In this issue of Blood, Vago and colleagues report an unexpected rapid increase of thymus-derived functional and protective T lymphocytes in adult patients after haploidentical transplantation of CD34+ stem cells.1
Aside from GVHD, poor and delayed recovery of the adaptive immune system with opportunistic infections is a major cause of death after allogeneic hematopoietic stem cell transplantation (HSCT). The length of the period of cellular immune deficiency is influenced by many variables, such as the occurrence of GVHD, the extent of thymic damage due to prior chemotherapy and/or the intensity of the conditioning regimens, and the degree of the age-related thymic involution of the patients.2 There are two different pathways for the regeneration of donor-derived T cells: The thymus-independent pathway involves expansion of graft-derived mature T cells, whereas the thymus-dependent pathway consists of the repopulation of the thymus with graft-derived precursor cells and the de novo generation of naive T cells with a more diverse T-cell receptor (TCR) repertoire. The expansion of graft-derived mature T cells is associated with a higher risk of GVHD, whereas the recovery of the thymus-dependent T cells is associated with a lower incidence of GVHD.3 The thymus-dependent recovery of naive T cells can be monitored by their expression of CD62L and CD45RA and by the measurement of single-joint T-cell receptor excision circles (sjTRECs), which are byproducts of the physiologic rearrangement of the α chain of the T-cell receptor. Because they are maintained as episomal DNA in newly generated T lymphocytes, the number of recent thymic emigrants (RTEs) can be quantified.4 In addition, the expression of the surface immunoglobulin-like receptor CD31 (PECAM-1) on CD4+ naive T cells can differentiate sjTREC-rich RTEs from aged naive T cells, and the number of CD4+/CD31+ naive T cells is a measure of the contribution of the thymic-dependent lymphopoiesis to the immune repertoire,5 which decreases during age-related thymic involution.
A delayed reconstitution of T lymphocytes has been observed after haploidentical transplantation of highly purified CD34+ stem cells in pediatric6 and adult patients.7 Because of the profound ex vivo T-cell depletion, the thymus-independent expansion of graft-derived mature T cells only marginally contributes to the T-cell recovery and the regeneration of the adaptive immune system is induced through the thymic-dependent pathway. Donor-derived bone marrow progenitor cells have to migrate to the thymus, where the generation of naive T cells and of RTEs tolerant to the host is orchestrated by a number of known and unknown factors, dependent on the function and the stage of involution of the thymus.
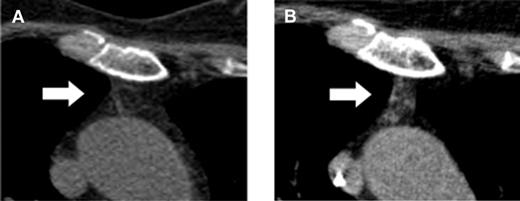
Here, Vago et al have made an unexpected observation and might have found a way to induce a surprisingly rapid de novo generation of naive RTEs with a broad and protective immune repertoire without an increased risk of GVHD.1 They report on 28 adult patients who participated in a multicenter phase 1/2 clinical trial, TK007. In this trial, purified mature donor-derived T cells that were retrovirally transduced with the Herpes Simplex Thymidine Kinase suicide gene (TKpos cells) were serially infused into adult patients with various hematologic malignancies after myeloablative conditioning and transplantation of highly purified CD34+ stem cells from haploidentical donors.8 In case of occurrence of GVHD, the suicide mechanism can be activated by ganciclovir to abrogate GVHD. Twenty-two of the 28 patients who received the purified TKpos cells showed a rapid recovery of T cells that was associated with a concomitant improvement of the clinical outcome and reduction of transplant-related mortality. Patients not receiving TKpos cells failed to attain T-cell immune reconstitution and had a dismal outcome mainly due to infectious complications. Therefore, the infusion of TKpos T cells seemed to induce a rapid and robust T-cell reconstitution. Interestingly, further follow-up of these patients revealed that the regenerating CD3+ T cells failed to express the TK suicide gene (TKneg cells), while donor-derived TKneg cells were not detected in patients who did not receive genetically modified T cells nor in those who failed to engraft TKpos cells. The TKneg cells were enriched in the CD4+ T-cell subset and an increase of the T-cell subset with a naive phenotype (CD62L+CD45RA+) was seen during the observation period even in elderly patients. In addition, measurement of the sjTRECs in patients treated with the TKpos cells demonstrated an increase of sjTREC counts parallel to the increase in the naive T-cell subset. Strinkingly, an increase of the serum levels of IL-7 was observed early after TKpos T-cell add-backs followed by a concomitant rise in peripheral T-cell counts. Further analysis of the reconstituting naive TKneg cells revealed that most of the TKneg naive CD4 cells coexpressed CD31, indicating a continuous output of RTEs, compatible with the computed tomography–based observation of increased thymic tissue even in elderly patients (see figure).
Computed tomography–based increase of the thymic density in a 64-year-old patient before (A) and after (B) add-backs of TKpos T cells. For detailed description see Figure 4 in the article by Vago et al that begins on page 1820.1
Computed tomography–based increase of the thymic density in a 64-year-old patient before (A) and after (B) add-backs of TKpos T cells. For detailed description see Figure 4 in the article by Vago et al that begins on page 1820.1
Additional experiments demonstrated that the antiviral activity of the newly generated TKneg cells was fully maintained even after elimination of the TKpos cells through the activation of the suicide mechanism.
The biologic mechanism for these unexpected findings is unclear. The transient increase of IL-7 might contribute, but is most likely not sufficient to induce long-lasting thymopoiesis.9 The role of the retroviral transduction of the T cells with the suicide gene remains to be shown and it might well be that the ex vivo stimulation of donor T cells with IL-2 and anti-CD3 antibody used for preparation of the cells for transduction induced a shift toward memory T cells, which have been shown to be less responsive to alloantigen stimulation compared with naive T cells.10 Because the incidence of GVHD was surprisingly low after add-back of high numbers of transduced T cells,8 further clinical dose-escalating studies with ex vivo activated but not transduced T cells might be envisioned to investigate the role of TK transduction on thymic function.
The impressive long-lasting increase of naive T cells and the continuous generation of RTEs warrant further clinical trials. The observations by Vago et al might offer new approaches to accelerate immune recovery in patients not only after haploidentical transplantation of CD34+ stem cells but also in other transplant settings, especially in elderly patients.
Conflict-of-interest disclosure: The author declares no competing financial interest. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal