Abstract
Abstract 4399
An increase in glycosylphosphatidylinositol-anchored protein-deficient (GPI-AP−) blood cells is a significant predictor of a good response to immunosuppressive therapy in patients with aplastic anemia (AA) and low-risk myelodysplastic syndrome (MDS). Immune mechanisms are therefore thought to be involved in the increase of GPI-AP− cells in such bone marrow (BM) failure, though the exact mechanisms are unknown. The lack of GPI-APs due to PIGA mutations may render hematopoietic stem cells (HSCs) resistant to myelosuppressive cytokines such as TGF-b. This may lead to preferential activation of PIGA mutant HSCs in immune-mediated BM failure, although little is known about the GPI-APs involved.
We assessed the roles of GPI-APs in TGF-b-mediated inhibition of cell proliferation, cell cycle, and signal transduction in the myeloid leukemia cell line TF-1, TF-1 with a PIGA mutation (PNH-TF-1), and TF-1 cells transfected with siRNA against CD109, a GPI-AP that serves as a TGF-b co-receptor in human keratinocytes. We used flow cytometry to study expression of CD109 in BM CD34 cells of healthy individuals and other hematopoietic cell lines.
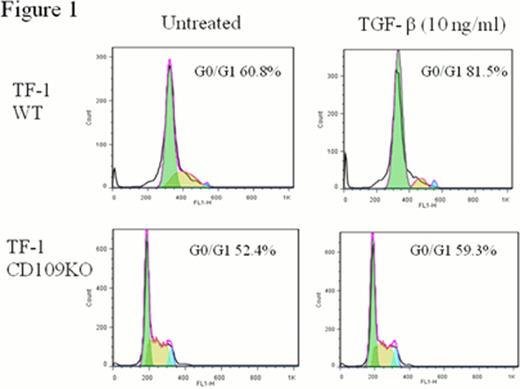
TGF-b inhibited PNH-TF-1 proliferation to a lesser degree (percentage of inhibition, 19±13%) than wild-type TF-1 cells (67±3%) in an MTT-based proliferation assay. Transfection of PIGA into PNH-TF-1 cells restored GPI-AP expression as well as sensitivity to TGF-b (53±10% versus 19±13% in PNH-TF-1 cells). CD109 coimmunoprecipitated with TGF-b in TF-1 cells, and its expression was confirmed on BM CD34+ cells of healthy individuals, particularly on CD34+CD38+CD123−CD45RA− megakaryocyte-erythroid progenitor cells, and also on TF-1 and KG1a cells. CD109 knockdown rendered TF-1 cells less sensitive to TGF-b-induced inhibition of proliferation (26±10% versus 67±3%), TGF-b induced cell cycle arrest (a relative increase in the G0/G1 phase of the cell cycle, 6.9% versus 20.7%, Figure) and TGF-b-induced SMAD2 phosphorylation (a relative increase in pSMAD2 MFI, 1±0.3% versus 16%±3%) compared to non-transfected TF-1 cells.
The lower sensitivity of PIGA mutant TF-1 cells to TGF-b compared to wild-type TF-1 cells is partially due to CD109 deficiency. This deficiency may also account for the preferential activation of PIGA mutant HSCs in immune-mediated BM failure, in which TGF-b suppresses activation of wild-type HSCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal