Neoplastic transformation requires the elimination of key tumor suppressors, which may result from E3 ligase-mediated proteasomal degradation. We previously demonstrated a key role for the E3 ubiquitin ligase E6AP in the regulation of promyelocytic leukemia protein (PML) stability and formation of PML nuclear bodies. Here, we report the involvement of the E6AP-PML axis in B-cell lymphoma development. A partial loss of E6AP attenuated Myc-induced B-cell lymphomagenesis. This tumor suppressive action was achieved by the induction of cellular senescence. B-cell lymphomas deficient for E6AP expressed elevated levels of PML and PML-nuclear bodies with a concomitant increase in markers of cellular senescence, including p21, H3K9me3, and p16. Consistently, PML deficiency accelerated the rate of Myc-induced B-cell lymphomagenesis. Importantly, E6AP expression was elevated in ∼ 60% of human Burkitt lymphomas, and down-regulation of E6AP in B-lymphoma cells restored PML expression with a concurrent induction of cellular senescence in these cells. Our findings demonstrate that E6AP-mediated down-regulation of PML-induced senescence is essential for B-cell lymphoma progression. This provides a molecular explanation for the down-regulation of PML observed in non-Hodgkin lymphomas, thereby suggesting a novel therapeutic approach for restoration of tumor suppression in B-cell lymphoma.
Introduction
A link between proteasomal degradation and cancer development has been established and a general proteasome inhibitor, Velcade (bortezomib), is in clinical use for the treatment of multiple myeloma and mantle cell lymphoma.1,2 Deregulation of E3 ubiquitin ligases can be sufficient to suppress the expression and function of key tumor suppressors. For example, the inhibition of p53 as a consequence of Mdm2 amplification is frequently observed in human sarcomas and retinoblastoma.3,–5 Interestingly, in human papilloma virus (HPV)–infected cells the suppression of p53 is not achieved by Mdm2, but rather by the cellular E6AP (E6-associated protein) ubiquitin ligase, which is recruited to p53 by the HPV-E6 protein.6,–8 E6AP is encoded by the UBE3A locus, which is mutated in Angelman syndrome (AS), a human neuro-developmental disorder.9 E6AP was the first mammalian ubiquitin E3 ligase to be identified. It is the prototype of the subfamily of E3 ligases that covalently bind ubiquitin and are characterized by a C-terminal HECT (homologous to the E6AP C terminus) domain.10
We recently demonstrated that E6AP regulates the stability of the promyelocytic leukemia (PML) protein and the formation of PML nuclear bodies (PML-NBs).11 PML is a tumor suppressor that was identified as a consequence of the chromosomal translocation of its gene in acute promyelocytic leukemia (APL).12 Consistent with the role of PML as a tumor suppressor, PML deficient mice showed abnormally increased susceptibility to carcinogen13,14 and oncogene-induced tumorigenesis.15 Importantly, PML expression was found to be down-regulated or lost in a variety of human cancer types, including prostate, breast, and colon adenocarcinomas.16,17 PML protein and the PML-NBs were found to play critical roles in cellular stress responses, including those that elicit apoptosis or cellular senescence.18,,–21 Cellular senescence is emerging as an important mechanism for tumor suppression.22,23 It represents a profound arrest of cellular proliferation, accompanied by a distinct set of alterations in the cellular phenotype, such as the formation of senescence-associated heterochromatin foci (SAHF, eg, H3K9me3) and up-regulation of certain inhibitors of cell growth, such as p21, PAI-1, and p16.24 In this study, we explored the role of the E6AP-PML axis in HPV-independent cancer development. We chose pre-B/B-cell lymphomagenesis as a model because of the high frequency of PML down-regulation in non-Hodgkin lymphomas (NHLs).16 For this purpose we used the well established Eμ-myc transgenic mice, a mouse model for Burkitt lymphoma and other NHLs.25 We found that the loss of one allele of E6AP significantly delayed Myc-driven B-cell lymphomagenesis and this was accompanied by elevated PML expression and the induction of cellular senescence. Importantly, E6AP expression was observed to be elevated in human Burkitt lymphoma and cell lines derived from these tumors. Our findings reveal a novel role for the E6AP-PML axis in B-cell lymphomagenesis. This insight may provide a rationale for novel approaches to the treatment of B lymphoma.
Methods
Mice
All mouse experiments were performed in accordance with guidelines administered by the Peter MacCallum Cancer Center Experimental Animal Ethics Committee. The generation and genotyping of Eμ-myc transgenic mice (backcrossed with C57BL/6 mice for > 30 generations) was previously described.25,26 E6AP+/− mice maintained on a C57BL/6 background11 were crossed with Eμ-myc mice to obtain Eμ-myc/E6AP+/− mice. When these mice were inter-crossed, most Eμ-myc/E6AP−/− mice were aborted, therefore making it impossible to obtain a cohort of Eμ-myc/E6AP−/− animals for analyses of tumor onset and preleukemic phenotype. Mice were monitored daily for palpable tumors and for systemic signs of illness, including apathy, breathing difficulties, weight loss, ruffled coats, immobility, or hunched posture. Sick mice showing any of these symptoms were euthanized and subjected to necropsy. Stem cell isolation, adoptive transfer, and lymphoma monitoring and analysis were undertaken as previously described.27,28 Eμ-myc transgenic mice and PML−/− mice were crossed, and the offspring was genotyped by allele-specific polymerase chain reaction. PML−/− and p53−/− mice were previously described.14,29
Flow cytometric analysis
Immunophenotyping.
Single-cell suspensions of freshly isolated lymphomas or lymphoid tissues of preleukemic mice were stained with the following antibodies: antigen presenting cell (APC)–conjugated monoclonal anti–mouse B220 (CD45R; eBioscience), fluorescein isothiocyanate (FITC)–conjugated anti–mouse IgM, phycoerythrin (PE)–conjugated anti–mouse IgD (BD Pharmingen), PE-conjugated anti–mouse Gr-1, FITC-conjugated anti–mouse Mac-1, and FITC-conjugated anti–mouse Thy 1.2 (eBioscience). Data were collected using BD FACS Canto 2 flow cytometer and analyzed on FlowJo Version 8.7 software.
Cell viability assay.
The percentages of viable cells were determined by propidium iodide (PI; 10 μg/mL) staining of freshly harvested lymphoid cells by fluorescence-activated cell sorter (FACS) analysis (10 000 single cell events were collected using BD FACSCanto 2 flow cytometer and FlowJo Version 8.7 software was used to analyze data).
Cell-cycle analysis.
Single-cell suspensions (0.5 × 106) of freshly isolated lymphoid cells were fixed by drop-wise addition of 5 mL of ice-cold 95% methanol while slowly vortexing and then placement at 4°C for 24 hours. Washed cells were resuspended in 300 μL of phosphate-buffered saline-2% fetal bovine serum containing 10 μg/mL of PI and 50 μg/mL RNAase A for 25 minutes before analysis (10 000 single-cell events were collected using BD FACS Canto 2 flow cytometer and analyzed using Modfit Version 3.2 software by Verity Software House). For cell-cycle analysis of premalignant B-lymphoid cells from bone marrow (BM), cells were fixed with 1% paraformaldehyde for 20 minutes and stained with anti-B220–APC for 30 minutes, followed by addition of PI and FACS analysis.
H3K9me3 analysis.
Freshly isolated cells were fixed with 1% paraformaldehyde for 20 minutes and stained with anti-B220–APC (eBiosceince) and anti-H3K9me3 antibodies (Abcam; ab8898) for 30 minutes, washed and incubated with goat anti–rabbit IgG antibodies conjugated with Alexa Fluor 488 (Molecular Probes; Invitrogen) for 1 hour, followed by FACS analysis.
White blood cell counts.
Total numbers of leukocytes in the blood were measured using an Advia 120 automated hematology analyzer (Bayer Diagnostics).
Cell culture
Human Burkitt lymphoma derived cell lines were cultured in RPMI supplemented with 10% heat-inactivated fetal calf serum. Cells were treated with 0.2 μg/μL doxycyclin (Sigma-Aldrich) for induction of shRNA expression vectors (see next 2 paragraphs).
Immunoblotting
Western blotting was performed as previously described.11 Equal amounts (20-50 μg) of protein lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Separated proteins were transferred to nitrocellulose membranes (Biorad), and probed with antibodies before detection by Odyssey Imager (LI-COR). Antibodies used included monoclonal antibodies against actin (clone AC-40, used as a loading control), E6AP (clone E6AP-330), and human PML (clone PML-97) were obtained from Sigma-Aldrich. Monoclonal antibody to mouse PML (clone 36.1-104) was purchased from Upstate Biotechnology, c-Myc antibody was from Cell Signaling (9402) and the Ki67-FITC antibody was obtained from Abcam (ab27619). Rabbit polyclonal antibodies (Santa Cruz) were used to detect p21 (sc-397), PAI-1 (sc-8979) and p16 (sc-759). Polyclonal rabbit antibody against H3K9me3 (ab8898) was obtained from Abcam. Secondary goat anti–rabbit IgG (926-32 211) or goat anti–mouse IgG (926-32 220) antibodies conjugated with infrared dyes (IRDye) were purchased from LI-COR.
Immunofluorescence
Cytospins of cells were immunostained as described previously.11 Briefly, cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized (0.1% Triton X) for 5 minutes, blocked (1% bovine serum albumin–PBS) for 20 minutes, then stained with anti-PML antibodies (Upstate/Sigma-Aldrich) and detected by Alexa Fluor 488–conjugated secondary antibodies (Molecular Probes; Invitrogen). DNA was stained with DAPI. Cells were visualized using a BX-51 microscope (Olympus). Pictures were acquired using SPOT Version 4.7 software (Diagnostic Instruments).
Lentivirus and retrovirus production and infection
Third-generation lentiviral vector packaging constructs were generously provided by Dr Marco Herold (The Walter and Eliza Hall Institute, Melbourne, Australia)30 and consisted of the 3 plasmids pMDLg/pRRE, pMD2.G-VSVg, and pRSV-REV. The FH1t-shE6AP expression vector (3 μg) and FH1t-shWobble (3 μg) were transfected along with the lentivirus packaging plasmids (2 μg of each plasmid) into HEK293T cells and the supernatants were collected 48 and 72 hours after transfection. Lymphoma cells (0.5 × 106) were incubated with the concentrated viral particles (250 μL) in the presence of polybrene (8 μg/mL) for 6 hours in a 12-well plate. Seventy-two hours after infection, cells were sorted based on the expression of reporter green fluorescent protein using FACS (Becton Dickinson FACS DiVa flow cytometer). Retroviral infections were performed as previously described.27
SA-β-galactosidase staining
Cryosections of lymphoid tissue embedded in Tissue-Tek OCT (Sakura Finetek) were cut (8 μm) or cytospins of cells were prepared and stained for SA-β-galactosidase as previously described.31
Analysis of human lymphoma samples
Slides were prepared from formalin-fixed paraffin embedded tissue blocks obtained from diagnostic biopsies. Cases were defined as having Burkitt lymphoma or diffuse large B-cell lymphoma according to the current WHO diagnostic criteria. Immunohistochemical staining was performed using anti-PML (Sigma-Aldrich) and anti-E6AP (Serotec). Images were captured using a BX-51 microscope (Olympus).
Statistical analysis
Animal survival curves were plotted using the Kaplan-Meier method. The log-rank test was used to assess differences and nominal P values were calculated. Statistical t test was used to calculate P values where indicated.
Results
A partial loss of E6AP significantly delayed the onset of Eμ-myc induced pre-B/B-cell lymphoma
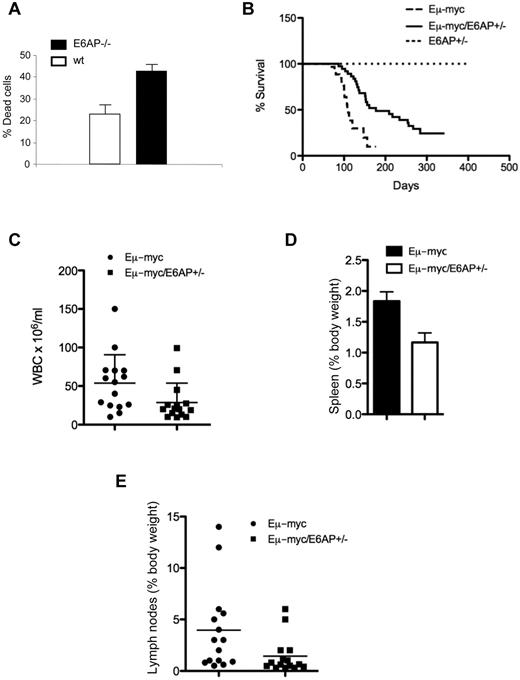
It was previously shown that PML expression is frequently down-regulated or lost in NHL.16 This down-regulation was shown to occur at the protein level.16 Recently, we demonstrated that E6AP functions as a physiologic E3 ligase of PML in a variety of cell types, including lymphoid cells.11 Therefore, we hypothesized that E6AP plays a critical role in the down-regulation of PML and cellular responses of lymphoid malignancies. Consistent with this notion, we found that B-lymphoid cells from the BM of E6AP deficient mice are more susceptible than wild-type (WT) B cells to γ-irradiation–induced apoptosis (Figure 1A). To examine the role of the E6AP-PML axis in B-cell lymphomagenesis, we used the Eμ-myc mice, a well-defined transgenic model for pre-B/B lymphoma development. For this purpose we crossed the Eμ-myc mice with E6AP heterozygote (E6AP+/−) mice to generate a cohort of Eμ-myc/E6AP+/− and as a control Eμ-myc/E6AP+/+ mice. (A cohort of Eμ-myc/E6AP−/− mice could not be produced because of breeding difficulties; see “Methods.”) Remarkably, loss of a single E6AP allele significantly extended the survival of the Eμ-myc mice, increasing the median survival from 103 days to 153 days (Figure 1B; P < .001). Moreover, the tumor burden in sick Eμ-myc/E6AP+/− mice was significantly reduced compared with control (E6AP+/+) Eμ-myc mice as determined by a reduction in white blood cell counts (Figure 1C), diminished enlargement of the spleens (Figure 1D) and lymph nodes (Figure 1E), and by an overall reduction in dissemination of lymphoma cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Collectively, these results show that the loss of one allele of E6AP delayed the onset and reduced the severity of Myc-induced pre-B/B-cell lymphoma. These results support a role for E6AP in the development of Myc-driven B-cell lymphoma. Interestingly, in the moribund Eμ-myc/E6AP+/− mice we observed invasive infiltrations of lymphoid cells in the liver, lung, kidney, and occasionally in the heart, which is probably the cause of death of these mice (supplemental Figure 2). Presumably this infiltration occurred because of the prolonged survival of the mice.
Myc-induced lymphomagenesis is significantly delayed in E6AP heterozygous mice. (A) Death of electronically gated B cells from BM of control wild-type (WT; E6AP+/+) and E6AP−/− mice, which had been irradiated with 5 Gy γ-irradiation was measured by PI staining and analyzed by FACS (n = 3, P < .001). Values represent mean ± SD. (B) Kaplan-Meier survival curves of Eμ-myc/E6AP+/− (n = 36, median survival, 153 days) and control (E6AP+/+) Eμ-myc transgenic mice (n = 30, median survival 103 days). Nontransgenic E6AP+/− mice were used as an additional control (n = 30). Lymphomas developed significantly later in Eμ-myc/E6AP+/− animals compared with the control Eμ-myc mice (P < .001; log-rank test). (C) Numbers of total leukocytes in the blood of sick, lymphoma-burdened mice (n = 15/genotype; P < .01). (D) Weights of spleens of sick, lymphoma burdened mice of the indicated genotypes (n = 15/genotype; P < .001). Values represent mean ± SEM. (E) Weights of combined lymph nodes of sick, lymphoma-burdened mice of the indicated genotypes (n = 15/genotype; P < .01).
Myc-induced lymphomagenesis is significantly delayed in E6AP heterozygous mice. (A) Death of electronically gated B cells from BM of control wild-type (WT; E6AP+/+) and E6AP−/− mice, which had been irradiated with 5 Gy γ-irradiation was measured by PI staining and analyzed by FACS (n = 3, P < .001). Values represent mean ± SD. (B) Kaplan-Meier survival curves of Eμ-myc/E6AP+/− (n = 36, median survival, 153 days) and control (E6AP+/+) Eμ-myc transgenic mice (n = 30, median survival 103 days). Nontransgenic E6AP+/− mice were used as an additional control (n = 30). Lymphomas developed significantly later in Eμ-myc/E6AP+/− animals compared with the control Eμ-myc mice (P < .001; log-rank test). (C) Numbers of total leukocytes in the blood of sick, lymphoma-burdened mice (n = 15/genotype; P < .01). (D) Weights of spleens of sick, lymphoma burdened mice of the indicated genotypes (n = 15/genotype; P < .001). Values represent mean ± SEM. (E) Weights of combined lymph nodes of sick, lymphoma-burdened mice of the indicated genotypes (n = 15/genotype; P < .01).
Eμ-myc/E6AP+/− mice predominantly develop mature (sIg+) B-cell lymphoma rather than (sIg-) pre-B lymphoma
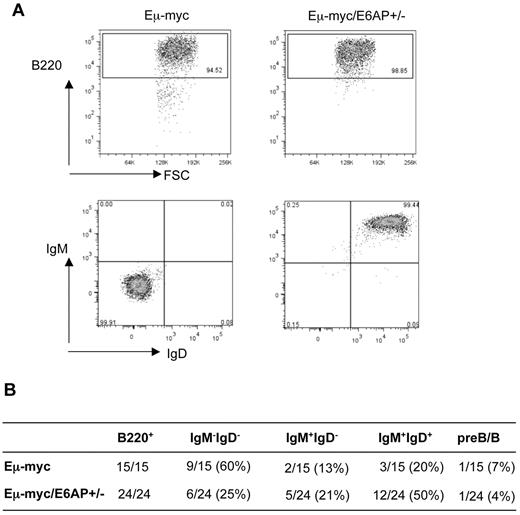
Eμ-myc mice develop mostly (∼ 60%-70%) sIg- pre-B lymphoma and to a lesser extent sIg+ mature B lymphoma.25,32 The relative frequency of these 2 types of B-lymphoid neoplasms can be affected by mutations that accelerate or delay lymphomagenesis, such as overexpression of antiapoptotic Bcl-233 or loss of the proapoptotic BH3-only proteins, Bim,34 Bmf,35 or Puma.36 Immunophenotyping of lymphomas from Eμ-myc/E6AP+/− and control Eμ-myc mice revealed a difference in their stage of maturation. Consistent with previous studies,32,35,36 we found that 9/15 (60%) of the analyzed lymphomas from Eμ-myc mice were IgM−IgD− indicative of pre-B lymphoma, whereas the remainder (5/15: 33%) of the represented more mature B lymphomas (3/15: 20% IgM+IgD+ and 2/15: 14% IgM+IgD−) or were mixed pre-B/B lymphomas (1/15 7%, Figure 2). In contrast, the majority of Eμ-myc/E6AP+/− lymphomas (17/24: 71%) were mature B lymphomas (12/24: 50% IgM+IgD+ and 5/24: 20% IgM+IgD−; Figure 2). This result suggests that the partial loss of E6AP favors the development of more mature B-cell lymphomas. We did not observe any abnormal expansion of other hematopoietic lineages in Eμ-myc/E6AP+/− mice (supplemental Figure 3). To test whether the delayed lymphomagenesis of Eμ-myc/E6AP+/− mice was solely because of the decreased number of B-lymphoid cells or because of the different maturation status of B lymphomas, we compared the rate of transplantability of lymphomas from both genotypes. Equal numbers of immunophenotypically identical sIg- lymphomas from Eμ-myc/E6AP+/− and control Eμ-myc mice were injected into recipient mice. Importantly, the rate of tumor formation on transplantation of the Eμ-myc/E6AP+/− lymphoma cells was slower than that of the Eμ-myc control lymphomas (supplemental Figure 4). This result suggests that neither the reduced number of B cells, nor the difference in the maturation status can solely explain the delayed B lymphomagenesis observed in the Eμ-myc/E6AP+/− mice.
The majority of lymphomas derived from Eμ-myc/E6AP+/− mice display a mature sIg+ B cell immunophenotype. (A) Representative dot plots of the most common immunophenotypic profiles obtained by flow cytometric analyses of Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphomas stained with the antibodies to B220, IgM, and IgD. (B) Summary of immunophenotypic analysis of Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphomas.
The majority of lymphomas derived from Eμ-myc/E6AP+/− mice display a mature sIg+ B cell immunophenotype. (A) Representative dot plots of the most common immunophenotypic profiles obtained by flow cytometric analyses of Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphomas stained with the antibodies to B220, IgM, and IgD. (B) Summary of immunophenotypic analysis of Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphomas.
Preleukemic Eμ-myc/E6AP+/− mice have reduced numbers of B-lymphoid cells
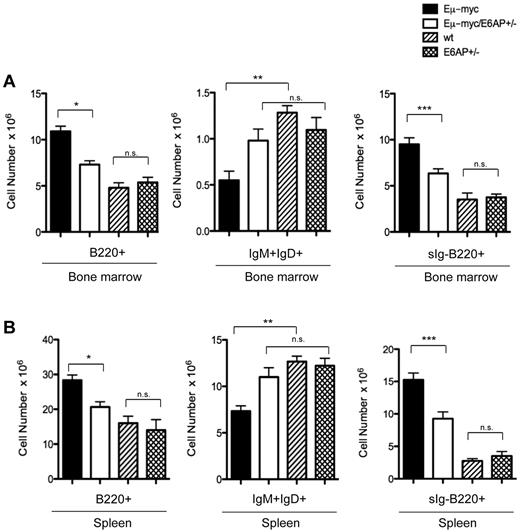
The aberrant expansion of preleukemic immature B-cell populations in the BM, spleen, lymph nodes, and blood represents a critical step in Myc-induced lymphomagenesis.32,37 It was therefore critical to assess whether loss of one allele of E6AP exerts its tumor suppressive effect during early stages of lymphomagenesis. Immunophenotypic analysis of cell suspensions from BM and spleen from 4-week-old animals revealed that Eμ-myc/E6AP+/− mice contained significantly lower numbers of B220+ B lymphoid cells compared with control Eμ-myc mice (Figure 3). Importantly, there was no statistically significant difference in the number of B220+ B-lymphoid cells in the absence of the Myc transgene, that is comparing E6AP+/+ (WT) and E6AP+/− mice (Figure 3). This indicates that the delayed onset of B-lymphomagenesis in Eμ-myc/E6AP+/− mice may be because of the phenomena that a reduction in E6AP diminishes the Myc driven expansion of preleukemic B220+ cells. Young (4-week-old) Eμ-myc mice display a characteristic reduction in the number of mature B cells because in addition to causing increased proliferation of pro-B and pre-B cells, Myc overexpression also inhibits further differentiation along the B lineage.25,26 Because the majority of the Eμ-myc/E6AP+/− lymphomas were sIg+ (ie, neoplastic counterparts of mature B cells; Figure 2) we investigated whether E6AP deficiency primarily reduces the numbers of preleukemic pro-B/pre-B cells while sparing the sIg+ B cells. In accordance with this hypothesis, we found that preleukemic Eμ-myc/E6AP+/− and control WT mice contained similar numbers of mature (IgM+IgD+) B cells (Figure 3). In contrast, and consistent with previous studies,25,26 the numbers of mature B cells were reduced in the BM and spleens of Eμ-myc mice compared with WT controls (Figure 3). These results indicate that E6AP deficiency attenuates Myc-induced lymphomagenesis already at the preleukemic stage and that the prime effect of E6AP loss is on the pro-B/pre-B cells (Figure 3).
Preleukemic Eμ-myc/E6AP+/− mice have abnormally reduced numbers of pro-B/pre-B cells but normal numbers of sIg+ B cells compared with control Eμ-myc mice. Cells extracted from the BM and spleens of 4-week-old WT (nontransgenic E6AP+/+; ie, WT), E6AP+/−, Eμ-myc, and Eμ-myc/E6AP+/− mice were stained with antibodies to B220, IgM, and IgD and analyzed by FACS. (A) Absolute numbers of total B cells (B220+; *P < .001), mature B cells (IgM+IgD+; **P < .001), and pro-B/pre-B cells (sIg-B220+; ***P < .001) in BM are presented (n = 3/genotype). Values represent mean ± SEM. (B) Absolute numbers of total B cells (B220+; *P < .001), mature B cells (IgM+IgD+; **P < .001), and pro-B/pre-B cells (sIg-B220+; ***P < .001) in the spleen are presented (n = 3/genotype). Values represent mean ± SEM; NS indicates not statistically significant.
Preleukemic Eμ-myc/E6AP+/− mice have abnormally reduced numbers of pro-B/pre-B cells but normal numbers of sIg+ B cells compared with control Eμ-myc mice. Cells extracted from the BM and spleens of 4-week-old WT (nontransgenic E6AP+/+; ie, WT), E6AP+/−, Eμ-myc, and Eμ-myc/E6AP+/− mice were stained with antibodies to B220, IgM, and IgD and analyzed by FACS. (A) Absolute numbers of total B cells (B220+; *P < .001), mature B cells (IgM+IgD+; **P < .001), and pro-B/pre-B cells (sIg-B220+; ***P < .001) in BM are presented (n = 3/genotype). Values represent mean ± SEM. (B) Absolute numbers of total B cells (B220+; *P < .001), mature B cells (IgM+IgD+; **P < .001), and pro-B/pre-B cells (sIg-B220+; ***P < .001) in the spleen are presented (n = 3/genotype). Values represent mean ± SEM; NS indicates not statistically significant.
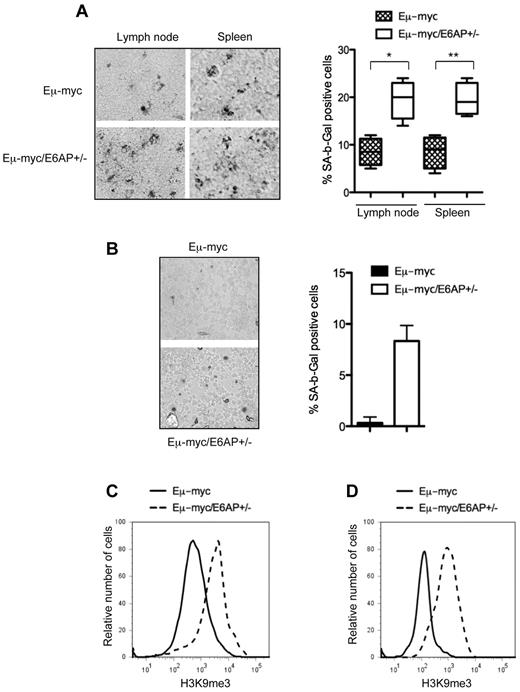
Attenuated lymphomagenesis in Eμ-myc/E6AP+/− mice is associated with enhanced cellular senescence but not apoptosis
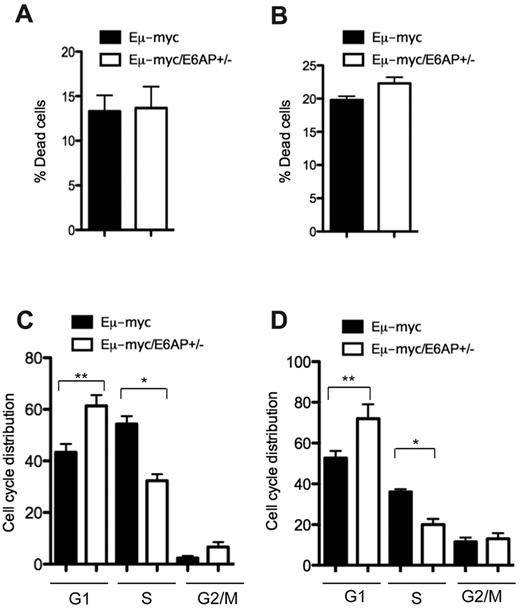
Two major tumor suppressor mechanisms, apoptotic cell death and cellular senescence, have been demonstrated to be associated with the attenuation of Myc-driven B-lymphomagenesis.38,–40 We therefore examined which of these processes was responsible for the extended lymphoma-free survival of Eμ-myc/E6AP+/− mice. Initially, we compared the extent of spontaneous cell death in the malignant lymphomas between the 2 genotypes of mice. This analysis was also extended to premalignant BM-derived B-lymphoid cells from young preleukemic mice of both genotypes. In both cases, this analysis revealed no significant differences in the extent of apoptosis between control Eμ-myc and Eμ-myc/E6AP+/− pre-B/B lymphomas (Figure 4A), or between BM-derived premalignant B cells from the 2 genotypes (Figure 4B). Next, we compared the effect of E6AP deficiency on the cell-cycle distribution of the lymphomas and the premalignant BM-derived B-lymphoid cells. This revealed a significant reduction in proliferating cells with a corresponding increase in cells within the G1 and G2/M phases of the cell cycle in the Eμ-myc/E6AP+/− pre-B/B lymphomas compared with the control Eμ-myc lymphomas (Figure 4C). Similarly, BM-derived premalignant B-lymphoid cells from young Eμ-myc/E6AP+/− mice had fewer cells in S phase and more cells within the G1 and G2/M stages of the cycle compared with their counterparts from young control Eμ-myc animals (Figure 4D). Given that cell-cycle arrest is the major hallmark of cellular senescence, we compared the extent of senescent cells between the pre-B/B lymphomas of the 2 genotypes using staining for β-Gal (SA-β-Gal), a commonly used marker for detection of senescent cells. Notably, we observed a dramatic increase in the proportion of cells positive for SA-β-Gal in Eμ-myc/E6AP+/− lymphoma cell populations (∼ 20%) compared with control Eμ-myc lymphoma cells (∼ 8%; Figure 5A). Remarkably, an increase in the proportion of senescent cells was already detected in the BM-derived premalignant B-lymphoid cells from young Eμ-myc/E6AP+/− mice (Figure 5B). It is important to note that senescent cells are rapidly cleared from healthy tissues41,42 ; hence detection of an 8% population of senescent premalignant B-lymphoid cells in young Eμ-myc/E6AP+/− mice is considerable, particularly in comparison to the 0.3% found in control Eμ-myc mice (Figure 5B). To further substantiate that a loss of E6AP increases cellular senescence in pre-B/B lymphoma cells and premalignant B-lymphoid cells, we examined a marker of SAHF. This revealed an approximately 3-fold increase of methylated histone H3 (H3K9me3) in both Eμ-myc/E6AP+/− lymphoma cells (Figure 5C, supplemental Figure 5) and BM-derived preleukemic B-lymphoid cells (Figure 5D) compared with their counterparts from control Eμ-myc mice. In addition we performed FACS analysis of combined staining for the cell proliferation marker Ki67 and the cellular senescence marker, p21, in BM-derived preleukemic B-lymphoid cells of both genotypes. Consistent with our hypothesis, we observed significantly increased numbers of Ki67neg/p21pos (senescent compartment) pre-B cells in BM of Eμ-myc/E6AP+/− mice compared with control Eμ-myc mice (supplemental Figure 6). Because we did not observe SA-β-Gal positive cells in the lymph nodes and spleens of E6AP+/− mice lacking the Eμ-myc transgene (supplemental Figure 7), we conclude that a loss of E6AP induces cellular senescence only in the context of oncogenic stress, such as Myc overexpression. Collectively, these results indicate that a partial loss of E6AP delays Myc-induced lymphomagenesis through induction of cellular senescence in premalignant B-lymphoid cells.
Growth arrest but not increased cell death is evident in lymphoma cells and premalignant B-lymphoid cells from Eμ-myc/E6AP+/− mice. (A) Spontaneous death of Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphoma cells was assayed by staining with PI followed by FACS analysis (n = 10/genotype). Values represent means ± SD. (B) Spontaneous death of BM derived premalignant B-lymphoid cells from 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice was assayed by staining with PI followed by FACS analysis (n = 3/genotype). Values represent mean ± SD. (C) Cell-cycle analysis of Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphoma cells showing percentages of cells in each of the phases of cell cycle (n = 10/genotype; *P < .001, **P < .01). Values represent mean ± SD. (D) Cell-cycle analysis of BM derived premalignant B-lymphoid cells of 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice was performed by staining with PI, showing percentages of cells in each of the phases of cell cycle (n = 3/genotype; *P < .001; **P < .01). Values represent mean ± SD.
Growth arrest but not increased cell death is evident in lymphoma cells and premalignant B-lymphoid cells from Eμ-myc/E6AP+/− mice. (A) Spontaneous death of Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphoma cells was assayed by staining with PI followed by FACS analysis (n = 10/genotype). Values represent means ± SD. (B) Spontaneous death of BM derived premalignant B-lymphoid cells from 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice was assayed by staining with PI followed by FACS analysis (n = 3/genotype). Values represent mean ± SD. (C) Cell-cycle analysis of Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphoma cells showing percentages of cells in each of the phases of cell cycle (n = 10/genotype; *P < .001, **P < .01). Values represent mean ± SD. (D) Cell-cycle analysis of BM derived premalignant B-lymphoid cells of 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice was performed by staining with PI, showing percentages of cells in each of the phases of cell cycle (n = 3/genotype; *P < .001; **P < .01). Values represent mean ± SD.
Enhanced cellular senescence in lymphomas and premalignant B-lymphoid cells from Eμ-myc/E6AP+/− mice. (A) Senescence-associated β-galactosidase (SA-β-gal) activity was assayed in lymph nodes and spleen sections at manifestation of lymphoma in Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice. Quantification of SA-β-gal positive cells: > 400 cells/tumor, n = 4/genotype were counted in 4 randomly selected fields; *P < .001,**P < .001. Values represent mean ± SD. Magnification ×200. (B) SA-β-gal activity was assayed in cytospins of premalignant B cells isolated from BM of 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice. Quantification of SA-β-gal positive cells: > 400 cells/mice, n = 3/genotype were counted in 4 randomly selected fields; P < .001. Values represent means ± SD. Magnification ×200. (C) The levels of H3K9me3 in Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphomas (n = 10/genotype) were measured by FACS analysis after staining with anti-H3K9me3 antibodies. Representative histograms are shown. Summary of results is shown in supplemental Figure 5. (D) The levels of H3K9me3 in BM derived premalignant B lymphoid cells of 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice were measured by flow cytometry after staining with anti-H3K9me3 antibodies (n = 3/genotype). Representative histograms are shown.
Enhanced cellular senescence in lymphomas and premalignant B-lymphoid cells from Eμ-myc/E6AP+/− mice. (A) Senescence-associated β-galactosidase (SA-β-gal) activity was assayed in lymph nodes and spleen sections at manifestation of lymphoma in Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice. Quantification of SA-β-gal positive cells: > 400 cells/tumor, n = 4/genotype were counted in 4 randomly selected fields; *P < .001,**P < .001. Values represent mean ± SD. Magnification ×200. (B) SA-β-gal activity was assayed in cytospins of premalignant B cells isolated from BM of 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice. Quantification of SA-β-gal positive cells: > 400 cells/mice, n = 3/genotype were counted in 4 randomly selected fields; P < .001. Values represent means ± SD. Magnification ×200. (C) The levels of H3K9me3 in Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphomas (n = 10/genotype) were measured by FACS analysis after staining with anti-H3K9me3 antibodies. Representative histograms are shown. Summary of results is shown in supplemental Figure 5. (D) The levels of H3K9me3 in BM derived premalignant B lymphoid cells of 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice were measured by flow cytometry after staining with anti-H3K9me3 antibodies (n = 3/genotype). Representative histograms are shown.
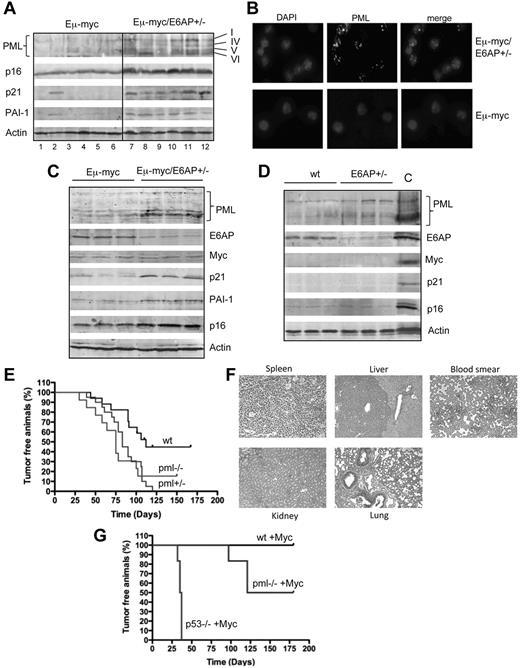
Up-regulation of PML and enhanced formation of PML-NB in lymphoid cells of Eμ-myc/E6AP+/− mice
In view of our previous findings that E6AP is the E3 ligase of PML,11 we hypothesized that a partial loss of E6AP expression in the Eμ-myc mice may result in the elevation of PML expression and enhanced formation of PML-NBs. We therefore compared PML expression in lymphoma cells derived from both genotypes of mice. As predicted, we found elevated levels of PML expression in Eμ-myc/E6AP+/− lymphoma cells compared with control Eμ-myc lymphoma cells (Figure 6A). This increase in PML protein levels in Eμ-myc/E6AP+/− lymphoma cells correlated with the accumulation of PML-NBs (Figure 6B). Furthermore, we found that the levels of PML were already elevated in the premalignant B-lymphoid cells of young Eμ-myc/E6AP+/− mice compared with their counterparts from control Eμ-myc mice (Figure 6C). Notably, the elevation of PML expression and accumulation of the PML-NBs in the Eμ-myc/E6AP+/− lymphoma cells and premalignant B-lymphoid cells is consistent with the induction of cellular senescence (Figure 5). Accordingly, we found an elevation in the expression of several senescence markers previously shown to be increased during PML-induced senescence.20 These include p16, p21, and PAI-1, which were all elevated in the lymphomas as well as the premalignant B-lymphoid cells from Eμ-myc/E6AP+/− mice compared with their counterparts from control Eμ-myc mice (Figure 6A-C). It is important to note that there was no apparent increase in p16 or p21 expression levels in B cells from E6AP+/− mice in the absence of the Eμ-myc transgene (Figure 6D), consistent with the lack of abnormally increased numbers of senescent B-lymphoid cells in these mice (supplemental Figure 7). These results reveal a critical link between partial loss of E6AP and induction of cellular senescence through the activation of PML and thereby provide a molecular explanation for the delay in lymphomagenesis observed in the Eμ-myc/E6AP+/−mice. To substantiate the role of PML as a tumor suppressor in Myc-induced B-lymphoma we intercrossed the Eμ-myc mice with PML−/− mice. The resultant Eμ-myc/PML+/− and Eμ-myc/PML−/− displayed a significantly accelerated lymphoma development compared with the control Eμ-myc mice (Figure 6E) and this was associated with the formation of highly invasive tumors infiltrating the liver (Figure 6F). The lymphomas arising from the Eμ-myc/PML+/− mice retained the WT allele, demonstrating a haploinsufficient tumor suppressor role for PML in this context (supplemental Figure 8). As a second approach to evaluate the impact of PML loss on Myc-induced lymphomagenesis, we performed hematopoietic reconstitution experiments in which lethally irradiated mice were transplanted with MSCV-myc transduced hematopoietic stem cells (HSCs) derived from PML−/− mice or control mice. Myc transduced HSCs from PML−/− mice promoted more rapid lymphoma formation in recipient mice compared with Myc transduced control (WT) HSCs (Figure 6G). As controls, we used Myc transduced HSCs from p53−/− mice, which also caused rapid lymphoma development in transplant recipients. These experiments provide solid evidence for the critical tumor suppressive function of PML in Myc-induced B-cell lymphomagenesis.
The critical role for PML in suppression of Myc-driven lymphomas: elevation of PML and PML-NB in lymphoma cells and premalignant B-lymphoid cells from Eμ-myc/E6AP+/− mice. (A) Immunoblot analysis of the indicated proteins in extracts from Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphomas. Probing for actin was used as a loading control. PML isoforms are indicated. (B) Immunofluorescence staining of PML nuclear bodies in lymphomas from Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice. Magnification ×1000. (C) Immunoblot analyses of the indicated proteins in premalignant B-lymphoid cells from 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice. Probing for actin was used as a loading control. (D) Immunoblot analyses of the indicated proteins in B lymphoid cells from 4-week-old E6AP+/− and control WT (E6AP+/+) mice. Probing for actin was used as a loading control. Lymphoma extract (A, lane 11) was used as a positive control (C). (E) PML loss accelerates tumor onset in Myc-expressing tumors. Eμ-myc transgenic mice were crossed to PML−/− mice. The resulting Eμ-myc mice that were either WT, Pml+/−, or Pml−/− were monitored for tumor onset by spleen as well as lymph node palpation and weekly blood smear analysis. The onset of lymphomas in both Eμ-myc/Pml+/− and Eμ–myc/Pml−/− mice was substantially accelerated compared with control Eμ-myc mice (P < .01). (F) Staining of tissue sections with hematoxylin and eosin revealed that Eμ-myc/Pml−/− lymphomas were highly invasive and infiltrated into the liver, but not kindey or lung. Magnification ×200. (G). HSCs derived from fetal livers of WT, p53−/−, and Pml−/− mice were retrovirally transduced with a MSCV-Myc construct coexpressing green fluorescent protein. The genetically modified stem cells were then used to reconstitute the hematopoietic system of lethally irradiated recipient (WT) animals, which were monitored for lymphoma onset by palpation, weekly blood smears, and whole body fluorescence imaging. Recipients of WT stem cells transduced with the Myc expression did not develop tumors over the observation period, whereas all of the recipients of Myc expression construct transduced p53−/− HSC developed very aggressive disease in a short time. Recipients of Myc expression construct transduced Pml−/− HSCs developed lymphoma more rapidly compared with the mice transplanted with the Myc expression construct transduced WT HSCs (P < .0003), again suggesting that PML can suppress Myc driven lymphoma development.
The critical role for PML in suppression of Myc-driven lymphomas: elevation of PML and PML-NB in lymphoma cells and premalignant B-lymphoid cells from Eμ-myc/E6AP+/− mice. (A) Immunoblot analysis of the indicated proteins in extracts from Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc lymphomas. Probing for actin was used as a loading control. PML isoforms are indicated. (B) Immunofluorescence staining of PML nuclear bodies in lymphomas from Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice. Magnification ×1000. (C) Immunoblot analyses of the indicated proteins in premalignant B-lymphoid cells from 4-week-old Eμ-myc/E6AP+/− and control (E6AP+/+) Eμ-myc mice. Probing for actin was used as a loading control. (D) Immunoblot analyses of the indicated proteins in B lymphoid cells from 4-week-old E6AP+/− and control WT (E6AP+/+) mice. Probing for actin was used as a loading control. Lymphoma extract (A, lane 11) was used as a positive control (C). (E) PML loss accelerates tumor onset in Myc-expressing tumors. Eμ-myc transgenic mice were crossed to PML−/− mice. The resulting Eμ-myc mice that were either WT, Pml+/−, or Pml−/− were monitored for tumor onset by spleen as well as lymph node palpation and weekly blood smear analysis. The onset of lymphomas in both Eμ-myc/Pml+/− and Eμ–myc/Pml−/− mice was substantially accelerated compared with control Eμ-myc mice (P < .01). (F) Staining of tissue sections with hematoxylin and eosin revealed that Eμ-myc/Pml−/− lymphomas were highly invasive and infiltrated into the liver, but not kindey or lung. Magnification ×200. (G). HSCs derived from fetal livers of WT, p53−/−, and Pml−/− mice were retrovirally transduced with a MSCV-Myc construct coexpressing green fluorescent protein. The genetically modified stem cells were then used to reconstitute the hematopoietic system of lethally irradiated recipient (WT) animals, which were monitored for lymphoma onset by palpation, weekly blood smears, and whole body fluorescence imaging. Recipients of WT stem cells transduced with the Myc expression did not develop tumors over the observation period, whereas all of the recipients of Myc expression construct transduced p53−/− HSC developed very aggressive disease in a short time. Recipients of Myc expression construct transduced Pml−/− HSCs developed lymphoma more rapidly compared with the mice transplanted with the Myc expression construct transduced WT HSCs (P < .0003), again suggesting that PML can suppress Myc driven lymphoma development.
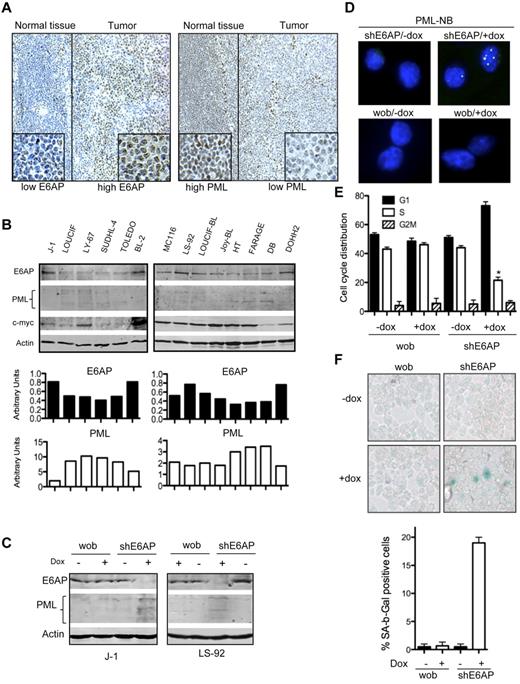
E6AP expression is elevated in Burkitt lymphomas
Our findings demonstrate the involvement of E6AP in pre-B/B lymphomagenesis in a mouse model. Analysis of E6AP expression levels revealed elevated expression of E6AP in a subset of Eμ-myc and Eμ-myc/E6AP+/− lymphomas (supplemental Figure 9), supporting the pro-oncogenic role of E6AP in B-cell lymphomagenesis. To investigate whether E6AP is also involved in human B-cell lymphoma development, we examined its expression in a cohort of primary Burkitt lymphomas. Immunohistochemical analysis revealed that in 12 of 20 samples examined (60%) the expression levels of E6AP were elevated compared with normal lymphoid tissues (Figure 7A, supplemental Figure 10). Interestingly, 6/12 (50%) of Burkitt patient lymphoma samples with high E6AP had correspondingly low levels of PML expression (Figure 7A). The analysis of diffuse large B-cell lymphoma (DLBCL) revealed that in 18% of the samples (7/39; 18%) moderate or elevated levels of E6AP inversely correlated with reduced PML levels (supplemental Figure 11). Furthermore, we also assessed the expression levels of E6AP in cell lines derived from human Burkitt lymphomas, DLBCLs, and follicular lymphomas. We found that in 4 of 14 cell lines (29%) analyzed, the expression levels of E6AP were elevated, whereas in 2 additional cell lines (14%) E6AP expression was moderately elevated (Figure 7B). Strikingly, elevated levels of E6AP expression inversely correlated with down-regulation of PML expression (Figure 7B). Interestingly, the observed correlation was found to be specifically associated with c-Myc overexpression in the majority of these lymphoma derived cell lines (Figure 7B). These results demonstrate the elevation of E6AP expression in a significant proportion of human Burkitt lymphoma cases, indicating that E6AP may play a role in the development and/or sustained growth of these hematologic cancers. Furthermore, the correlation between elevated E6AP expression and down-regulation of PML supports our finding that E6AP negatively regulates PML, at least within certain B-cell lymphomas.
E6AP levels are elevated in human Burkitt lymphomas and down-regulation of E6AP restores PML-induced cellular senescence. (A) Representative images of E6AP (left panel) and PML (right panel) immunostaining in human Burkitt lymphomas. Expression of E6AP is relatively low in normal lymphoid tissue, but the infiltrating Burkitt lymphoma cells show elevated levels of E6AP accompanied by reduced levels of PML; N = 20; Magnification ×200. (B) Immunoblot analysis to determine the levels of E6AP, PML, and c-Myc in a panel of cell lines derived from Burkitt lymphoma derived (J-1, LOUCIF, LY-67, BL-2, MC116, LS-92, LOUCIF-BL, JOY-BL), DLBCL (SUDHL-4, FARAGE, TOLEDO, DB, HT), or follicular lymphoma (DOHH2). Probing for actin was used as a loading control. The expression levels of E6AP and PML normalized against the levels of actin were quantified and are presented on the graphs (bottom panel). (C) Immunoblot analysis of the indicated proteins in J-1 and LS-92 Burkitt lymphoma derived cells transduced with inducible lentiviral constructs containing wobble E6AP or shRNA for E6AP that were treated with (+) or without (−) doxycyclin. Cells were analyzed 7 days after doxycyclin (dox) induction. Probing for actin was used as a loading control. (D) Immunofluorescence staining of PML-NBs in J-1 Burkitt lymphoma cells transduced with the aforementioned shRNA expression constructs on day 7 after dox treatment. Magnification ×1000. (E) Cell-cycle analyses of J-1 Burkitt lymphoma cells transduced with the aforementioned shRNA expression constructs on performed on day 7 after dox induction; *P < .001. Values represent means ± SD. (F) SA-β-gal levels were determined in cytospins of J-1 Burkitt lymphoma cells transduced with the aforementioned shRNA expression constructs on day 7 after dox treatment. Magnification ×400. Quantification of SA-β-gal positive cells: > 400 cells/cytospin, n = 3, cells were counted in 4 randomly selected fields. Values represent means ± SD, and were derived from 3 independent experiments performed in triplicate.
E6AP levels are elevated in human Burkitt lymphomas and down-regulation of E6AP restores PML-induced cellular senescence. (A) Representative images of E6AP (left panel) and PML (right panel) immunostaining in human Burkitt lymphomas. Expression of E6AP is relatively low in normal lymphoid tissue, but the infiltrating Burkitt lymphoma cells show elevated levels of E6AP accompanied by reduced levels of PML; N = 20; Magnification ×200. (B) Immunoblot analysis to determine the levels of E6AP, PML, and c-Myc in a panel of cell lines derived from Burkitt lymphoma derived (J-1, LOUCIF, LY-67, BL-2, MC116, LS-92, LOUCIF-BL, JOY-BL), DLBCL (SUDHL-4, FARAGE, TOLEDO, DB, HT), or follicular lymphoma (DOHH2). Probing for actin was used as a loading control. The expression levels of E6AP and PML normalized against the levels of actin were quantified and are presented on the graphs (bottom panel). (C) Immunoblot analysis of the indicated proteins in J-1 and LS-92 Burkitt lymphoma derived cells transduced with inducible lentiviral constructs containing wobble E6AP or shRNA for E6AP that were treated with (+) or without (−) doxycyclin. Cells were analyzed 7 days after doxycyclin (dox) induction. Probing for actin was used as a loading control. (D) Immunofluorescence staining of PML-NBs in J-1 Burkitt lymphoma cells transduced with the aforementioned shRNA expression constructs on day 7 after dox treatment. Magnification ×1000. (E) Cell-cycle analyses of J-1 Burkitt lymphoma cells transduced with the aforementioned shRNA expression constructs on performed on day 7 after dox induction; *P < .001. Values represent means ± SD. (F) SA-β-gal levels were determined in cytospins of J-1 Burkitt lymphoma cells transduced with the aforementioned shRNA expression constructs on day 7 after dox treatment. Magnification ×400. Quantification of SA-β-gal positive cells: > 400 cells/cytospin, n = 3, cells were counted in 4 randomly selected fields. Values represent means ± SD, and were derived from 3 independent experiments performed in triplicate.
Down-regulation of E6AP restores PML expression and induces cellular senescence in human Burkitt lymphoma derived cell lines
To establish a functional interaction between E6AP and PML in human Burkitt lymphoma, it was important to ascertain whether the down-regulation of the relatively high levels of E6AP in the cell lines derived from these tumors (Figure 7B) could restore PML expression. For this purpose we used an inducible shRNA expression vector to knockdown E6AP expression in the J-1 and LS-92 Burkitt lymphoma derived cell lines, which express elevated levels of E6AP. Strikingly, E6AP knockdown resulted in restoration of PML expression (Figure 7C) and formation of PML-NBs in these cells (Figure 7D). To examine the consequences of PML restoration on these Burkitt lymphoma derived cell lines, we compared their cell-cycle distribution in the presence or absence of E6AP shRNA expression. As shown in Figure 7E, down-regulation of E6AP increased the proportion of cells in G1 with a corresponding decrease of cycling cells (in the S phase), resulting in a reduction in cell proliferation with no impact on cell viability (supplemental Figure 12). This effect coincided with a significant number of cells showing hallmarks of senescence (Figure 7F). These results support our hypothesis that elevated levels of E6AP with the resulting down-regulation of PML and inhibition of cellular senescence promote the growth of human Burkitt lymphoma cells.
Discussion
Restoration of the activity of tumor suppressors is an attractive approach for cancer treatment.23,43 One avenue by which this may be achieved is by protecting key tumor suppressors from proteasomal degradation. Indeed, deregulation of E3 ligases can be sufficient to block the activity of tumor suppressors. This is best exemplified by the amplification of the E3 ligase Mdm2, which negates the pressure for p53 mutations in one-third of human sarcomas.4,5 This study builds on our recent finding that E6AP functions as an E3 ligase for PML.11 Because PML expression is frequently down-regulated or lost in many cancer types,16,44 we examined the role of the E6AP-PML regulatory pathway in tumorigenesis, particularly the development of pre-B/B lymphoma. Using the Eμ-myc transgenic mouse model, in which overexpressed Myc deregulates proliferation of B-lymphoid cells, thereby promoting pre-B/B lymphoma development,25,26 we show that haplo-insufficiency of E6AP reduces disease burden and significantly prolongs the survival of these mice (Figure 1). Effects on apoptotic cell death are well known to impact on Myc-induced lymphomagenesis, in particular when p53 activity has been triggered, as in the case of haplo-insufficient Mdm2 mice.36,38,45 Surprisingly, however, the partial loss of E6AP sensitized Myc-expressing B cells to undergo senescence rather than apoptosis (Figures 5–6). Thus far, cellular senescence has been demonstrated in Myc-driven B lymphomas in the context of the p53R172P mutation, which disables the proapoptotic activity of p53, and in response to TGF-β.39,40 Interestingly, the induction of senescence by E6AP haplo-insufficiency is already evident in the premalignant B lymphoid cells (Figure 5). Therefore, this response probably contributes to the observed decrease in the number of premalignant B lymphoid cells (Figure 3) and the prolonged survival of these animals (Figure 1). The detection of only relatively low numbers of senescent cells in Eμ-myc/E6AP+/− mice is probably because of the rapid clearance of these cells by the immune system.41,42
Consistent with our previous finding that E6AP regulates PML stability,11 we found that Eμ-myc lymphoma cells as well as premalignant Myc overexpressing B lymphoid cells with a partial loss of E6AP had elevated levels of PML and PML-NBs, consistent with their increased propensity to undergo cellular senescence (Figure 6). The PML tumor suppressor plays a key role in the induction of cellular senescence in response to diverse stimuli.19,–21,46 Remarkably, multiple key markers implicated in PML-induced senescence,20 including p16, p21, and PAI-1, were elevated in E6AP deficient pre-B/B lymphoma cells (Figure 6). PML-NBs are critical for the activation of chromatin remodeling molecules (eg, HIRA, HP1) required for the formation of SAHF that are responsible for permanent growth arrest.47,,–50 Importantly, consistent with a role of PML in heterochromatin silencing, we detected enhanced methylation of histone H3 (H3K9me3) in Eμ-myc/E6AP+/− lymphoma cells compared with control Eμ-myc lymphoma cells (Figure 5). Our results therefore support a role for E6AP in the regulation of PML-mediated senescence in Myc-induced B-lymphomagenesis. The critical role of PML as a tumor suppressor in Myc-induced lymphoma was confirmed by the accelerated B-lymphomagenesis caused by a loss of one or both alleles of PML (Figure 6).
To extend our findings to human malignancy, we screened a cohort of primary human Burkitt lymphoma cases and a set of cell lines derived from such tumors, for levels of E6AP and PML. Remarkably, we found that E6AP expression levels were elevated in 43% of the human Burkitt lymphoma derived cells lines (Figure 7B) and in 60% of the primary human Burkitt lymphomas (Figure 7A). In line with our previous report11 and the observations in the Eμ-myc pre-B/B lymphomas, we found that in a subset of these samples with elevated expression of E6AP, the levels of PML expression were correspondingly low (Figure 7). This supports our notion that E6AP regulates cellular senescence by controlling the levels of PML, at least in a subset of B-cell lymphomas. To demonstrate this functional interaction more directly, we down-regulated E6AP in 2 Burkitt lymphoma cell lines that over-expressed this protein. Remarkably, this E6AP down-regulation restored PML expression levels and the formation of PML-NBs, leading to a concomitant increase in cellular senescence (Figure 7C-F). This strongly supports the involvement of the E6AP-PML axis in the regulation of cellular senescence in B lymphoma cells. Induction of cellular senescence is emerging as an important mechanism for cancer therapy.23,24 Recent studies demonstrated that senescence is a key process underlying tumor regression in response to oncogene inactivation, reactivation of tumor suppressors or certain chemotherapeutic interventions.23,24 Moreover, restoration of PML expression has been shown to attenuate tumor development in a xenograft model.51,52 Our findings provide a rationale for targeting the E6AP-PML axis for treating B-cell lymphomas by triggering PML-mediated cellular senescence. This is of particular interest, given that PML expression is down-regulated in a variety of human cancers, including NHL, and that this occurs largely at the protein level.16,44
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank David Huang for the Burkitt lymphoma–derived cell lines and Marco Herold and Luke Lambeth for the inducible shRNA to E6AP lentivirus.
This work was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia to Y.H. (NHMRC 509196, 509197, 1026990, and 1026988) and A.S. (Program Grant 461221); by a grant from the Cancer Council Victoria; the Leukemia and Lymphoma Society (SCOR grant 7413 to A.S.); and by the VESKI award. Y.H. is an NHMRC Senior Research Fellow and A.S. an NHMRC Australia Fellow. This work was made possible by operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS.
Authorship
Contribution: K.W. performed all of the experimental work on mice and human cells, with technical assistance from V.C. and S.J.W.; K.W. and Y.H. wrote the paper and designed the experiments; E.d.S and S.W.L conceived, designed, and performed the experiment with PML KO mice and cells; Burkitt lymphoma cell lines were provided by D.S., and the human Burkitt lymphoma samples by J.S, R.W.J, and S.O.; samples were analyzed by J.S., B.K., and S.F.; the cell death analysis of B cells was performed by Y.L.-C., O.A.-B., and I.L.-H.; Ube3A mice were provided by Y.-H.J.; PML KO mice were provided by P.P.P.; and A.S. and C.L.S. provided advice and protocols related to the Eμ-myc mouse model; and S.H. and Y.H. conceived and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for I.L.-H. is Samuel Lunenfeld Research Institute, Mt Sinai Hospital, Toronto, ON.
Correspondence: Ygal Haupt, The Peter MacCallum Cancer Centre, St Andrew's Place, East Melbourne, 3002, Victoria, Australia; e-mail: ygal.haupt@petermac.org.