Abstract
Tyrosine kinase inhibitor therapy with imatinib (IM), dasatinib (DAS), or nilotinib is very effective in chronic-phase chronic myeloid leukemia. Two hundred fifty-three patients with newly diagnosed chronic-phase chronic myeloid leukemia were randomized to IM 400 mg/day or DAS 100 mg/day. The proportion of patients achieving a complete cytogenetic remission rate was superior with DAS (84% vs 69%), as was the 12-month molecular response by the proportions of patients achieving > 3-log, > 4-log, and > 4.5-log reduction in BCR-ABL transcript levels. Overall and progression-free survival was similar in the 2 arms. Among patients who achieved hematologic CR, 3-year relapse-free survival was 91% with DAS and 88% with IM 400 mg. Grade 3 and 4 toxicities were most commonly hematologic, including thrombocytopenia in 18% and 8% of DAS and IM patients, respectively. DAS induced more complete cytogenetic response and deeper molecular responses after 12 months, compared with IM 400 mg, and with a median follow-up of 3.0 years there have been very few deaths, relapses, or progressions in the 2 arms. In summary, DAS compared with IM appeared to have more short-term cytogenetic and molecular response, more hematologic toxicity, and similar overall survival. This trial is registered at www.clinicaltrials.gov as NCT00070499.
Introduction
The treatment of chronic myeloid leukemia (CML) has been revolutionized by the tyrosine kinase inhibitor imatinib mesylate (IM), which inhibits the constitutively active fusion gene BCR-ABL, found in virtually all cases of CML.1,2 In the IRIS (International Randomized Study of Interferon and STI571) trial, IM at 400 mg/d yielded a complete cytogenetic response (CCyR) rate of 69% at 12 months after starting treatment, and at 5 years the estimated probabilities of freedom from progression to accelerated phase/blast crisis (AP/BC) and of overall survival (OS) were 97% and 89%, respectively.3 Moreover, no patients with documented major molecular response (MMR) at 12 months progressed to AP/BC.4 IM 400 mg/d is currently recommended for first-line treatment of CML by the National Comprehensive Cancer Network (NCCN)5 and European LeukemiaNet (ELN).6
Imatinib, however, is not universally effective in chronic-phase CML, as 31% of patients in the IRIS trial randomized to IM discontinued therapy or crossed over, mainly for lack of efficacy or toxicity.3 The multikinase inhibitor dasatinib (DAS) has been found to be effective in treating patients resistant or intolerant to imatinib, producing CCyR in roughly 40% of cases with IM resistance.7-9 Several single-center studies have suggested that in newly diagnosed chronic-phase CML, DAS produces superior cytogenetic and molecular responses compared with IM 400 mg/day.10,11 In addition, 2 randomized phase 3 trials of DAS and nilotinib in newly diagnosed chronic-phase CML demonstrated the superior short-term efficacy of these second-generation tyrosine kinase inhibitor (TKIs) compared with IM, with the second-generation TKIs yielding superior 1-year molecular and cytogenetic responses, as well as fewer progressions to advanced-phase disease.12,13
In 2005, 4 North American cooperative groups (Southwestern Oncology Group [SWOG], Eastern Cooperative Oncology Group [ECOG], Cancer and Leukemia Group B [CALGB], and the National Cancer Institute [NCI] Canada Clinical Trials Group) initiated study S0325, a trial of IM 400 versus 800 mg/d in newly diagnosed chronic-phase CML (CML-CP). In the second phase of the trial, patients were enrolled on IM 400 mg/d versus daily DAS. This report focuses on the results of the second phase of the trial, the comparison of DAS versus standard dose IM.
Methods
Patient population and study design
Eligible patients were required to have chronic-phase CML (CML-CP) diagnosed no more than 6 months before enrollment. CML was defined by the presence of the Philadelphia chromosome (Ph) by peripheral blood or bone marrow cytogenetics or FISH, or the detection of BCR-ABL by RT-PCR. Chronic phase (CP) was defined by < 15% blasts in peripheral blood and bone marrow, < 30% blasts plus promyelocytes in peripheral blood and bone marrow, < 20% basophils in peripheral blood, and at least 100 × 109/L platelets. No prior CML therapy was allowed except for hydroxyurea and/or anagrelide. Patients were at least 18 years old and had adequate liver, kidney, and cardiac function and Zubrod performance status 0-2. This study was conducted in accordance with the Declaration of Helsinki. The ethics committee or institutional review board at each participating center was responsible for reviewing the study protocol. All participants were required to give written informed consent before study entry in accordance with institutional regulations.
Study design and treatment arms
Patients with CML-CP were randomized 1:1 to IM 400 mg once daily or DAS 100 mg once daily. Randomization was stratified by Hasford risk category (low vs intermediate vs high)14 Patients were to remain on treatment until treatment failure or unacceptable toxicity, initially for a maximum of 1 year (later extended to 5 years). Treatment failure was defined as failure to achieve complete hematologic response (CHR) by 3 months, loss of CHR, loss of partial (PCyR) or complete cytogenetic response (CCyR), or progression to accelerated or blastic phase of CML. Patients with > 95% Ph+ metaphases at 6 months could escalate IM to 600 mg/d, which if tolerated for 2 weeks could be further increased to 800 mg/d. DAS could be escalated to 140 mg/d.
Adverse events (AEs) were graded according to Version 3.0 of the Common Terminology Criteria for Adverse Events (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm). Dose interruptions were specified as an AE management strategy before dose reduction. For grade 2-4 nonhematologic toxicity or grade 3-4 hematologic toxicity (absolute neutrophil count [ANC] or platelets only), therapy was held and then resumed on recovery to grade 1 or less. Dose reduction to IM 300 mg/d or DAS 70 mg/d was required for hematologic toxicity of grade 3-4 or of grade 2 persisting 28 days, and for recurring toxicities; further reduction to IM 200 mg/d or DAS 50 mg/d was allowed for recurrent grade 3-4 hematologic toxicity. Dose re-escalation was allowed after 1 month without grade 2-4 nonhematologic toxicity or recurrence of reduction-causing toxicity. Management of AEs persisting longer than 28 days required guidance from the clinical trial leader. Treatment with study drug continued until disease progression or patient intolerance.
Disease monitoring
Hematologic assessments (hemoglobin, hematocrit, white blood cells [WBCs], platelets, percentage of eosinophils, percentage of basophils, and percentage of blasts) were measured weekly for the first month and then, absent grade 2 or higher hematologic toxicity, every 2 weeks until week 12, then monthly until month 12 and every 3 months thereafter until end of study. Metaphase chromosome analyses of the bone marrow were performed before therapy and at 6 and 12 months. Complete hematologic response (CHR) required all of the following for at least 28 days: WBC < 10.0 × 109/L; platelets < 450 × 109/L; normal peripheral blood differential with absence of blasts and promyelocytes, and with myelocytes plus metamyelocytes < 5%; and absence of hepatosplenomegaly. Stable and increasing disease were defined by failure to achieve CHR because of persistence of CP and because of progression to accelerated or blast phase, respectively. A CCyR was defined by absence of Ph+ metaphases in a minimum of 20 marrow cells. Each patient's hematologic and cytogenetic response was based on his or her best responses during the first 12 months. Relapse from CHR was defined by any of the following: WBC count > 2000/mm3, platelets ≥ 600,000/mm3, myelocytes + metamyelocytes > 5% in peripheral blood, appearance of blasts in peripheral blood, or appearance of extramedullary disease. Molecular response was based on quantitative RT-PCR (QPCR) taken from peripheral blood at 3, 6, 9, and 12 months.15 Each patient's relative BCR-ABL mRNA level at any given assessment was calculated as the ratio of his/her level to the appropriate baseline level. In this study, the baseline was defined as the Cooperative Group–specific median pretreatment mRNA level. The central CALGB and NCI Canada laboratories performed the molecular studies on patients enrolled in their own cooperative groups; the central SWOG laboratory performed studies on all SWOG and ECOG patients. The log reduction of BCR-ABL mRNA was calculated by comparison to group-specific BCR-ABL baseline level, similar conceptually to the IRIS trial. Before initiation of the trial, tests were performed to test for comparability of assay performance between the laboratories. Cell-line dilution and sample exchange studies were performed in the 3 laboratories before the initiation of the protocol. Cell-line experiments had intralaboratory and interlaboratory correlations of R ≥ 0.97. Results on CML samples had intralaboratory and interlaboratory correlations of R ≥ 0.92-0.96.
Mutational analyses
Peripheral blood samples from patients who failed to achieve CHR or who relapsed from CHR or CCyR were sequenced for mutations in the ABL tyrosine kinase domain. Samples nearest to the times that resistant disease or relapse, as appropriate, were collected and studied by direct nucleotide sequencing.
Statistical analyses
The primary outcome measure for this study was a 4-log reduction of BCR-ABL levels at 12 months, although CHR, CCyR, and the variation of BCR-ABL mRNA levels over time were also investigated. Time-to-event outcomes included: overall survival (OS) from the date of randomization until death from any cause, with observation censored at the date of last contact for patients last known to be alive; progression-free survival (PFS) from the date of randomization until CML progression to accelerated phase or blast crisis; relapse from CHR or death from any cause, with observation censored at the date of last contact for patients last known to be alive without report of progression or relapse; and relapse-free survival (RFS) from the date of CHR until relapse or death from any cause, with observation censored at the date of last contact for patients last known to be alive without report of relapse. Analyses of CHR, OS, and PFS were based on all eligible randomized patients (RFS was limited to patients who achieved CHR), while molecular response and CCyR were based on patients with follow-up assessments. Distributions of OS, PFS, and RFS were estimated using the Kaplan-Meier method.16 Variation of BCR-ABL expression, which was to be measured at 3, 6, 9 and 12 months, was analyzed using mixed models of the form Yi(T) = αi + βDi + γ(Di,T), where Yi(T) is the log-transformed relative mRNA level of patient i at time T (days since randomization); αi is a random coefficient reflecting patient-to-patient variability (and introducing within-patient correlation); Di = 1 for dasatinib, 0 for imatinib; β is a nonrandom coefficient representing the treatment difference; and γ(Di,T) is a polynomial function to model the pattern of average relative mRNA levels as a possibly treatment-dependent function of time. mRNA levels reported as nondetects were left-censored at 10−6. The planned number of patients was 240 (120 per arm). Power calculations were based on the end point of a 4-log reduction from the baseline BCR-ABL level after 12 months of therapy. A 2-sided test at the 5% critical level comparing the 4-log reduction rates at 12 months would have 93% power if the true 4-log reduction rates at 12 months were 15% in one arm and 35% in the other, and 91% power of the true rates were 20% and 40%. Analyses were based on data available December 12, 2011.
Results
From November 2006 through February 2009, 253 patients with newly diagnosed CML-CP were randomized to IM 400 mg po/day or DAS 100 mg po/day. Five patients were ineligible because of absence of Ph or BCR-ABL (n = 2), CML in accelerated phase (n = 2), or cardiac symptoms (n = 1). One patient who withdrew consent and another who received no protocol treatment because of cost were not evaluable. Pretreatment characteristics of the remaining 246 patients were generally balanced between the arms, although patients randomized to DAS were somewhat younger and had significantly higher WBC (Table 1). One patient randomized to DAS was treated with the IM regimen.
Characteristics of 246 patients with previously untreated chronic-phase CML, by treatment arm
| . | Imatinib 400 mg/d, N = 123 . | Dasatinib 100 mg/d, N = 123 . | P* . |
|---|---|---|---|
| Median age, y (min-max) | 50 (19-89) | 47 (18-90) | .042 |
| Median WBC, 109/L (min-max) | 51.9 (0.3-401) | 89.0 (3.0-410) | .0097 |
| Median basophils, % (min-max) | 3 (0-18) | 3 (0-17) | .37 |
| Median platelets, 109/L (min-max) | 378 (109-1390) | 363 (100-1810) | .25 |
| Median BM blasts, % (min-max) | 1 (0-9) | 2 (0-12) | .56 |
| Sex, no. of patients (%) | .90 | ||
| Female | 51 (41) | 49 (40) | |
| Male | 72 (59) | 74 (60) | |
| Hasford risk category, no. of patients (%) | .73 | ||
| Low | 44 (36) | 44 (36) | |
| Intermediate | 45 (37) | 40 (33) | |
| High | 34 (28) | 39 (32) | |
| Performance status, no. of patients (%) | .36 | ||
| 0 | 76 (63) | 71 (58) | |
| 1 | 44 (36) | 47 (39) | |
| 2 | 1 (1) | 4 (3) | |
| NA | 2 (—) | 1 (—) | |
| Palpable splenomegaly, no. of patients (%) | .25 | ||
| Yes | 53 (44) | 63 (51) | |
| No | 68 (56) | 60 (49) | |
| NA | 2 (—) | 0 (—) | |
| Palpable hepatomegaly, no. of patients (%) | .75 | ||
| Yes | 4 (3) | 6 (5) | |
| No | 112 (97) | 111 (95) | |
| NA | 7 (—) | 6 (—) |
| . | Imatinib 400 mg/d, N = 123 . | Dasatinib 100 mg/d, N = 123 . | P* . |
|---|---|---|---|
| Median age, y (min-max) | 50 (19-89) | 47 (18-90) | .042 |
| Median WBC, 109/L (min-max) | 51.9 (0.3-401) | 89.0 (3.0-410) | .0097 |
| Median basophils, % (min-max) | 3 (0-18) | 3 (0-17) | .37 |
| Median platelets, 109/L (min-max) | 378 (109-1390) | 363 (100-1810) | .25 |
| Median BM blasts, % (min-max) | 1 (0-9) | 2 (0-12) | .56 |
| Sex, no. of patients (%) | .90 | ||
| Female | 51 (41) | 49 (40) | |
| Male | 72 (59) | 74 (60) | |
| Hasford risk category, no. of patients (%) | .73 | ||
| Low | 44 (36) | 44 (36) | |
| Intermediate | 45 (37) | 40 (33) | |
| High | 34 (28) | 39 (32) | |
| Performance status, no. of patients (%) | .36 | ||
| 0 | 76 (63) | 71 (58) | |
| 1 | 44 (36) | 47 (39) | |
| 2 | 1 (1) | 4 (3) | |
| NA | 2 (—) | 1 (—) | |
| Palpable splenomegaly, no. of patients (%) | .25 | ||
| Yes | 53 (44) | 63 (51) | |
| No | 68 (56) | 60 (49) | |
| NA | 2 (—) | 0 (—) | |
| Palpable hepatomegaly, no. of patients (%) | .75 | ||
| Yes | 4 (3) | 6 (5) | |
| No | 112 (97) | 111 (95) | |
| NA | 7 (—) | 6 (—) |
CML indicates chronic myeloid leukemia; WBC, white blood cell; NA, not available; and —, not calculated.
Two-sided P value from the Wilcoxon test (continuous variables) or the Fisher exact test (sex, organomegaly). P value from the Pearson χ2 test (Hasford risk category, performance status).
Hematologic, cytogenetic, and molecular responses
Treatment outcomes are summarized in Table 2, and are shown by Hasford risk category in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CHR rates were 82% in the IM arm and 81% in the DAS arm (P = 1.00). Another 19 patients met the criteria for CHR but without confirmation that the CHR continued for at least 28 days; if these unconfirmed CHRs are included, the rates increased to 92% and 87% in the IM and DAS arms, respectively (P = .30). Only 13 patients had stable disease (IM 6%, DAS 5%, P = 1.00) and none had increasing disease. Thirteen patients (IM 2%, DAS 8%) had incomplete (8 patients) or no (5 patients) assessments of clinical response. Among the 131 patients (IM 61, DAS 70) whose cytogenetic responses could be assessed, the proportion achieving CCyR was higher in patients receiving DAS (84%) compared with IM (69%, P = .04).
Treatment outcomes of 246 patients with chronic-phase CML, by treatment arm
| . | Imatinib 400 mg/d . | Dasatinib 100 mg/d* . | P† . | ||
|---|---|---|---|---|---|
| No. of patients (%) . | 95% CI . | No. of patients (%) . | 95% CI . | ||
| n = 123 | n = 123 | ||||
| Complete hematologic response (confirmed) | 101 (82) | 74-88 | 100 (81) | 73-88 | 1.00 |
| Complete hematologic response (any) | 113 (92) | 86-96 | 107 (87) | 80-92 | .30 |
| Resistant disease | 7 (6) | 2-11 | 6 (5) | 2-10 | 1.00 |
| n = 61 | n = 70 | ||||
| Complete cytogenetic response | 42 (69) | 56-80 | 59 (84) | 74-92 | .040 |
| n = 91 | n = 99 | ||||
| Molecular response at 1 year‡ | |||||
| 3-log decrease | 40 (44) | 34-55 | 58 (59) | 48-68 | .059 |
| 4-log decrease | 19 (21) | 13-31 | 27 (27) | 19-37 | .32 |
| 4.5-log decrease | 14 (15) | 9-24 | 21 (21) | 14-31 | .35 |
| . | Imatinib 400 mg/d . | Dasatinib 100 mg/d* . | P† . | ||
|---|---|---|---|---|---|
| No. of patients (%) . | 95% CI . | No. of patients (%) . | 95% CI . | ||
| n = 123 | n = 123 | ||||
| Complete hematologic response (confirmed) | 101 (82) | 74-88 | 100 (81) | 73-88 | 1.00 |
| Complete hematologic response (any) | 113 (92) | 86-96 | 107 (87) | 80-92 | .30 |
| Resistant disease | 7 (6) | 2-11 | 6 (5) | 2-10 | 1.00 |
| n = 61 | n = 70 | ||||
| Complete cytogenetic response | 42 (69) | 56-80 | 59 (84) | 74-92 | .040 |
| n = 91 | n = 99 | ||||
| Molecular response at 1 year‡ | |||||
| 3-log decrease | 40 (44) | 34-55 | 58 (59) | 48-68 | .059 |
| 4-log decrease | 19 (21) | 13-31 | 27 (27) | 19-37 | .32 |
| 4.5-log decrease | 14 (15) | 9-24 | 21 (21) | 14-31 | .35 |
CML indicates chronic myeloid leukemia; CI, confidence interval; DAS, dasatinib; IM, imatinib mesylate; CHR, complete hematologic response; and CCyR, complete cytogenetic response.
One patient randomized to DAS received IM 400; this patient achieved CHR (confirmed), was in CCyR at 12 months, and remains alive without report of CHR at 35 months; but did not achieve 3-log molecular response in 12 months (1.34-log at 90 days, 1.73 log at 174 days, 2.05 log at 266 days, 2.39 log at 363 days).
Two-sided P value from the Fisher exact test.
Based on blood specimens collected 295-406 days after randomization.
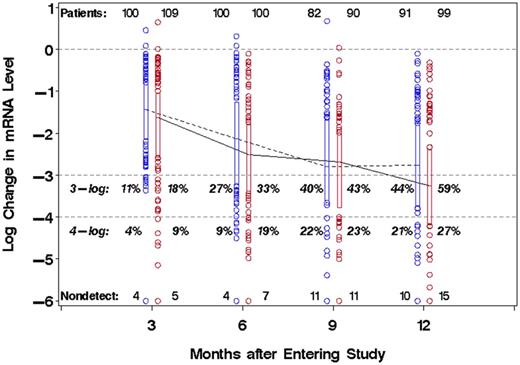
The molecular responses in the IM and DAS arms are shown in Table 2 and Figure 1. Molecular response was deeper in the DAS arm, judging by the proportion of patients achieving 3-log (1000-fold), 4-log (10 000-fold), and 4.5-log (31 623-fold) reduction in BCR-ABL transcript levels. At 1 year, 59% of the DAS arm compared with 44% of the IM arm achieved a 3-log reduction of BCR-ABL (P = .059). The corresponding rates of 4- and 4.5-log reduction of the DAS versus IM arms were 27% versus 21%, and 21% versus 15%, respectively. If the rate of 3-log reduction is calculated as in the Dasatinib versus Imatinib Study in Treatment-Naive CML Patients (DASISION) trial (designated as the major molecular response, or MMR), where the absence of a test is counted as a negative, the rate of MMR in the IM group was 33%, compared with 47% in the DAS arm. The median BCR-ABL mRNA reduction at 1 year was 3.3 log in the DAS arm versus 2.8 log in the IM arm (P = .063).
Molecular responses of CML-CP patients, by treatment arm and approximate time on study. Changes of BCR-ABL mRNA level, relative to group-specific median baseline values, are shown on a common log (log10) scale for patients randomized to DAS (solid line) or IM (dashed line) therapy. Boxplots showing the 25th and 75th percentiles are connected at the median values (IM, blue; DAS, red). Horizontal dashed lines indicate no change, 3-log and 4-log reduction from baseline. Month 3: days 43-126; month 6: days 127-210; month 9: 211-294; month 12: days 295-420.
Molecular responses of CML-CP patients, by treatment arm and approximate time on study. Changes of BCR-ABL mRNA level, relative to group-specific median baseline values, are shown on a common log (log10) scale for patients randomized to DAS (solid line) or IM (dashed line) therapy. Boxplots showing the 25th and 75th percentiles are connected at the median values (IM, blue; DAS, red). Horizontal dashed lines indicate no change, 3-log and 4-log reduction from baseline. Month 3: days 43-126; month 6: days 127-210; month 9: 211-294; month 12: days 295-420.
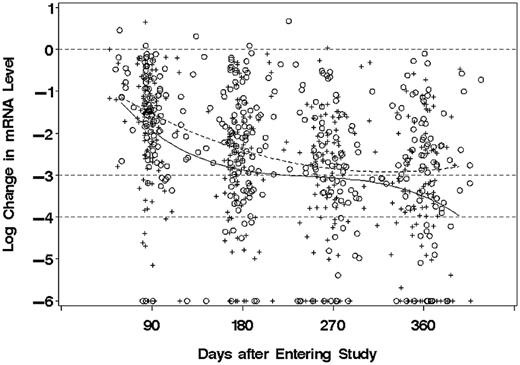
In the mixed model analysis in which BCR-ABL mRNA levels during the first year on study were allowed to vary as a cubic function of time, the levels were on average 0.433 log (2.7-fold) lower for patients in the DAS arm, compared with the IM arm (P = .026). The pattern of decrease over time differed somewhat between the DAS and IM patients (P = .026), although as shown in Figure 2 the average level was consistently lower in the DAS arm.
Molecular responses of CML-CP patients, by treatment arm and time on study. Changes of BCR-ABL mRNA level, relative to group-specific median baseline values, are shown on a common log (log10) scale for patients randomized to DAS (+) or IM (○) therapy. Values reported as “non-detect” are assigned 6-log reduction. Curved lines are fitted models for mean change from baseline for DAS (solid) or IM (dashed). Horizontal dashed lines indicate no change, 3-log and 4-log reduction from baseline.
Molecular responses of CML-CP patients, by treatment arm and time on study. Changes of BCR-ABL mRNA level, relative to group-specific median baseline values, are shown on a common log (log10) scale for patients randomized to DAS (+) or IM (○) therapy. Values reported as “non-detect” are assigned 6-log reduction. Curved lines are fitted models for mean change from baseline for DAS (solid) or IM (dashed). Horizontal dashed lines indicate no change, 3-log and 4-log reduction from baseline.
Mutation analyses
Samples for mutation analysis were sought for 10 DAS and 15 IM patients with evidence of resistant disease (11 patients), or with hematologic or cytogenetic relapse or progression (14 patients). No sample was available in 1 DAS case, and the RNA was not amplifiable for 2 IM cases. The number and type of Abl mutations in the remaining 22 patients are shown in Table 3. There was 1 mutation in a DAS patient at the time of relapse from CHR (V299L), and 2 mutations in IM patients (M244V, E453K). One of these cases (M244V) was tested because of stable disease, while the other 2 were tested because of hematologic relapse.
Mutational analyses of resistant disease or relapse/progression specimens
| Outcome . | Patients, N = 25 . | Patients with mutation data, N = 22 . | Specimen collection times . |
|---|---|---|---|
| Imatinib 400 mg/d | |||
| Stable disease | 6 | 1Mut: M244V | 31 days before removal from protocol treatment |
| 3 WT | 22 days before and 103 days after removal from protocol treatment; day 371 of protocol treatment | ||
| Relapse from CHR | 8 | 1 Mut: E453K | 63 days before relapse |
| 7 WT | 6, 7, 14, 24, 78, and 672 days before; 658 days after relapse | ||
| Progression | 1 | 1 WT | 4 days before removal from protocol treatment due to progression (patient was not adequately assessed for response) |
| Dasatinib 100 mg/d | |||
| Stable disease | 5 | 4 WT | 111 days before removal from protocol treatment; days 96, 329 and 374 of protocol treatment |
| Relapse from CHR | 4 | 1Mut: V299L | Day of relapse |
| 3 WT | 52, 264, and 364 days before relapse | ||
| Cytogenetic relapse | 1 | 1 WT | Day of cytogenetic relapse |
| Outcome . | Patients, N = 25 . | Patients with mutation data, N = 22 . | Specimen collection times . |
|---|---|---|---|
| Imatinib 400 mg/d | |||
| Stable disease | 6 | 1Mut: M244V | 31 days before removal from protocol treatment |
| 3 WT | 22 days before and 103 days after removal from protocol treatment; day 371 of protocol treatment | ||
| Relapse from CHR | 8 | 1 Mut: E453K | 63 days before relapse |
| 7 WT | 6, 7, 14, 24, 78, and 672 days before; 658 days after relapse | ||
| Progression | 1 | 1 WT | 4 days before removal from protocol treatment due to progression (patient was not adequately assessed for response) |
| Dasatinib 100 mg/d | |||
| Stable disease | 5 | 4 WT | 111 days before removal from protocol treatment; days 96, 329 and 374 of protocol treatment |
| Relapse from CHR | 4 | 1Mut: V299L | Day of relapse |
| 3 WT | 52, 264, and 364 days before relapse | ||
| Cytogenetic relapse | 1 | 1 WT | Day of cytogenetic relapse |
CHR indicates complete hematologic response; Mut, mutation; and WT, wild type.
Toxicity
There were no fatal toxicities. Among the 245 patients who received their assigned regimens, 15% (18 of 122) of DAS patients have experienced grade 4 toxicities, compared with only 2% (2 of 123) of IM 400 mg patients (P = .0001). Eight patients, all in the DAS arm, had grade 4 nonhematologic toxicities including febrile neutropenia (2 patients), cardiac ischemia, asystole, pericardial effusion, sensory neuropathy, proteinuria, and metabolic abnormality (LDH elevation). Altogether 58% of DAS patients and 35% of IM 400 mg patients have had grade 3-4 toxicities (P = .0001), most commonly hematologic, including thrombocytopenia (< 50 × 109/L) in 18% and 8%, respectively (P = .024; Table 4). The patient who was randomized to DAS but treated with IM 400 mg had no toxicities of grade 3 or 4.
Toxicities of 245 patients with CML in chronic phase, by treatment arm
| . | Imatinib 400 mg/d, N = 123, n (%) . | Dasatinib 100 mg/d, N = 122, n (%) . | ||
|---|---|---|---|---|
| All grades . | Grade 3-4 . | All grades . | Grade 3-4 . | |
| Hematologic toxicities | ||||
| Hemoglobin | 87 (71) | 5 (4) | 87 (71) | 12 (10) |
| Neutrophils | 45 (37) | 15 (12) | 45 (37) | 18 (15) |
| Febrile neutropenia | 0 (0) | 0 (0) | 3 (2) | 3 (2) |
| Platelets | 42 (34) | 10 (8) | 71 (58) | 22 (18) |
| Fluid retention | ||||
| Edema (any) | 61 (50) | 3 (2) | 28 (23) | 1 (1) |
| Pleural effusion | 2 (2) | 1 (1) | 21 (17) | 3 (2) |
| Pericardial effusion | 0 (0) | 0 (0) | 4 (3) | 3 (2) |
| Gastrointestinal toxicities | ||||
| Diarrhea | 51 (41) | 2 (2) | 41 (34) | 6 (5) |
| Nausea | 63 (51) | 1 (1) | 34 (28) | 0 (0) |
| Vomiting | 25 (20) | 0 (0) | 20 (16) | 1 (1) |
| Anorexia | 12 (10) | 0 (0) | 22 (18) | 0 (0) |
| Other nonhematologic toxicities | ||||
| Fatigue | 67 (54) | 1 (1) | 67 (55) | 1 (1) |
| Musculoskeletal pain (any) | 54 (44) | 2 (2) | 39 (32) | 3 (2) |
| Rash | 35 (28) | 2 (2) | 41 (34) | 0 (0) |
| Headache | 23 (19) | 3 (2) | 34 (28) | 3 (2) |
| Prolonged QTc interval | 1 (1) | 0 (0) | 2 (2) | 1 (1) |
| . | Imatinib 400 mg/d, N = 123, n (%) . | Dasatinib 100 mg/d, N = 122, n (%) . | ||
|---|---|---|---|---|
| All grades . | Grade 3-4 . | All grades . | Grade 3-4 . | |
| Hematologic toxicities | ||||
| Hemoglobin | 87 (71) | 5 (4) | 87 (71) | 12 (10) |
| Neutrophils | 45 (37) | 15 (12) | 45 (37) | 18 (15) |
| Febrile neutropenia | 0 (0) | 0 (0) | 3 (2) | 3 (2) |
| Platelets | 42 (34) | 10 (8) | 71 (58) | 22 (18) |
| Fluid retention | ||||
| Edema (any) | 61 (50) | 3 (2) | 28 (23) | 1 (1) |
| Pleural effusion | 2 (2) | 1 (1) | 21 (17) | 3 (2) |
| Pericardial effusion | 0 (0) | 0 (0) | 4 (3) | 3 (2) |
| Gastrointestinal toxicities | ||||
| Diarrhea | 51 (41) | 2 (2) | 41 (34) | 6 (5) |
| Nausea | 63 (51) | 1 (1) | 34 (28) | 0 (0) |
| Vomiting | 25 (20) | 0 (0) | 20 (16) | 1 (1) |
| Anorexia | 12 (10) | 0 (0) | 22 (18) | 0 (0) |
| Other nonhematologic toxicities | ||||
| Fatigue | 67 (54) | 1 (1) | 67 (55) | 1 (1) |
| Musculoskeletal pain (any) | 54 (44) | 2 (2) | 39 (32) | 3 (2) |
| Rash | 35 (28) | 2 (2) | 41 (34) | 0 (0) |
| Headache | 23 (19) | 3 (2) | 34 (28) | 3 (2) |
| Prolonged QTc interval | 1 (1) | 0 (0) | 2 (2) | 1 (1) |
CML indicates chronic myeloid leukemia; and QTc, corrected QT interval.
Selected nonhematologic toxicities are shown in Table 4. There were more toxicities of edema, nausea, and muscle pain in the IM group compared with DAS; in contrast, the DAS group had more pleural effusions. Of the 21 cases of pleural effusions in the DAS cohort (compared with 2 in the IM arm), 3 were judged to be grade 3-4.
Dose reductions and escalations
As shown in Table 5, 59 patients were removed from protocol treatment in the first year, including 16 DAS and 12 IM patients because of toxicity, most frequently edema or pleural effusion (4 DAS and 2 IM patients), rash/pruritis (4 IM patients), and cardiac toxicities (3 DAS patients; see also supplemental Table 2). Nine others (3 DAS, 6 IM) were removed because of refusal. Nearly half of the patients in each arm (43% DAS, 46% IM) are known to have completed 1 year of treatment with no dose reduction or interruption. Another 52 patients had temporary discontinuations of protocol treatment (20 DAS, 9 IM) or dose reductions (16 DAS, 7 IM) in the first year. DAS was reduced to 70 mg/d and 50 mg/d permanently for 6 and 3 patients, respectively, and temporarily for 5 and 2 patients; IM was reduced to 300 mg/d and 200 mg/d permanently for 1 and 3 patients, and temporarily for 2 and 1 patients. Only 3 patients had dose escalations in the first year, 1 DAS patient to 180 mg/d, and 2 IM patients to 600 mg/d and 800 mg/d.
Reasons for removal from protocol treatment and dose reduction or discontinuation within 12 months, by treatment arm
| . | Imatinib 400 mg/d, N = 123, n (%) . | Dasatinib 100 mg/d, N = 123, n (%) . |
|---|---|---|
| Reason for removal from treatment | Patients removed from treatment within 12 mo | |
| Toxicity | 12 (10) | 16 (13) |
| Refusal | 6 (5) | 3 (2) |
| Failure to achieve CHR | 4 (3) | 1 (1) |
| Relapse or progression | 4 (3) | 1 (1) |
| Other* | 8 (7) | 4 (3) |
| Total | 34 (28) | 25 (20) |
| Dose reduction or discontinuation within 12 mo | Patients not removed from treatment within 12 mo | |
| None | 57 (46) | 53 (43) |
| Temporary discontinuation | 9 (7) | 20 (16) |
| Permanent reduction† | 4 (3) | 9 (7) |
| Temporary reduction | 3 (2) | 7 (6) |
| Indeterminate | 16 (13) | 8 (7) |
| Received wrong treatment | 0 (0) | 1 (1) |
| . | Imatinib 400 mg/d, N = 123, n (%) . | Dasatinib 100 mg/d, N = 123, n (%) . |
|---|---|---|
| Reason for removal from treatment | Patients removed from treatment within 12 mo | |
| Toxicity | 12 (10) | 16 (13) |
| Refusal | 6 (5) | 3 (2) |
| Failure to achieve CHR | 4 (3) | 1 (1) |
| Relapse or progression | 4 (3) | 1 (1) |
| Other* | 8 (7) | 4 (3) |
| Total | 34 (28) | 25 (20) |
| Dose reduction or discontinuation within 12 mo | Patients not removed from treatment within 12 mo | |
| None | 57 (46) | 53 (43) |
| Temporary discontinuation | 9 (7) | 20 (16) |
| Permanent reduction† | 4 (3) | 9 (7) |
| Temporary reduction | 3 (2) | 7 (6) |
| Indeterminate | 16 (13) | 8 (7) |
| Received wrong treatment | 0 (0) | 1 (1) |
CHR indicates complete hematologic response; DAS, dasatinib; and IM, imatinib mesylate.
Other reasons include patient/physician dissatisfaction with response and/or decision to change treatment (7 IM patients, 1 DAS patient), loss of insurance (1 IM patient), and other medical reasons (3 DAS patients: myocardial infarction, circulatory complications, and pregnancy).
Includes 8 patients (2 IM, 6 DAS) who were last known to be on treatment at reduced doses.
Survival and deaths
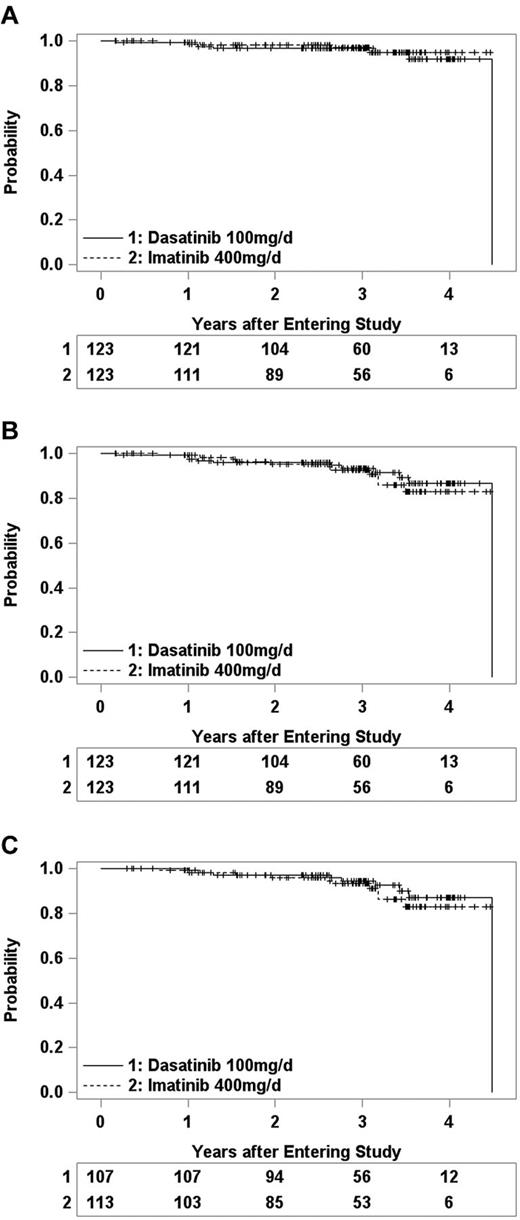
There have been very few deaths, relapses, or progressions, and consequently OS, PFS, and RFS are similar in the 2 arms (Figure 3). Eleven patients have died (Table 6), and the other 235 were last known to be alive between 2 months and 4.5 years (median 3.0 years) after entering the study. OS at 3 years was 97% (91%-99%) in the DAS arm and 97% (95% CI 90%-99%) in the IM arm. Fifteen patients (6 DAS, 9 IM) relapsed from CHR (5 DAS, 7 IM) or progressed to CML-AP (1 IM) or CML-BC (1 DAS, 1 IM), and 7 others (5 DAS, 2 IM) died without report of relapse or progression. PFS at 3 years was 93% (86%-96%) in DAS arm and 90% (82%-95%) in the IM arm. Among the 220 patients who achieved CHR, 14 (6 DAS, 8 IM) had CML relapse and 5 others (3 DAS, 2 IM) died in CHR. RFS at 3 years after achieving CHR was 91% (82%-95%) and 88% (78%-94%) in the DAS and IM arms, respectively.
Kaplan-Meier estimates of treatment outcomes for CML-CP patients randomized to DAS (1) or IM (2) therapy. Tickmarks indicate censored observations. Numbers remaining at risk are shown beneath each plot. (A) Overall survival. (B) Progression-free survival. (C) Relapse-free survival of patients who achieved complete hematologic remission.
Kaplan-Meier estimates of treatment outcomes for CML-CP patients randomized to DAS (1) or IM (2) therapy. Tickmarks indicate censored observations. Numbers remaining at risk are shown beneath each plot. (A) Overall survival. (B) Progression-free survival. (C) Relapse-free survival of patients who achieved complete hematologic remission.
Summary of deaths on Study S0325
| Patient no. . | Age at entry, y . | Day of death . | Cause of death . | Remark . |
|---|---|---|---|---|
| Dasatinib 100 mg/d | ||||
| 1 | 90 | 60 | Not specified | Off protocol treatment at day 17 due to treatment-related pulmonary embolism |
| 2 | 55 | 368 | Not specified | Off protocol treatment at day 133 due to relapse from CHR to blast crisis |
| 3 | 69 | 406 | Lung cancer (diagnosed at 10 mo) | Off protocol treatment at day 39 due to neuromotor toxicity |
| 4 | 46 | 472 | Motor vehicle accident | On protocol treatment until death |
| 5 | 68 | 1150 | Congestive heart failure, not related to protocol treatment | On protocol treatment until death |
| 6 | 67 | 1290 | Metastatic bladder cancer | Off protocol treatment at day 944 due to diagnosis of bladder cancer |
| 7 | 40 | 1639 | GVHD from postrelapse stem cell transplantation | Off protocol treatment at day 721 due to relapse from CHR |
| Imatinib 400 mg/d | ||||
| 8 | 50 | 246 | CML | Off protocol treatment at day 134 due to relapse from CHR |
| 9 | 63 | 415 | CML | Off protocol treatment at day 186 due to relapse from CHR |
| 10 | 75 | 961 | Cardiopulmonary arrest, not related to protocol treatment or CML | On protocol treatment until death |
| 11 | 70 | 1126 | Cardiopulmonary arrest, not related to protocol treatment or CML | On protocol treatment until death |
| Patient no. . | Age at entry, y . | Day of death . | Cause of death . | Remark . |
|---|---|---|---|---|
| Dasatinib 100 mg/d | ||||
| 1 | 90 | 60 | Not specified | Off protocol treatment at day 17 due to treatment-related pulmonary embolism |
| 2 | 55 | 368 | Not specified | Off protocol treatment at day 133 due to relapse from CHR to blast crisis |
| 3 | 69 | 406 | Lung cancer (diagnosed at 10 mo) | Off protocol treatment at day 39 due to neuromotor toxicity |
| 4 | 46 | 472 | Motor vehicle accident | On protocol treatment until death |
| 5 | 68 | 1150 | Congestive heart failure, not related to protocol treatment | On protocol treatment until death |
| 6 | 67 | 1290 | Metastatic bladder cancer | Off protocol treatment at day 944 due to diagnosis of bladder cancer |
| 7 | 40 | 1639 | GVHD from postrelapse stem cell transplantation | Off protocol treatment at day 721 due to relapse from CHR |
| Imatinib 400 mg/d | ||||
| 8 | 50 | 246 | CML | Off protocol treatment at day 134 due to relapse from CHR |
| 9 | 63 | 415 | CML | Off protocol treatment at day 186 due to relapse from CHR |
| 10 | 75 | 961 | Cardiopulmonary arrest, not related to protocol treatment or CML | On protocol treatment until death |
| 11 | 70 | 1126 | Cardiopulmonary arrest, not related to protocol treatment or CML | On protocol treatment until death |
CHR indicates complete hematologic response; and CML, chronic myeloid leukemia.
Discussion
This randomized trial of IM 400 mg/d versus DAS 100 mg/d demonstrated that (1) DAS was associated with better short-term response as reflected by improved cytogenetic and molecular response, as well as fewer relapses and progression compared with IM; (2) but DAS was also associated with greater toxicity compared with IM, particularly hematologic toxicities. Overall survivals were equally outstanding for both arms, with a 3-year OS of 97%. These results are similar to phase 2 single institution trials and the industry sponsored-phase 3 DASISION trial.
By all measures of response our results are very similar to the DASISION trial.12 In that trial, the probabilities of CCyR by 12 months were 72% and 83% for the IM and DAS arms, respectively; in this trial, the corresponding results were 69% and 84%. In addition, the molecular response rates at 12 months calculated as in the DASISION trial, that is, the proportions with at least 3-log reductions of BCR-ABL transcript among all patients (including those without molecular data), were quite similar: 28% and 46% (IM and DAS) in the DASISION trial, compared with 33% and 47%, respectively, in the current trial. Similarly, both trials had fewer progressions to advanced-phase disease in the DAS arm, as the PFS probabilities at 12 months in the DASISION trial were 97% and 96% in the IM and DAS arms, compared with 95% and 98%, respectively. Perhaps the most important response results are the total fraction of patients who fail to obtain a CHR or who relapse or progress because these patients will need a new intervention. In the DASISION trial, these patients accounted for 7% of the IM cases and 3% in the DAS arm; in this trial, the respective values were 6% and 3%, respectively.
For reasons that are unclear, the compliance with sample submission, particularly cytogenetics, was hardly optimal, as nearly 50% of potentially informative cytogenetic data were missing. This may reflect the large shift in practice patterns, where molecular testing is increasingly being used as the primary method to measure response. However unsatisfying, it is unlikely that this influenced the study conclusions, given the missing data were distributed evenly across the 2 study arms, and that the results so closely parallel those from other second-generation TKI trials.
A major difference in this study, compared with DASISION, was the toxicity experience in both the IM and DAS arms. Overall, significantly fewer patients treated with IM in our study had any toxicities of grades 3-4 compared with DAS (35% vs 58%; these analogous values are not reported in the DASISION trial). In addition, 10% of the IM and 13% of the DAS arm patients had to discontinue the trial because of treatment toxicity; the respective values in the DASISION trial were 4% and 5%. While grade 3-4 hematologic toxicity was more common in the DAS arm (8% IM vs 18% DAS), the values are quite similar to those in DASISION (10% IM vs 19% DAS), and this was not a common reason for study discontinuation. Indeed, of the 28 total patients who discontinued because of toxicity, only 2 (1 in each arm) were taken off protocol treatment for hematologic toxicity.
These data, taken together with very similar data from the nilotinib Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) trial,13 tell a very similar story: second-generation TKIs show a superior short-term response, and fewer progressions compared with IM. However, in all 3 studies, there is no statistically significant difference in survival of DAS/nilotinib (NIL) versus IM. Whether this is an artifact of insufficient follow-up time, insufficient patient/events, or actual natural history of chronic-phase CML treated with these TKIs is yet to be determined.
Thus, for newly diagnosed chronic-phase CML, we now have an embarrassment of riches—a “standard” frontline therapy (IM) with a long-term track record with regard to response and toxicity, and 2 more potent second-generation drugs (DAS and NIL), with an improved short-term response that may translate into long-term benefits, but without the long-term toxicity experience as IM (an example is the recent description of rare cases of pulmonary arterial hypertension associated with DAS). How do a physician and patient choose the “best” of the 3 available TKIs? Both the ELN and the NCCN recommendations allow for any of the 3 as frontline therapy for chronic-phase disease. In the absence of such guidance, the choice of drugs can be individualized considering at least the following 4 factors: (1) risk of progression: patients with higher Sokal/Hasford scores might benefit from DAS/NIL; (2) preexisting comorbidity: while there are no strong data, it would be sensible for example, to avoid NIL in the presence of significant cardiac disease, and DAS with pulmonary disease, etc; (3) compliance: adequate TKI exposure is clearly essential to optimize response.17 It is not clear that compliance is influenced greatly by drug schedule, but once a day (IM, DAS) versus twice a day (NIL) might be an important issue for a small subset of patients; (4) physician experience: in the United States, most CML patients are cared for in the community, and given its low incidence, most physicians treat only small numbers of CML patients, and thus, a physician may have much more practical experience with one agent than another.
IM will soon become generic, so it may become increasing relevant to determine how patients might be optimally managed in treatment strategies combining the cheaper TKI (IM) with the more potent, but more expensive, alternative (DAS/NIL). Could a strategy of starting with a generic IM, and switching to DAS or NIL only if the patient does not hit aggressive treatment milestones, be a superior cost-effective strategy than starting and staying with a more expensive second-generation drug? Or alternatively, could we treat with a second-generation TKI, and switch to a generic IM “maintenance” if the patient enjoys a deep molecular response?
CML has ushered in “targeted” therapy, the use of molecular markers as surrogate treatment end points, the development of second-generation TKIs, and recently, the phenomena of TKI discontinuation in patients who are persistently free of molecular disease. This could be a very interesting next few years as the complementary yet competing worlds of molecular biology, drug development, and health policy vie to cure CML.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Public Health Service Cooperative Agreement grant numbers CA32102-31, CA38926-25; CA31946, CA41287, CA32291, and CA077202 from the National Institutes of Health; and Canadian Cancer Society Research Institute Grant 21039.
National Institutes of Health
Authorship
Contribution: J.P.R., K.J.K., and B.J.D. oversaw analysis and writing of the manuscript; J.P.R., S.K.-R., W.S., G.M., and E.P. performed BCR-ABL monitoring; J.P.R., K.J.K., F.R.A., S.K.-R., W.S., E.P., M.W., R.A.L., P.E., M.T., J.L., A.R.T., and B.J.R. contributed to study design, study implementation, and writing of the manuscript; K.J.K. performed statistical analysis; and B.J.D. was the principal investigator.
Conflict-of-interest disclosure: J.P.R. received honoraria (consulting) from Novartis, Bristol-Myers Squibb (BMS), Pfizer, and Ariad, and research support from Novartis and BMS. K.J.K. received salary support for this trial from BMS as did the SWOG Statistical Center. R.A.L. received honoraria for consulting from Novartis, BMS, and Pfizer. W.S. received research support from Novartis and BMS. M.D. received honorarium (consulting) from BMS and Novartis, and research support from Novartis. B.J.D.'s institution received clinical trial support from Novartis and BMS. Oregon Health & Science University (OHSU) and B.J.D. have a financial interest in MolecularMD. OHSU has licensed technology used in some of these clinical trials to MolecularMD. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. J.L. received consulting fees from BMS, Novartis, and Pfizer, and research support from BMS and Novartis. A.R.T. received honoraria and research support form BMS and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Jerald P. Radich, Fred Hutchinson Cancer Research Center, 1400 Fairview Ave N, C2-023, Seattle, WA 98109; e-mail: jradich@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal