Abstract
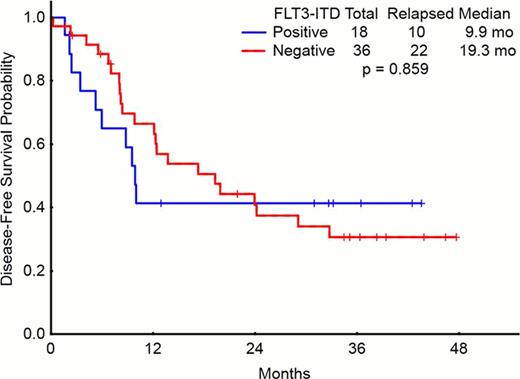
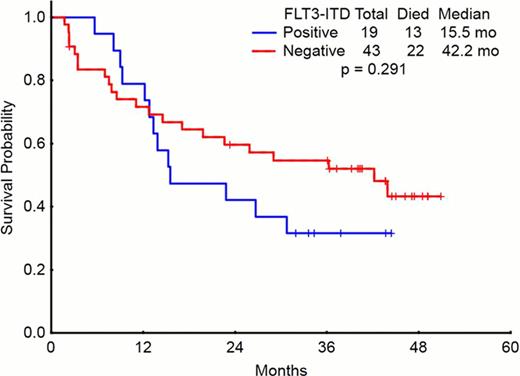
Sorafenib is a potent inhibitor of FLT3 kinase with demonstrable clinical activity in patients with acute myeloid leukemia (AML) and FLT3-ITD mutation, but not those with FLT3-D835 mutation. Objectives: To determine the long term outcome of patients with AML treated with the combination of Sorafenib, cytarabine and idarubicin, and in particular those with FLT3-ITD mutation. Method: Between October 2007 to March 2010, 62 patients with newly diagnosed, previously untreated AML, were treated with Sorafenib 400 mg orally twice daily (BID) for 7 days, cytarabine 1.5 g/m2 by continuous intravenous (IV) infusion daily for 4 days (3 days if > 60 years of age), and idarubicin at 12 mg/m2 IV daily for 3 days on an IRB-approved clinical trial. After achieving remission, patients received up to 5 cycles of consolidation with sorafenib 400 mg BID continuously and abbreviated doses of cytarabine and idarubicin given at approximately one month intervals. Results: 62 patients were treated on the phase II portion of the study. Median age was 53 years (range, 18 – 66) and 12 (19%) patients were > 60 years. 23 (37%) had FLT3 mutations including 17 with FLT3-ITD, 4 with FLT3-D835, and 2 with both mutations. Cytogenetics was diploid in 36 (58%), chromosome 5 and 7 deletion in 4 (6%) and 1 (2%) respectively, complex in 8 (13%), miscellaneous in 13 (21%). Median white blood cell count (WBC) at diagnosis was 7.25 × 109/L (range, 0.6 – 225), and 28 (45%) patients had WBC > 10 × 109/L; among these, 12 (43 %) had FLT3-ITD. After induction, 49 (79 %) patients achieved CR and 5 (8%) CR with incomplete platelet recovery (CRp). 1 (2%) patient died before response assessment could be performed and 7 (11%) were non responders. Patients with FLT3-ITD were more likely to achieve a CR/CRp than patients without FLT3-ITD [18/19 (95%) patients vs. 36/43 (83%) patients, respectively (p=0.23)]. To date, 32 (59%) of the responders have relapsed including 10 of 18 (56%) patients with FLT3-ITD and 22 of 36 (61%) patients without FLT3-ITD (P=0.86). 35 patients received salvage therapy; 14 died after receiving salvage therapy, 11 achieved a second CR and 10 were refractory. After a median follow up of 40.6 months (range, 5.7 to 50 months), the median DFS and OS were 13 months and 29 months, respectively. Hematopoietic stem cell transplantation (HSCT) was performed in 34 (55 %) patients, including 23 in CR, and 11 with active disease. Stem cell source was from related donors in 15 (44%), unrelated donors in 9 (26%), cord blood in 7 (21%), and haploidentical donors in 3 (9%) patients. Overall, 35 (56%) patients have died; 16 (26%) had infectious complications, 12 (19%) multi-organ failure, 9 (15%) graft versus host disease, and 10 (16%) other causes with some patients having overlapping causes. Three-year disease free survival (DFS)(in patients achieving CR, n=54) and overall survival (OS) (n=62) were 34% and 47%, respectively (Figures 1 and 2). Conclusion: Combination of sorafenib, idarubicin and cytarabine is an effective regimen with durable responses in patients with newly diagnosed AML, particularly those with FLT3-ITD.
Ravandi:Bayer: Research Funding; Onyx: Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal