Abstract
Abstract  933
933
Cytogenetics findings are the most important pronostic factor in multiple myeloma (MM) with age and ISS. IMW recommends to test t(4-14), del17p and t(14-16) for all patients at diagnosis. However this initial screening doesn't represent patientÕs heterogeneity and many others cytogenetic abnormalities may impact on prognosis as shown in recent studies with SNParrays. Chromosome 1 appeared as a critical region in MM pathogenesis: 1q gain is associated with a poor outcome and few studies have analyzed 1p deletions as a pejorative prognostic factor (especially 1p22 and 1p32 deletions). There's a lack of data on the real impact of 1p abnormalities on an important and homogeneous group of patients. To address this issue we studied the incidence and prognosis impact of 1p22 and 1p32 deletions in 1195 patients from the IFM (Institut Francophone du Myélome) cell collection, homogeneously treated with induction (VAD or VD) and autologous transplantation. We used BAC/PAC clones for screening by FISH method 1195 patients from the IFM cell collection. Data for del17p, t(4;14) del13, ISS, survival data were from the IFM database. Patients were treated with VAD or VD induction followed by autologous transplantation. Survival analysis were performed by Kaplan-Meier curves (logrank test), Cox logistic regression (adjusted to age at diagnosis, induction treatment, initial beta2M, del17p and t(4;14)). Median PFS was 30.9 months (Mo) and median OS was 88.2 Mo. ISS distribution was ISS1 :33.6%, ISS2 :37.4% and ISS3 29%, median age at diagnosis was 56.5 years. Chromosome 1p deletion was present in 23.3% of the patients (271) : 15.1% (176) for 1p22 and 7.3% (85) for 1p32 deletions. Both deletions were simultaneously present for only 1 patient. In contrast we found a statistic link by chi2 test between presence of 1p22 or 1p32 and t(4;14), del17p and high beta2M. Incidence of other cytogenetic abnormalities was: 18.2% (178) for del17p, 12.5% (126) for t4;14) and 58.5% (529) for del13.
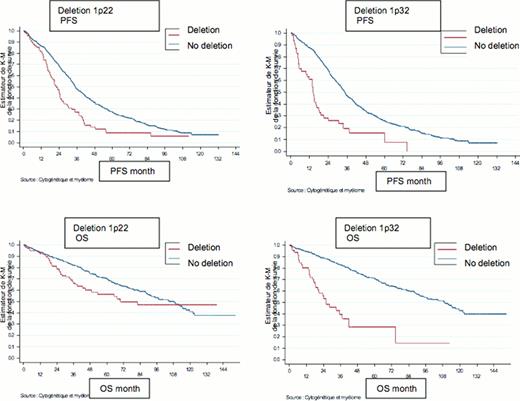
In univariate analyses, 1p22 and 1p32 appeared as negative prognostic factors for :
- PFS : for 1p22 : 22 Mo vs 35 Mo p<0.001 and for 1p32 : 14.1 Mo vs 34.4 Mo p<0.001;
- OS : for 1p22: 69.6 Mo vs 108.3 Mo p=0.002 and for 1p32: 25.9 Mo vs 109.1 Mo p<0.001.
Presence of del17p, t(4;14) or patients with ISS3 were associated with a poor outcome as commonly admitted in the literature.
In multivariate analyses 1p22 and 1p32 deletions appear as independent negative prognostic factors regarding to t(4;14), del17p, del13, ISS or treatment arm. Hazard ratio adjusted to cytogenetic data, ISS and treatment show that progression risk is increased 1.7 times for 1p22 and 2.8 times for 1p32, and death risk was increased 2 times for 1p22 and 4.2 times for 1p32. In multivariate analysis the negative impact of 1p22 decreased during the time, it may be explained by a lack of follow-up for those patients. Interestingly, when del17p and 1p32 were both present, 1p32 deletion appear to worsen the prognosis of patient.
Conclusion: our data show that 1p22 and 1p32 deletions are major negative prognostic factors for PFS and OS for patients with MM independently of t(4;14), del17p, del13, ISS or treatment arm. We thus suggest that 1p22 and 1p32 deletions should be tested for all patients at diagnosis even if prospective studies are necessary to confirm these data.
Roussel:celgene: Honoraria; janssen: Honoraria. Hulin:celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; janssen: Membership on an entity's Board of Directors or advisory committees. Facon:onyx: Membership on an entity's Board of Directors or advisory committees; celgene: Membership on an entity's Board of Directors or advisory committees; janssen: Membership on an entity's Board of Directors or advisory committees; millenium: Membership on an entity's Board of Directors or advisory committees. Attal:celgene: Membership on an entity's Board of Directors or advisory committees; janssen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal