Anemia caused by insufficient renal production of erythropoietin (EPO) can be overcome by reactivating hepatic EPO expression with systemically administered siRNA targeting cellular oxygen sensors according to the report by Querbes et al in this issue of Blood.1
Many readers, familiar with the field of EPO research, will recall the stimulating article “Why the Kidney?” by Erslev and colleagues with the insightful analysis of the unique match-up between blood flow and oxygen consumption in the renal peritubular region.2 Erslev had opened intensive decades of research on humoral regulation of erythropoiesis by an article that appeared in Blood in 1953.3 Since then, we have learned from hypoxia/anemia-induced expression of the EPO gene that cells have hydroxylases as oxygen sensors that posttranslationally control the abundance and activity of hypoxia-inducible transcription factor 1 (HIF-1).4 HIF-1 is formed as a heterodimer from oxygen-sensitive α-subunits (HIF-α) and a constitutive nuclear HIF-1β-subunit. HIF-1α and its paralogue HIF-2α are hydroxylated only in well-oxygenated cells by prolyl hydroxylase PHD1 (synonym EglN2), PHD2 (EglN1), and PHD3 (EglN3), which are all members of oxygen- and 2-oxoglutarate–dependent deoxygenases.4 Once hydroxylated, HIF-α's become poly-ubiquitinated and are prone to degradation by the proteasomes.4,5 Hypoxia decreases PHD activity, HIF-αs can accumulate, dimerize with HIF-1β in the nucleus, and drive hypoxia-induced expression of several hundred HIF-1–dependent genes, in particular that encoding for EPO.6
In adults, EPO is produced by the kidneys and this is why end-stage renal disease with loss of endocrine function of the kidneys is accompanied by anemia.7 Recombinant EPO therapy has been proven to be a very successful treatment of this anemia.8 Recently, first clinical experiences indicate that it is also possible to therapeutically address the upstream oxygen sensors in control of EPO expression by inhibiting the enzymatic activity of PHDs.9 Even anephric patients treated with PHD inhibitors showed an erythropoietic response that was due to increased HIF-α levels and EPO expression in the liver.9
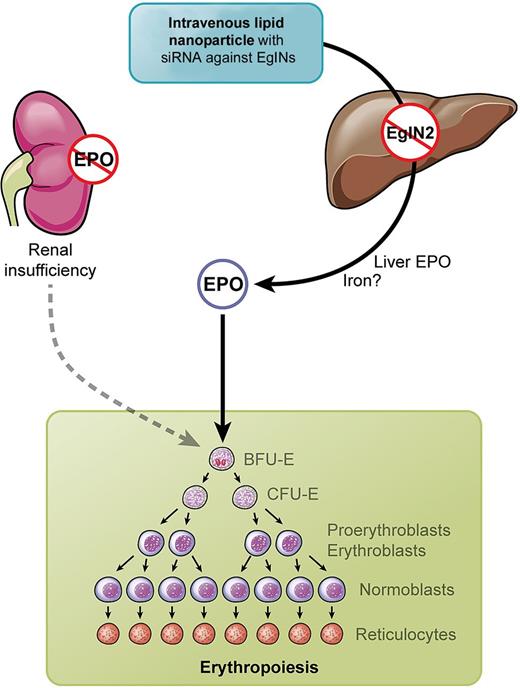
Why in the liver? The liver is prenatally the primary site of EPO synthesis and the production is switched to the kidneys around birth.10 Querbes et al succeed in specifically targeting the PHD oxygen sensors in the liver and were able to reactivate hepatic EPO synthesis by knocking down PHDs with siRNA packed in lipid nanoparticles (LNPs).1 LNPs when injected intravenously specifically target the liver, and the authors used this approach for organ-specific inactivation of PHDs (see figure). Compared with systemic treatment by inhibitors of enzymatic PHD activity, this may, indeed, have the advantage of avoiding effects on HIF target genes in other organs, which is a concern in clinical studies. On the other hand, PHD inhibitors can be taken orally, and among the “desired” side effects, iron metabolism may also be positively affected, including down-regulation of hepcidin.11 LNPs in the present study had the same effect when liver hepcidin expression was reduced, although it remains to be clarified whether this was a direct HIF-1 effect or due to increased erythropoiesis.1
Kidneys in renal insufficiency fail to produce erythropoietin (EPO). Intravenous injection of lipid nano particles (LNPs) containing siRNA against EglNs activate hepatic EPO synthesis and stimulate erythropoiesis. Professional illustration by Kenneth X. Probst.
Kidneys in renal insufficiency fail to produce erythropoietin (EPO). Intravenous injection of lipid nano particles (LNPs) containing siRNA against EglNs activate hepatic EPO synthesis and stimulate erythropoiesis. Professional illustration by Kenneth X. Probst.
The approach by Querbes et al using systemic LNPs with siRNA against oxygen sensors was tested in a rat model and was successful in compensating renal anemia.1 Future studies will prove whether it is worth considering “why not the liver instead of the kidney?” when the kidney fails to produce EPO.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal