the tumor:bone microenvironment was provided by the enhanced cytotoxicity of PCI-32765 when myeloma cells were cultured in the presence of BMSCs and the reduction in adhesion and migration of myeloma cells to BMSCs in response to BTK inhibition.
These in vitro studies provide compelling evidence to support the potential for targeting both seed and soil by BTK inhibition. However, it is impossible to fully recreate the complexities of the bone marrow microenvironment in an in vitro setting, necessitating the use of animal models that accurately reflect human disease. The response to PCI-32765 was evaluated in a severe combined immunodeficient (SCID)–hu model, in which myeloma cell growth is restricted to human bone implanted into imunodeficient mice and associated with osteolysis of the human bone fragment. BTK inhibition was found to reduce both tumor burden and osteolytic bone disease, associated with a reduction in osteoclast number and activity. Although strongly supportive of a positive effect of BTK inhibition in the treatment of myeloma disease, the study design, treatment from time of tumor detection, does not reflect the clinical situation where the majority of patients present with osteolytic bone disease on diagnosis with myeloma. Thus, it remains to be determined whether BTK inhibition is still effective in a preventative setting when osteolytic lesions are already present, particularly because this approach appears to have minimal effect on osteoblastic bone formation.
The conclusions by Tai and colleagues both identify a novel role for BTK in myeloma pathogenesis and provide strong support for the rationale for targeting BTK in this hematologic malignancy. These studies are made all the more exciting by the use of the BTK inhibitor PCI-32765, which is currently undergoing clinical evaluation in CLL, with initial reports demonstrating good safety profiles and response rates. Furthermore, the ability of PCI-32765 to target both tumor growth and osteolysis render it an extremely attractive approach for the treatment of myeloma and the associated bone disease.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal