Abstract
The cytoprotective effects of activated protein C (aPC) are well established. In contrast, the receptors and signaling mechanism through which aPC conveys cytoprotection in various cell types remain incompletely defined. Thus, within the renal glomeruli, aPC preserves endothelial cells via a protease-activated receptor-1 (PAR-1) and endothelial protein C receptor-dependent mechanism. Conversely, the signaling mechanism through which aPC protects podocytes remains unknown. While exploring the latter, we identified a novel aPC/PAR-dependent cytoprotective signaling mechanism. In podocytes, aPC inhibits apoptosis through proteolytic activation of PAR-3 independent of endothelial protein C receptor. PAR-3 is not signaling competent itself as it requires aPCinduced heterodimerization with PAR-2 (human podocytes) or PAR-1 (mouse podocytes). This cytoprotective signaling mechanism depends on caveolin-1 dephosphorylation. In vivo aPC protects against lipopolysaccharide-induced podocyte injury and proteinuria. Genetic deletion of PAR-3 impairs the nephroprotective effect of aPC, demonstrating the crucial role of PAR-3 for aPC-dependent podocyte protection. This novel, aPC-mediated interaction of PARs demonstrates the plasticity and cell-specificity of cytoprotective aPC signaling. The evidence of specific, dynamic signaling complexes underlying aPC-mediated cytoprotection may allow the design of cell type specific targeted therapies.
Introduction
The cytoprotective effects of activated protein C (aPC) are well established, but the underlying mechanism remains a matter of debate.1,2 The uncertainty of aPC-dependent signaling stems from the initial observation that aPC conveys cytoprotective effects via protease-activated receptor-1 (PAR-1), the same receptor through which thrombin (at concentrations > 0.1nM) mediates opposing effects.1 The physiologic relevance of aPC-dependent PAR-1 activation has been further questioned based on kinetic studies showing that aPC is approximately 104-fold less potent than thrombin in regard to PAR-1 cleavage.2 Specificity of aPC-mediated cytoprotection has been attributed to the coreceptor endothelial protein C receptor (EPCR).3 The recent identification of further coreceptors mediating aPC-dependent effects, such as EDG1 or ApoER2, and the organization of these receptor complexes in lipid rafts provided new insight into mechanisms of aPC-dependent cytoprotection.4-7
We and others have recently identified a nephroprotective role of aPC.8-10 In experimental diabetic nephropathy, aPC prevents apoptosis of endothelial cells and podocytes, the cellular constituents of the glomerular filtration barrier.8 Glucose-induced endothelial cell apoptosis is prevented by aPC involving activation of PAR-1 and EPCR.8 Conversely, the receptors and the signaling mechanism underlying the cytoprotective, antiapoptotic effect of aPC in podocytes remain elusive.
In the course of our studies, we observed that podocytes lack EPCR but express PAR-3. In renal glomeruli, expression of PAR-3 is predominantly localized to podocytes. PAR-3 is a potential receptor through which aPC may convey cytoprotection, as aPC-mediated neuroprotection depends at least in part on PAR-3 in in vivo and in vitro models of neuronal damage or N-methyl-D-aspartate–induced apoptosis.11-13 However, insights into the mechanism of aPC/PAR-3 mediated cytoprotection are lacking. This may be attributable to PAR-3's apparent inability to directly alter cellular signaling. In regard to thrombin/PAR-3 signaling, this dogma has been recently challenged.14 Other potential mechanism of thrombin–PAR-3–dependent intracellular signaling include interaction of PAR-3′ tethered ligand with other PARs (PAR-1, PAR-2), activation of PAR-4 after binding of thrombin to the hirudin-like sequence of PAR-3, or allosteric modulation of G-protein function in constitutively present PAR heterodimers.15-20 These insights into mechanisms of PAR-3–dependent signaling are derived from studies using either thrombin or receptor activating peptides as PAR-3 agonists, whereas studies evaluating the mechanism of aPC signaling via PAR-3 are lacking.
To identify the receptors and signaling mechanism involved in aPC-mediated podocyte protection, we used immortalized human and mouse podocytes. We show that aPC-dependent inhibition of podocyte apoptosis requires cleavage of the extracellular N-terminal end of PAR-3 and heterodimerization of PAR-3 with PAR-2 (human podocytes) or with PAR-1 (mouse podocytes). Using the lipopolysaccharide (LPS)–induced podocyte injury model, we demonstrate that aPC requires PAR-3 for maximal podocyte protection in vivo. These findings identify a new mechanism of aPC-mediated cytoprotection, which supports podocyte survival and depends on a novel aPC-inducible, PAR-3–dependent signaling mechanism.
Methods
See supplemental Methods for further details (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell culture
Conditionally immortalized human and mouse wild-type podocytes were cultured as described elsewhere.21,22 In brief, podocytes were routinely grown on plates coated with collagen type 1 at 33°C in the presence of IFN-γ (10 U/mL) to enhance expression of a thermosensitive T antigen. Under these conditions, cells proliferate and remain undifferentiated. To induce differentiation, podocytes were grown at 37°C in the absence of IFN-γ for 14 days. Experiments were performed after 14 days of differentiation. Differentiation was confirmed by determining expression of synaptopodin and Wilms tumor-1 protein. Mouse mesangial cells were obtained from ATCC and cultured according to the distributor's recommendations. Briefly, cells were maintained at 37°C in a humidified 5% CO2 incubator in a 3:1 mixture of DMEM and Ham's F12 medium with 14mM HEPES and 5% FBS. Immortalized mouse glomerular endothelial cells were grown in RPMI 1640 medium with 10% heat-inactivated FCS as described.23
In vivo podocyte injury models
Animal experiments were conducted following standards and procedures approved by the local Animal Care and Use Committee (Regierungspräsidium Karlsruhe, Germany). For the LPS-induced podocyte injury model, we used 8- to 10-week-old littermates with an at least 98% C57BL/6-derived genetic background. A subset of mice was intraperitoneally injected with ultrapure LPS (200 μg/mice in a total volume of 200 μL sterile PBS, InvivoGen). Recombinant human aPC (20 μg/mice) was intravenously injected 6 hours and 18 hours after the LPS challenge. Mice were placed in metabolic cages for 4 hours to collect urine before kidneys were harvested 24 hours after LPS injection. Proteinuria was determined using the Bradford reagent.
Determination of apoptosis
Podocytes were serum-starved overnight in serum-free medium and pretreated with aPC or activating peptides for 3 hours before the addition of puromycin aminonucleoside (PAN; 30 μg/mL). aPC was added after every 12 hours. Cells were incubated with cleavage blocking anti–PAR-1 (human podocytes: ATAP-2, 10 μg/mL and WEDE15 10 μg/mL; mouse podocytes: H-111, 10 μg/mL), anti–PAR-2 (SAM-11, 10 μg/mL), anti–PAR-3 (H-103, 20 μg/mL), or anti–PAR-4 (H-120, 10 μg/mL) antibodies for 30 minutes before treatment with APC. PAR agonist peptides (PAR-APs) and control peptide were used at 20μM concentration. After 36 hours of PAN treatment, cells were fixed in 4% neutral buffered formalin, washed in PBS, and apoptosis was determined using the TUNEL method as previously described.24 Briefly, cells were incubated with terminal deoxynucleotidyl transferase in the presence of fluorescein-labeled dUTP (60 minutes at 37°C) and counterstained with Hoechst 33258 (3.5 μg/mL). Random images were obtained, and the frequency (in percentage) of TUNEL-positive cells was determined by a blinded investigator.
Mouse mesangial cells were cultured as described in “Cell culture” in 6-well plates. At 50% to 60% confluence, cells were transiently transfected with wild-type or mutant PAR-3 expression constructs using Hifect transfection reagent. Cells were treated 24 hours after transfection with either 1μM staurosporine for 6 hours in the presence or absence of aPC. Cells were fixed and apoptosis was determined using TUNEL method as described in the previous paragraph.
Determination of aPC induced PAR-3 cleavage
To determine aPC-induced PAR-3 cleavage, mesangial cells were transiently transfected with PAR-3 expression constructs (for further information see supplements). Twenty-four hours after transfection, aPC (20 μg/mL) was added to the cells. After 1 hour, culture supernatants were collected and immunoprecipitated with 3 μg of rabbit polyclonal anti-V5 antibody. Immunoprecipitates were purified with protein A/G agarose beads and separated by SDS-PAGE (15%), transferred to membranes, and subjected to immunoblotting with anti–V5-HRP–conjugated antibody.
Statistical analysis
The data are summarized as the mean ± SEM. The Kolmogorov-Smirnov test was used to determine whether the data are consistent with a Gaussian distribution. Statistical analyses were performed with Student t test, ANOVA, or Kruskal-Wallis test as appropriate. Posthoc comparisons of ANOVA were corrected with the method of Tukey. StatistiXL (http://www.statistixl.com) and Prism Version 5 software (GraphPad) was used for statistical analyses. Statistical significance was accepted at values of P < .05.
Results
aPC inhibits apoptosis in podocytes independent of its anticoagulant properties
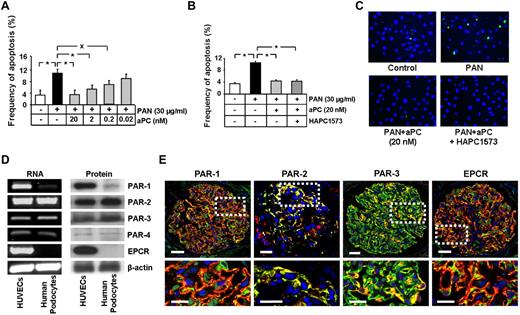
We have recently shown that aPC prevents podocyte apoptosis.8 To determine whether aPC inhibits podocyte apoptosis independent of its anticoagulant properties, we induced apoptosis in human podocytes in vitro. Treatment of podocytes with PAN (30 μg/mL)–induced apoptosis (10.7% vs 3.1% in control; P < .001, Figure 1A,C). Treatment with aPC reduced PAN-induced podocytes apoptosis, even at concentrations as low as 0.2nM (6.8% vs 10.7% in PAN only, P < .01, Figure 1A,C). The antiapoptotic effect was not impaired after preincubation of aPC with the antibody HAPC1573, which inhibits specifically its anticoagulant function without impairing the cytoprotective effect (4.4% vs 10.5% in PAN only, P < .001, Figure 1B-C). Similar results were obtained when inducing podocyte apoptosis with high glucose (30mM, data not shown). These data establish that aPC cell autonomously inhibits podocyte apoptosis independent of its anticoagulant properties.
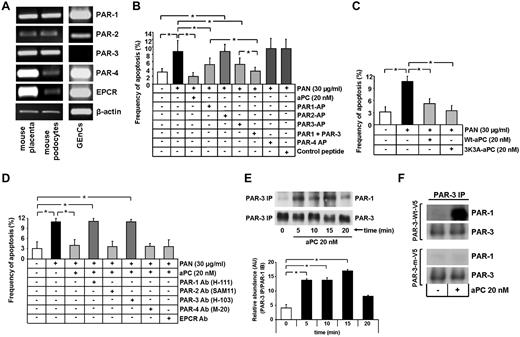
aPC, independent of its anticoagulant property, inhibits apoptosis in human podocytes, which predominantly express PAR-2 and PAR-3. (A-C) aPC reduces dose-dependently and independent of its anticoagulant effect PAN-induced apoptosis in podocytes. Bar graphs summarizing the frequency of apoptosis in PAN-stressed human podocytes treated with different concentrations of aPC (A) or treated with aPC preincubated 1:1 with the antibody HAPC1573, which specifically blocks the anticoagulant properties of aPC (B). Representative images of TUNEL assay with fluorescent-labeled nucleotides (green) and Hoechst 33258 nuclear counterstain (blue, C). (D) PAR-2 and PAR-3 are expressed in human podocytes. Representative images show expression of PARs and EPCR in human podocytes and HUVECs (positive control) as determined by semiquantitative RT-PCR (left panel) and Western blot (right panel). (E) Immunofluorescence images showing strong colocalization (yellow) of PAR-2 and PAR-3, but not of PAR-1 or EPCR (all red) with the podocyte specific marker synaptopodin (green) in normal human kidney samples. PAR-2, conventional fluorescence microscopy on frozen section; PAR-1, PAR-3, and EPCR, confocal microscopy on paraffin sections; Hoechst 33258 nuclear counterstain (blue). Top: Bar represents 50 μm. Bottom: Bar represents 10 μm. Data are mean ± SEM of at least 3 independent experiments performed in duplicates. *P < .001 vs control. XP < .01 vs control (ANOVA).
aPC, independent of its anticoagulant property, inhibits apoptosis in human podocytes, which predominantly express PAR-2 and PAR-3. (A-C) aPC reduces dose-dependently and independent of its anticoagulant effect PAN-induced apoptosis in podocytes. Bar graphs summarizing the frequency of apoptosis in PAN-stressed human podocytes treated with different concentrations of aPC (A) or treated with aPC preincubated 1:1 with the antibody HAPC1573, which specifically blocks the anticoagulant properties of aPC (B). Representative images of TUNEL assay with fluorescent-labeled nucleotides (green) and Hoechst 33258 nuclear counterstain (blue, C). (D) PAR-2 and PAR-3 are expressed in human podocytes. Representative images show expression of PARs and EPCR in human podocytes and HUVECs (positive control) as determined by semiquantitative RT-PCR (left panel) and Western blot (right panel). (E) Immunofluorescence images showing strong colocalization (yellow) of PAR-2 and PAR-3, but not of PAR-1 or EPCR (all red) with the podocyte specific marker synaptopodin (green) in normal human kidney samples. PAR-2, conventional fluorescence microscopy on frozen section; PAR-1, PAR-3, and EPCR, confocal microscopy on paraffin sections; Hoechst 33258 nuclear counterstain (blue). Top: Bar represents 50 μm. Bottom: Bar represents 10 μm. Data are mean ± SEM of at least 3 independent experiments performed in duplicates. *P < .001 vs control. XP < .01 vs control (ANOVA).
PAR-2 and PAR-3 are predominantly expressed in podocytes within human glomeruli
To identify the receptors through which aPC inhibits podocyte apoptosis, we first determined the expression of PARs and EPCR in human podocytes. Surprisingly, immortalized human podocytes do not express EPCR (mRNA and protein) and express very low levels of PAR-1 and PAR-4 (mRNA and protein), whereas PAR-2 and PAR-3 are readily detectable (Figure 1D). In human glomeruli, PAR-3 and PAR-2 colocalized predominantly with synaptopodin, a podocyte-specific marker25 (Figure 1E; supplemental Figure 1, yellow indicates colocalization). Conversely, on immunohistochemical analyses, PAR-1 and EPCR poorly colocalized with synaptopodin, which is consistent with the absence of EPCR and the low expression of PAR-1 in podocytes in vitro (Figure 1E; supplemental Figure 1). Thus, PAR-2 and PAR-3 are the PARs predominantly expressed in human podocytes.
PAR-3 is required for aPC-dependent inhibition of human podocyte apoptosis
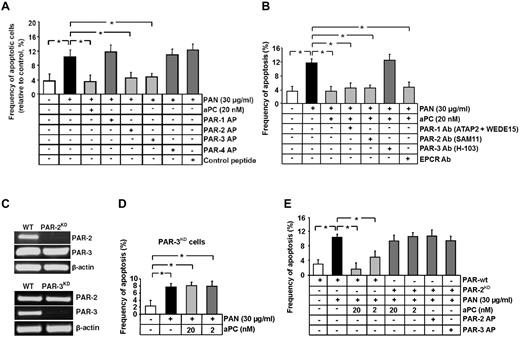
This expression pattern raises the question whether aPC mediates its antiapoptotic effects through a mechanism entirely distinct from the established receptor-dependent mechanism in human endothelial cells, which requires PAR-1 and EPCR.3,8 We next evaluated the functional relevance of PARs and EPCR for the antiapoptotic effect of aPC in human podocytes. PAR-2 and PAR-3 agonist peptide efficiently prevented PAN-induced podocyte apoptosis (4.6% and 4.7% vs 10.2% in PAN only, P < .001 and P < .001, respectively, Figure 2A), whereas exposure to either PAR-1 or PAR-4 agonist peptide was not protective (11.8% and 10.3% vs 10.2% in PAN only, P = .38 and P = .95, respectively, Figure 2A).
Inhibition of podocyte apoptosis depends on PAR-3. (A-B) Frequency of apoptosis in PAN stressed podocytes treated with PAR-APs (A) or preincubated with PAR or EPCR blocking antibodies before treatment with aPC (B). Activation of PAR-2 and PAR-3 protects podocytes against PAN-induced apoptosis. Of note, blocking PAR-3 activation, but not PAR-2 activation, abolishes the protective effect of aPC. (C) Representative image showing shRNA-mediated knockdown of PAR-2 and PAR-3 in human podocytes (RT-PCR). (D) Bar graph summarizing the frequency of apoptosis in PAN-stressed PAR-3KD human podocytes. aPC fails to prevent PAN-induced apoptosis in PAR-3KD podocytes. (E) Bar graph summarizing the frequency of apoptosis in PAN-stressed wild-type and PAR-2KD human podocytes treated with aPC or PAR-APs. aPC as well as the activating peptides for PAR-2 and PAR-3 fail to prevent PAN-induced apoptosis in PAR-2KD podocytes. Data are mean ± SEM of at least 3 independent experiments. *P < .001 vs control (ANOVA).
Inhibition of podocyte apoptosis depends on PAR-3. (A-B) Frequency of apoptosis in PAN stressed podocytes treated with PAR-APs (A) or preincubated with PAR or EPCR blocking antibodies before treatment with aPC (B). Activation of PAR-2 and PAR-3 protects podocytes against PAN-induced apoptosis. Of note, blocking PAR-3 activation, but not PAR-2 activation, abolishes the protective effect of aPC. (C) Representative image showing shRNA-mediated knockdown of PAR-2 and PAR-3 in human podocytes (RT-PCR). (D) Bar graph summarizing the frequency of apoptosis in PAN-stressed PAR-3KD human podocytes. aPC fails to prevent PAN-induced apoptosis in PAR-3KD podocytes. (E) Bar graph summarizing the frequency of apoptosis in PAN-stressed wild-type and PAR-2KD human podocytes treated with aPC or PAR-APs. aPC as well as the activating peptides for PAR-2 and PAR-3 fail to prevent PAN-induced apoptosis in PAR-2KD podocytes. Data are mean ± SEM of at least 3 independent experiments. *P < .001 vs control (ANOVA).
We next used blocking antibodies to inhibit aPC-dependent activation of PARs or interaction with EPCR. Preincubation of human podocytes with blocking antibodies against PAR-1, PAR-2, or EPCR did not impair the antiapoptotic effect of aPC. Only a PAR-3 blocking antibody efficiently abolished the antiapoptotic effect of aPC in podocytes (12.5% vs 3.5% in aPC-treated, P < .001, Figure 2B). These results indicate that aPC inhibits apoptosis in human podocytes by activation of PAR-3.
Considering that the PAR-2 agonist peptide was sufficient to prevent apoptosis in human podocytes and that PAR-3 is generally considered to be signaling incompetent, we evaluated whether the aPC–PAR-3-mediated antiapoptotic effect depends on PAR-2. To this end, we generated stable PAR-2 and PAR-3 knockdown podocytes (PAR-2KD, PAR-3KD podocytes). Knockdown of PAR-2 did not reduce PAR-3 expression, and knockdown of PAR-3 did not reduce PAR-2 expression (Figure 2C). In agreement with results obtained using blocking antibodies, the antiapoptotic effect of aPC was completely abolished in PAR-3KD podocytes (7.6% PAN-treated PAR-3KD podocytes vs 8.0% in aPC-treated PAR-3KD podocytes, P = .728, Figure 2D). Of note, the antiapoptotic effects of aPC or of the agonist peptides for PAR-2 and PAR-3 were also completely abolished in PAR-2KD podocytes (9.5% in 20nM aPC-treated PAR-2KD and 8.9% in PAR-3 AP-treated PAR-2KD vs 1.7% aPC-treated PAR-wt podocytes, P < .001 and P < .001, respectively, Figure 2E). In addition, preincubation of podocytes with the PAR-2 cleavage blocking antibody did not impede inhibition of apoptosis by the PAR-2 agonist peptide (supplemental Figure 2), indicating that signaling through, but not cleavage of, PAR-2 is required for the antiapoptotic effect in human podocytes.
Taken together, the antiapoptotic effect of aPC depends primarily on PAR-3. Nevertheless, PAR-2 is required in addition to PAR-3 for the antiapoptotic effect of aPC in podocytes, indicating an interaction of PAR-2 and PAR-3 in podocytes.
The antiapoptotic effect of aPC requires PAR-2/PAR-3 dimerization in human podocytes
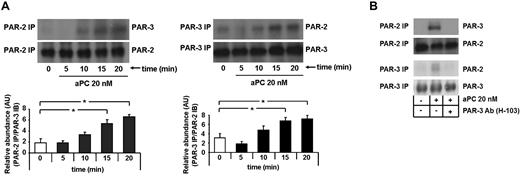
To determine whether aPC induces dimerization of PAR-2 and PAR-3, human podocytes were treated with aPC, and PAR-2/PAR-3 complex formation was determined by coimmunoprecipitation at various time points. aPC induced complex formation of PAR-2 and PAR-3, which was first detectable after 10 minutes and further increased at later time points (P = .014 in PAR-2 immunoprecipitation and P = .025 in PAR-3 immunoprecipitation, respectively, at 15 minutes; P < .001 in PAR-2 immunoprecipitation and P = .026 in PAR-3 immunoprecipitation, respectively, at 20 minutes vs 0 minutes, Figure 3A). Pretreatment of podocytes with an antibody blocking PAR-3 (20 μg/mL) prevented dimerization of PAR-3 and PAR-2 (Figure 3B). Thus, aPC induces heterodimerization of PAR-2 and PAR-3, which are both required for its antiapoptotic effect in human podocytes.
aPC induces dimerization of PAR-2 and PAR-3, which is required for apoptosis inhibition in human podocytes. (A) Representative immunoblots of immunoprecipitates showing heterodimerization of PAR-2 and PAR-3 after treatment with aPC (20nM) at different time points as indicated (top panel) and bar graphs summarizing the results (bottom panel). (B) Representative immunoblots of immunoprecipitates showing heterodimerization of PAR-2 and PAR-3 after treatment with aPC (20nM) for 15 minutes in the presence or absence of PAR-3 blocking antibody. PAR-2 IP indicates PAR-2 immunoprecipitation; PAR-3 IP, PAR-3 immunoprecipitation; anti–PAR-3, blocking antibody, 20 μg/mL; and AU, arbitrary units. Data are mean ± SEM of at least 3 independent experiments. *P < .05 vs control (ANOVA).
aPC induces dimerization of PAR-2 and PAR-3, which is required for apoptosis inhibition in human podocytes. (A) Representative immunoblots of immunoprecipitates showing heterodimerization of PAR-2 and PAR-3 after treatment with aPC (20nM) at different time points as indicated (top panel) and bar graphs summarizing the results (bottom panel). (B) Representative immunoblots of immunoprecipitates showing heterodimerization of PAR-2 and PAR-3 after treatment with aPC (20nM) for 15 minutes in the presence or absence of PAR-3 blocking antibody. PAR-2 IP indicates PAR-2 immunoprecipitation; PAR-3 IP, PAR-3 immunoprecipitation; anti–PAR-3, blocking antibody, 20 μg/mL; and AU, arbitrary units. Data are mean ± SEM of at least 3 independent experiments. *P < .05 vs control (ANOVA).
PAR-3 cleavage by aPC is required, but not sufficient to inhibit apoptosis
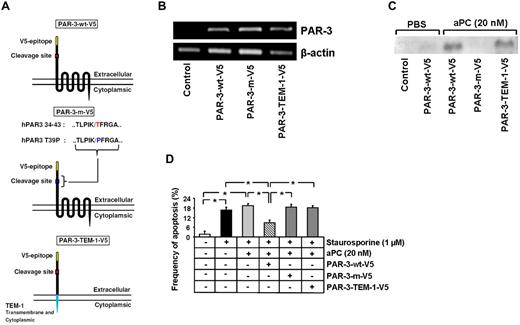
Blocking PAR-3 is sufficient to abolish the antiapoptotic effect of aPC, and both aPC and the PAR-3 agonist peptide failed to inhibit apoptosis in PAR-2KD podocytes. Based on these observations, we hypothesized that aPC binds to PAR-3, cleaving its N-terminal end and liberating a tethered ligand, which interacts with and activates PAR-2. To determine whether aPC cleaves the N-terminal end of PAR-3, we transfected mesangial cells, which do not express PAR-3 (supplemental Figure 3), transiently with V5-tagged PAR-3 (PAR-3-wt–V5) or a V5-tagged PAR-3 mutant (T39P), lacking the known cleavage site (PAR-3-m–V5, Figure 4A-B).26 After treatment with aPC for 1 hour, only the wild-type PAR-3 was cleaved, resulting in easily detectable free V5 epitope in the cellular supernatant (Figure 4C).
Cleavage of the PAR-3 N-terminal end by aPC is required, but not sufficient, to inhibit apoptosis. (A) Schematic representation of the V5-tagged wild-type and V5-tagged mutant PAR-3 expression constructs. (B) Representative image showing the expression of V5-tagged PAR-3 wild-type (PAR-wt–V5) and V5-tagged mutant expression constructs (PAR-m–V5, PAR-3–TEM-1–V5) in transiently transfected mesangial cells (semiquantitative RT-PCR). (C) Representative immunoblot showing V5 levels in the culture supernatant after treatment with PBS or aPC (20nM) for 1 hour of cells transfected with V5-tagged PAR-3 wild-type and V5-tagged mutant PAR-3 expression constructs. The detection of the V5 epitope in the supernatant reflects proteolytic activation of PAR-3. (D) Bar graph summarizing the frequency of apoptosis in staurosporine-treated mesangial cells transfected with V5-tagged PAR-3 wild-type and V5-tagged mutant PAR-3 expression constructs. Only full-length PAR-3 with a nonmutated cleavage site enables aPC to inhibit apoptosis. Data are mean ± SEM of at least 3 independent experiments. *P < .01 vs control (ANOVA).
Cleavage of the PAR-3 N-terminal end by aPC is required, but not sufficient, to inhibit apoptosis. (A) Schematic representation of the V5-tagged wild-type and V5-tagged mutant PAR-3 expression constructs. (B) Representative image showing the expression of V5-tagged PAR-3 wild-type (PAR-wt–V5) and V5-tagged mutant expression constructs (PAR-m–V5, PAR-3–TEM-1–V5) in transiently transfected mesangial cells (semiquantitative RT-PCR). (C) Representative immunoblot showing V5 levels in the culture supernatant after treatment with PBS or aPC (20nM) for 1 hour of cells transfected with V5-tagged PAR-3 wild-type and V5-tagged mutant PAR-3 expression constructs. The detection of the V5 epitope in the supernatant reflects proteolytic activation of PAR-3. (D) Bar graph summarizing the frequency of apoptosis in staurosporine-treated mesangial cells transfected with V5-tagged PAR-3 wild-type and V5-tagged mutant PAR-3 expression constructs. Only full-length PAR-3 with a nonmutated cleavage site enables aPC to inhibit apoptosis. Data are mean ± SEM of at least 3 independent experiments. *P < .01 vs control (ANOVA).
Because aPC fails to prevent apoptosis in mesangial cells,8 we used these cells to determine whether expression and proteolytic activation of PAR-3 are sufficient to confer the antiapoptotic effect of aPC. To this end, we transiently transfected mesangial cells with PAR-3-wt–V5 or PAR-3-m–V5. After exposure to staurosporine (1μM), aPC failed to inhibit apoptosis in nontransfected mesangial cells or in mesangial cells expressing the PAR-3 mutant (T39P; 19.5% and 18.5% in nontransfected and PAR-3-m–V5 transfected cells, respectively, vs 1.73% in control cells, P < .001 and P < .001, respectively, Figure 4D). Conversely, aPC reduced apoptosis in staurosporine-treated mesangial cells expressing wild-type PAR-3 (8.7% in PAR-3-wt–V5 transfected vs 19.5% in nontransfected mesangial cells, P < .001, Figure 4D). Hence, ectopic expression and proteolytic activation of PAR-3 is sufficient for aPC-dependent apoptosis inhibition in mesangial cells.
To gain insight into the structures of PAR-3 required for cross-coupling with PAR-2, we expressed a chimeric protein consisting of the V5-epitope tagged extracellular N-terminal end of PAR-3 and the transmembrane and cytoplasmic domain of endosialin (TEM-1), a type 1 transmembrane molecule (PAR-3–TEM-1–V5, Figure 4A-B). After treatment with aPC, the V5 epitope was easily detectable in the supernatant, reflecting cleavage of the N-terminal end (Figure 4C). Nevertheless, aPC failed to inhibit apoptosis in mesangial cells expressing PAR-3–TEM-1–V5, establishing that the N-terminal extracellular domain of PAR-3 is not sufficient to mediate the antiapoptotic effect of aPC (Figure 4D).
Taken together, cleavage of the N-terminal end of PAR-3 is required for the antiapoptotic effect of aPC via PAR-3, but other domains of PAR-3 are additionally required for cross-coupling with PAR-2 and apoptosis inhibition.
aPC-dependent apoptosis inhibition in podocytes requires caveolin-1
These data identify a novel, PAR-3-dependent mechanism through which aPC inhibits apoptosis in podocytes. In endothelial cells, the organization in lipid rafts and the interaction with caveolin-1 are important for aPC-mediated cytoprotection via PAR-1.6,27 To determine whether the aPC/PAR-3–mediated cytoprotection is likewise dependent on lipids rafts and caveolin-1 in podocytes, we first performed coimmunoprecipitation experiments. In resting podocytes, an interaction of PAR-2 and PAR-3 with caveolin-1 is readily detectable (Figure 5A-B). After treatment with aPC (20nM, 15 minutes), PAR-3 dimerized with PAR-2, as shown in Figure 3A, but the association of these receptors with caveolin-1 was decreased, reflecting dissociation of PAR-2 and PAR-3 from caveolin-1 (Figure 5A-B). This aPC-induced dissociation required intact lipid rafts, as the cholesterol-depleting substance methyl-β-cyclodextrin (MβCD) impaired the aPC-induced dissociation of PAR-2 and PAR-3 from caveolin-1 and the dimerization of PAR-3 with PAR-2 (Figure 5A-B).
Caveolin-1 (Cav-1) is required for aPC-dependent apoptosis inhibition in human podocytes. (A-B) Representative immunoblot images showing dissociation of PAR-2 and PAR-3 from Cav-1 and reorganization of Cav-1, PAR-2, and PAR-3 after treatment with aPC (20nM) in the presence or absence of MβCD (10mM). (C) Bar graph summarizing the frequency of apoptosis in PAN-treated Cav-1 knockdown human podocytes (Cav-1KD, left) and representative image showing shRNA-mediated knockdown of Cav-1 in human podocytes (semiquantitative RT-PCR, right). aPC and activating peptides (AP) for PAR-2 or PAR-3 fail to prevent PAN-induced apoptosis in Cav-1–deficient podocytes. (D) Representative immunoblots showing levels of Phospho-Cav-1 (pCav-1 [Tyr-14]) after treatment with aPC (20nM) at different time points in control (wild-type, wt) and PAR-3KD podocytes (top panel) and bar graphs summarizing the results (bottom panel). APC induces dephosphorylation of Cav-1 only in the presence of PAR-3. (E) Bar graph summarizing the frequency of apoptosis in PAN-stressed Cav-1KD human podocytes infected with adenoviral expression constructs, wild-type (Ad-Cav-1wt), or a Cav-1 mutant (Ad-Cav-1Y14A) and treated with aPC. aPC fails to protect against PAN-induced apoptosis in podocytes infected with phosphorylation-deficient Cav-1 mutant (Ad-Cav-1Y14A). (F) Representative immunoblots showing expression of Cav-1 in Cav-1KD podocytes transfected with adenoviral expression constructs (Ad-Cav-1wt, Ad-Cav-1Y14A). Data are mean ± SEM of at least 3 independent experiments. XP < .05 vs control. *P < .01 vs control (ANOVA).
Caveolin-1 (Cav-1) is required for aPC-dependent apoptosis inhibition in human podocytes. (A-B) Representative immunoblot images showing dissociation of PAR-2 and PAR-3 from Cav-1 and reorganization of Cav-1, PAR-2, and PAR-3 after treatment with aPC (20nM) in the presence or absence of MβCD (10mM). (C) Bar graph summarizing the frequency of apoptosis in PAN-treated Cav-1 knockdown human podocytes (Cav-1KD, left) and representative image showing shRNA-mediated knockdown of Cav-1 in human podocytes (semiquantitative RT-PCR, right). aPC and activating peptides (AP) for PAR-2 or PAR-3 fail to prevent PAN-induced apoptosis in Cav-1–deficient podocytes. (D) Representative immunoblots showing levels of Phospho-Cav-1 (pCav-1 [Tyr-14]) after treatment with aPC (20nM) at different time points in control (wild-type, wt) and PAR-3KD podocytes (top panel) and bar graphs summarizing the results (bottom panel). APC induces dephosphorylation of Cav-1 only in the presence of PAR-3. (E) Bar graph summarizing the frequency of apoptosis in PAN-stressed Cav-1KD human podocytes infected with adenoviral expression constructs, wild-type (Ad-Cav-1wt), or a Cav-1 mutant (Ad-Cav-1Y14A) and treated with aPC. aPC fails to protect against PAN-induced apoptosis in podocytes infected with phosphorylation-deficient Cav-1 mutant (Ad-Cav-1Y14A). (F) Representative immunoblots showing expression of Cav-1 in Cav-1KD podocytes transfected with adenoviral expression constructs (Ad-Cav-1wt, Ad-Cav-1Y14A). Data are mean ± SEM of at least 3 independent experiments. XP < .05 vs control. *P < .01 vs control (ANOVA).
To evaluate the mechanistic relevance of caveolin-1 for the aPC–PAR-3–mediated antiapoptotic effect in podocytes, we generated stable caveolin-1 knockdown podocytes using shRNA (Cav-1KD, Figure 5C). aPC, PAR-2 AP, and PAR-3 AP failed to inhibit apoptosis in PAN-stressed Cav-1KD podocytes (8.5% in no aPC vs 8.7% in aPC-treated, 8.8% in PAR-2 AP-treated and 8.3% in PAR-3 AP-treated podocytes, P = .91, P = .87, and P = .85, respectively, Figure 5C). In addition to modulating the PAR-2/PAR-3 dimerization and interaction with caveolin-1, aPC time-dependently reduces caveolin-1 Tyr-14 phosphorylation (10.5 arbitrary units [AU] at 15 minutes and 6.4 AU at 20 minutes in aPC-treated podocytes vs 18.5 AU in controls [0 minutes], P = .02 and P < .01, respectively, Figure 5D). After exposure to aPC, caveolin-1 Tyr-14 phosphorylation was maintained in PAR-3kd podocytes (Figure 5D), establishing that PAR-3 is required for aPC induced Tyr-14 caveolin-1 dephosphorylation.
We next infected Cav-1KD podocytes with caveolin-1 wild-type (Ad-Cav-1wt, control) and a phosphorylation-deficient caveolin-1 mutant (Ad-Cav-1Y14A, Figure 5E-F) using adenoviral particles. After exposure to PAN, aPC failed to inhibit apoptosis in Cav-1KD cells infected with the Ad-Cav1Y14A mutant (11.1% in PAN + aPC vs 10.7% in PAN only treated podocytes, P = .79, Figure 5E), whereas the antiapoptotic effect of aPC was restored in Cav-1KD cells after reconstitution of caveolin-1 (6.1% in Cav1wt infected + PAN-treated vs 10.7% in PAN only treated podocytes, P < .005, Figure 5E). Thus, caveolin-1, which is dephosphorylated by aPC and dissociates from PAR-2 and PAR-3, is required for aPC-dependent apoptosis inhibition in podocytes.
The antiapoptotic effect of aPC requires PAR-1/PAR-3 dimerization in mouse podocytes
We next intended to demonstrate the pathophysiologic relevance of aPC-PAR-3 signaling in a murine in vivo model. Hence, we first evaluated aPC signaling in immortalized and differentiated mouse podocytes. Similar to human podocytes, mouse podocytes express PAR-3, and PAR-3 expression is predominantly localized to podocytes in mouse glomeruli (Figure 6A; and data not shown). Of note, based on the analyses of established cell lines, only mouse podocytes, but not mouse mesangial or mouse glomerular endothelial cells, express PAR-3 (Figure 6A; supplemental Figure 3). Unlike in human podocytes, mouse podocytes only weakly express PAR-2, whereas PAR-1 expression is readily detectable (Figure 6A). Thus, expression of PARs in podocytes varies in humans and mice, implying a different mechanism of receptor activation through aPC in mouse and human podocytes.
In mouse podocytes, aPC induces heterodimerization of PAR-1 and PAR-3, which are both required for aPC-mediated apoptosis inhibition. (A) Representative images show expression of PARs and EPCR in mouse podocytes, mouse GEnCs, and mouse placenta (positive control) as determined by semiquantitative RT-PCR. PAR-1 and PAR-3 are the receptors predominantly expressed in mouse podocytes. (B-D) Bar graphs summarizing the frequency of apoptosis in PAN-stressed mouse podocytes treated with PAR-APs (B), murine wild-type or mutant aPC (3K3A aPC, which lacks anticoagulant function, C), or PAR-blocking antibodies (D). Activation of PAR-1 and PAR-3 conveys the antiapoptotic effect of aPC independent of its anticoagulant function. (E) Representative immunoblots of immunoprecipitates showing heterodimerization of PAR-1 and PAR-3 after treatment with aPC (20nM) at different time points (top panel) and bar graph summarizing results (bottom panel). aPC induces heterodimerization of PAR-1 and PAR-3 in mouse podocytes. (F) Representative immunoblots of immunoprecipitates showing heterodimerization of PAR-1 and PAR-3 in mesangial cells transiently transfected with V5-tagged wild-type PAR- 3 (PAR-3-wt–V5) or V5-tagged mutant PAR-3 lacking the known cleavage site (PAR-3-m–V5). Proteolytic activation of PAR-3 by aPC is required for PAR-1/PAR-3 heterodimerization. PAR AP indicates protease-activated receptor agonist peptide; and PAR Ab, protease-activated receptor blocking antibody. Data are mean ± SEM of at least 3 independent experiments performed in duplicates. *P < .01 vs control (ANOVA).
In mouse podocytes, aPC induces heterodimerization of PAR-1 and PAR-3, which are both required for aPC-mediated apoptosis inhibition. (A) Representative images show expression of PARs and EPCR in mouse podocytes, mouse GEnCs, and mouse placenta (positive control) as determined by semiquantitative RT-PCR. PAR-1 and PAR-3 are the receptors predominantly expressed in mouse podocytes. (B-D) Bar graphs summarizing the frequency of apoptosis in PAN-stressed mouse podocytes treated with PAR-APs (B), murine wild-type or mutant aPC (3K3A aPC, which lacks anticoagulant function, C), or PAR-blocking antibodies (D). Activation of PAR-1 and PAR-3 conveys the antiapoptotic effect of aPC independent of its anticoagulant function. (E) Representative immunoblots of immunoprecipitates showing heterodimerization of PAR-1 and PAR-3 after treatment with aPC (20nM) at different time points (top panel) and bar graph summarizing results (bottom panel). aPC induces heterodimerization of PAR-1 and PAR-3 in mouse podocytes. (F) Representative immunoblots of immunoprecipitates showing heterodimerization of PAR-1 and PAR-3 in mesangial cells transiently transfected with V5-tagged wild-type PAR- 3 (PAR-3-wt–V5) or V5-tagged mutant PAR-3 lacking the known cleavage site (PAR-3-m–V5). Proteolytic activation of PAR-3 by aPC is required for PAR-1/PAR-3 heterodimerization. PAR AP indicates protease-activated receptor agonist peptide; and PAR Ab, protease-activated receptor blocking antibody. Data are mean ± SEM of at least 3 independent experiments performed in duplicates. *P < .01 vs control (ANOVA).
In agreement with the expression of PARs, PAR-1 and PAR-3 agonist peptide significantly reduced PAN induced mouse podocyte apoptosis (5.3% and 5.2% vs 8.9% in PAN only, P < .001 and P < .001, respectively, Figure 6B), whereas exposure to either PAR-2 or PAR-4 agonist peptide failed to protect against PAN-induced apoptosis (8.8% and 9.8% vs 8.9% in PAN only, respectively, Figure 6B). Combined usage of PAR-1 and PAR-3 agonists showed a synergistic effect, further reducing PAN-induced apoptosis to levels observed in controls (3.8% PAR-1 + PAR-3 vs 5.3% in PAR-1 and 5.2% in PAR-3, P = .007 and P = .003, respectively, Figure 6B). Treatment of PAN-stressed podocytes with the murine aPC variant 3K3A-aPC, which provides cytoprotective, but not anticoagulant, effects,28 efficiently prevented apoptosis (10.6% in PAN treated vs 5.1% Wt-aPC and 3.4% 3K3A-aPC-treated, P < .003 and P < .001, respectively, Figure 6C).
We next used blocking antibodies to inhibit aPC-dependent signaling through PARs. Preincubation of mouse podocytes with blocking antibodies against PAR-2, PAR-4, or EPCR did not impair the antiapoptotic effect of aPC. However, both PAR-1 and PAR-3 blocking antibodies efficiently abolished the antiapoptotic effect of aPC in podocytes (11.1% and 10.9% vs 4.01% in aPC-treated, P < .001, Figure 6D). Similar results were obtained when apoptosis was induced using high glucose (data not shown).
Because the antiapoptotic effect of aPC in human podocytes required heterodimerization of PAR-2 and PAR-3, we tested whether in murine podocytes aPC induces heterodimerization of PAR-3 with PAR-1. Indeed, in murine podocytes, aPC induced heterodimerization of PAR-3/PAR-1 (17.0 AU at 15 minutes vs 4.1 AU at 0 minutes, P < .005, Figure 6E). To determine whether aPC cleaves in analogy to the situation in human podocytes mouse PAR-3, we generated a mouse PAR-3 mutant lacking the known cleavage site (S38P, PAR-3-m–V5). In mouse mesangial cells, which lack PAR-3 (supplemental Figure 3), murine aPC cleaved mouse V5-tagged wild-type PAR-3 (PAR-3-wt–V5), but not the mutant PAR-3 (PAR-3-m–V5, supplemental Figure 4).
Although lacking PAR-3, mouse mesangial cells endogenously express PAR-1 (supplemental Figure 3), permitting us to determine whether proteolytic cleavage of PAR-3 is required for the interaction with PAR-1. Indeed, PAR-1/PAR-3 heterodimerization was easily detectable in mesangial cells transfected with wild-type PAR-3 (PAR-3-wt–V5), but not in those transfected with the mutant PAR-3 (PAR-3-m–V5, Figure 6F).
These results establish that, similar to human PAR-3, mouse PAR-3 plays a crucial role in exerting aPC-mediated antiapoptotic effect in murine podocytes. However, unlike in human podocytes, where PAR-3 forms a heterodimer with PAR-2, PAR-3 dimerizes with PAR-1 in mouse podocytes.
aPC-PAR-3 signaling is essential in protecting against podocyte injury and proteinuria in vivo
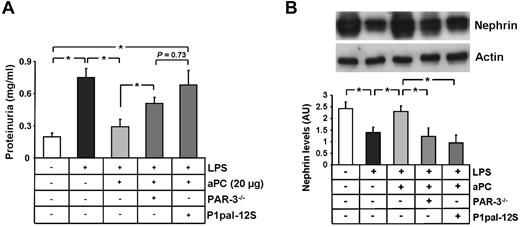
To evaluate the in vivo relevance of PAR-3 for aPC-mediated nephroprotection, we conducted studies in PAR-3 knockout (PAR-3−/−) mice. Because C57BL/6 mice are resistant to PAN-induced nephropathy,29 we used a mouse model of LPS-induced podocyte injury and proteinuria.30,31 Administration of LPS (200 μg intraperitoneally once) resulted in marked proteinuria within 24 hours in wild-type mice (0.74 mg/mL in LPS-treated vs 0.19 mg/mL PBS-treated controls, P < .005, Figure 7A). Intravenous administration of recombinant human aPC (20 μg/mice) 6 hours after LPS administration markedly reduced proteinuria in wild-type mice (0.74 in LPS only vs 0.29 mg/mL in LPS + aPC-treated mice, P < .005, Figure 7A). Proteinuria levels remained significantly elevated in aPC-treated PAR-3−/− mice (0.50 vs 0.29 mg/mL in aPC-treated wild-type mice, P < .005, Figure 7A) or in mice pretreated with the cell penetrating lipid-conjugated PAR-1 antagonist Ppal12S (0.68 mg/mL vs 0.29 mg/mL in aPC-treated mice, P < .005, Figure 7A).32-34
aPC-PAR-3 signaling protects against podocyte injury and proteinuria in vivo. (A) Bar graph summarizing proteinuria levels (milligrams per milliliter) in urine obtained 24 hours after LPS treatment. Loss of PAR-3 (PAR-3−/− mice) or blocking PAR-1 (P1pal-12S) reduces aPCs' protective effect. (B) Representative immunoblot and bar graph showing nephrin levels analyzed from renal cortex samples in LPS-treated mice, illustrating the failure of aPCs to maintain nephrin expression in PAR3−/− or P1pasl-12S–treated mice. Ppal-12S indicates PAR-1 antagonist. Data are mean ± SEM of at least 6 animals per group. *P < .05 vs control or LPS + aPC-treated (ANOVA).
aPC-PAR-3 signaling protects against podocyte injury and proteinuria in vivo. (A) Bar graph summarizing proteinuria levels (milligrams per milliliter) in urine obtained 24 hours after LPS treatment. Loss of PAR-3 (PAR-3−/− mice) or blocking PAR-1 (P1pal-12S) reduces aPCs' protective effect. (B) Representative immunoblot and bar graph showing nephrin levels analyzed from renal cortex samples in LPS-treated mice, illustrating the failure of aPCs to maintain nephrin expression in PAR3−/− or P1pasl-12S–treated mice. Ppal-12S indicates PAR-1 antagonist. Data are mean ± SEM of at least 6 animals per group. *P < .05 vs control or LPS + aPC-treated (ANOVA).
Expression of nephrin was markedly reduced in LPS-treated wild-type mice, reflecting podocyte injury (1.4 AU vs 2.4 AU in PBS-treated controls, P = .02, Figure 7B).22 Nephrin expression was maintained in aPC-treated wild-type mice (2.3 AU in LPS + aPC-treated mice vs 1.4 AU in LPS only mice, P = .03, Figure 7B), but not in aPC-treated PAR-3−/− mice or in mice concomitantly treated with aPC and the PAR-1 antagonist Ppal12S (1.2 AU in PAR-3−/− and 0.9 AU in Ppal12S-treated vs 2.3 AU in aPC-treated mice, P = .03 and P = .01, respectively, Figure 7B). These in vivo data establish a functional role of both PAR-1 and PAR-3 for aPC-dependent protection against LPS-induced proteinuria and podocyte injury in mice.
Taken together, the in vitro and in vivo results presented within this study identify a novel aPC-dependent signaling mechanism protecting against podocyte injury and proteinuria, which is distinct from endothelial cells and requires aPC-mediated PAR-3 activation (supplemental Figure 5).
Discussion
It is firmly established that aPC mediates cytoprotection in acute and chronic disease through receptor-dependent signaling.8,35 How aPC mediates cytoprotection in various tissues, such as the vasculature, the brain, or the kidney, despite activating the same receptor as thrombin, PAR-1, remained enigmatic for a long time. Recent studies shed light on to this conundrum, as they identified a number of coreceptors through which aPC mediates cytoprotection and activates intracellular signaling pathways, which differ from those activated by thrombin.4,5,7,36 In addition, it was recognized that the temporal activation pattern and the concentration of the activating protease determine the consequences of PAR-dependent signaling.37 In the current study, we evaluated aPC-dependent signaling in nonendothelial cells. We identify a novel signaling mechanism underlying the cytoprotective effect of aPC in podocytes. aPC binds to and cleaves PAR-3 in podocytes, which induces heterodimerization of PAR-3 with PAR-2 (human) or PAR-1 (mouse) in podocytes. The newly identified, aPC-dependent PAR interaction in podocytes differs from previously described pathways though which aPC exerts cytoprotection in other cell types. This demonstrates the plasticity and the cellular specificity of the phenomenon aPC-mediated cytoprotection.
In agreement with the expression pattern observed in human glomeruli, we failed to detect EPCR and observed only weak expression of PAR-1 in immortalized human podocytes in vitro. In immortalized human podocytes, aPC must signal independent of EPCR and PAR-1, as blocking either EPCR or PAR-1 does not abolish the antiapoptotic effect of aPC. In addition, PAR-1–agonism fails to inhibit podocyte apoptosis. Conversely, PAR-2 and PAR-3, the 2 receptors predominantly colocalizing with the podocyte specific marker synaptopodin in human glomeruli, are required for the antiapoptotic effect of aPC in human podocytes in vitro. This is in striking contrast to the situation in human endothelial cells, where EPCR and PAR-1 are the established bona fide receptors for aPC.
A recent report demonstrated that aPC can inhibit apoptosis in human endothelial cells independent of EPCR.36 Unlike in human podocytes, the antiapoptotic effect of aPC in endothelial cells did, however, require PAR-1. In nonendothelial cells, the human leukemic monocytic U937 cell line aPC can modulate Dab1-dependent signaling through ApoER2, apparently independent of PAR-1 and EPCR.7 However, these cells do express EPCR and PAR-1; thus, a functional role of these receptors in monocytes cannot be excluded, in particular in regard to physiologic relevant endpoints, such as apoptosis or cytokine production, which were not evaluated. Given the lack of EPCR and the low expression and functional irrelevance of PAR-1 in regard to apoptosis inhibition in human podocytes, the current observations establish, for the first time, that aPC can mediate cytoprotection independent of both PAR-1 and EPCR, at least in vitro. These findings strongly suggest that the cytoprotective effects of aPC are not depending on the engagement of a specific set of receptors. Rather, aPC engages cell-specific receptors to mediate cytoprotection. Further studies are required to identify coreceptors that may be required for aPC-dependent cytoprotection in podocytes.
A functional role of murine and human PAR-3 as a thrombin receptor has been previously proposed, but with the exception of mouse platelets, the exact mechanism through which thrombin–PAR-3 mediates cellular signaling remains unknown.11,13-16,18-20 In mouse platelets, thrombin cleaves the N-terminal extracellular PAR-3 domain, which is sufficient to enhance PAR-4–dependent platelet activation in the absence of PAR-3/PAR-4 heterodimerization.26 The aPC-dependent mechanism identified in podocytes differs, as PAR-3–mediated cellular signaling requires in addition to the PAR-3 N-terminal extracellular domain other structures of PAR-3 and depends on heterodimerization with PAR-1/PAR-2. Although the heterodimerization partner of PAR-3 after activation by aPC is species specific in podocytes, the function of PAR-3 is comparable in human and murine podocytes. These observations suggest that the expression and function of PAR-3 in podocytes are evolutionarily conserved across species. The future availability and analyses of mice with podocyte-specific PAR-3 deficiency will be invaluable to provide further in vivo evidence for the pivotal role of PAR-3 in podocytes.
In a recent report, it was concluded that PAR-3 can signal autonomously in human kidney cells (HEK-293) transfected with PAR-3.14 While considering a potential role of PAR-1, this study did not investigate a potential role of PAR-2, which, given the current results, is required to conclude that PAR-3 can signal autonomously. Likewise, our observation that ectopic expression of PAR-3 in mesangial cells renders these cells “aPC-sensitive” does not allow concluding that PAR-3 itself is sufficient. Murine mesangial cells lack EPCR but express PAR-1 and PAR-2, which may function as the required coreceptor. The dependence of PAR-3 signaling on a coreceptor is consistent with the absence of a cytoplasmic domain in PAR-3 known to couple with G-proteins.38
How does PAR-3 modulate intracellular signaling without being directly signaling competent? Heterordimerization of PAR-2 or PAR-1 with PAR-3 in podocytes may regulate the recruitment of G-protein, a mechanism established for other 7-transmembrane receptors.39 Thus, in human endothelial cells, thrombin-mediated PAR-1 activation in preexisting PAR-1/PAR-3 heterodimers promotes Gα13 signaling.18 In endothelial cells, modulation of intracellular signaling via PAR-1/PAR-3 is independent of PAR-3 cleavage by thrombin, and the function of PAR-3 was proposed to be confined to that of an allosteric regulator within constitutively present PAR-1/PAR-3 heterodimers.18 The mechanism in podocytes differs, as (1) aPC directly cleaves PAR-3 and (2) aPC induces heterodimerization of PAR-3 with PAR-2 and PAR-1.
aPC-induced PAR heterodimerization is associated with dissociation from and dephosphorylation of caveolin-1. This reflects a dynamic, caveolin-1–associated rearrangement of the receptor complex, as previously shown for other G-protein coupled receptors.40-42 Intriguingly, this is reminiscent of the dissociation of EPCR from caveolin-1 and recruitment of PAR-1 to an endothelial barrier-protective signaling pathway in endothelial cells.43 Thus, we propose that podocyte PAR-3 has a function similar to that of EPCR in other cells. Both receptors, EPCR and PAR-3, are required for cytoprotection yet are not signaling competent themselves. Furthermore, both receptors (EPCR and PAR-3) dissociate from caveolin-1 after ligand interaction43 (current results; and supplemental Figure 5). A striking difference between EPCR and PAR-3 is, however, that ligand occupancy is sufficient in the case of EPCR, whereas proteolytic activation is required for PAR-3–mediated cytoprotection in podocytes43 (current results). Thus, although a common theme appears to be the dynamic, caveolin-1-dependent rearrangement of receptor complexes into a cytoprotective signaling unit, the receptors involved and the dynamic rearrangement of these receptors into “operational receptor units” appear to be cell-specific.
In conclusion, the current study identifies a novel mechanism of aPC-mediated receptor activation and cytoprotection in podocytes. Through proteolytic activation of PAR-3 and dimerization of PAR-3 with PAR-2/PAR-1, aPC prevents PAN-induced podocyte apoptosis in vitro as well as LPS-induced podocyte injury and proteinuria in vivo. The identification of this novel signaling mechanism supports a concept of cell-specific signaling complexes, through which coagulation proteases regulate cellular function. This cellular specificity may enable the rational design of cell-targeted cytoprotective therapies (eg, specific activation of the PAR complex in renal disease caused by podocyte dysfunction).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Hans-Joachim Anders (Department of Nephrology, University of Munich, Germany) for kindly providing us glomerular endothelial cells and Simone Schmidt for excellent technical support.
This work was supported by Deutsche Forschungsgemeinschaft (grant IS 67/2-4; B.I.), the Dietmar Hopp Stiftung (B.I. and P.P.N.), the EFSD (European Foundation for the Study of Diabetes; B.I.), the DDS (Deutsche Diabetes Stiftung; B.I.), and the Stiftung Pathobiochemie (B.I.). T.M. has a postdoctoral fellowship from the Medical Faculty at the University of Heidelberg. K.S. has a scholarship from the German Academic Exchange Service (DAAD). Q.Z. has scholarships from the China Scholarship Council.
Authorship
Contribution: T.M. and H.W. designed, interpreted, and performed experiments; B.K.S., E.G., and H.-J.G. performed histologic analyses; Q.Z. conducted cloning work; K.S. assisted in ex vivo analyses; S.M.-K. assisted with in vitro work; V.S., B.G., B.W.G., J.H.G., J.R., and C.T.E. provided reagents; P.P.N. assisted in preparing the manuscript; and B.I. conceptually designed, conducted, and interpreted the experimental work and prepared the manuscript.
Conflict-of-interest disclosure: B.G. is employed by Lilly Research Laboratories, a division of Eli Lilly & Co, which produces recombinant human aPC for the treatment of severe sepsis. The remaining authors declare no competing financial interests.
Correspondence: Berend Isermann, Department of Clinical Chemistry and Pathobiochemistry, Otto-von-Guericke-University Magdeburg, Leipziger Str 44, 39120 Magdeburge, Germany; e-mail: berend.isermann@med.ovgu.de or berend_isermann@yahoo.de.
References
Author notes
T.M. and H.W. contributed equally to this study.

![Figure 5. Caveolin-1 (Cav-1) is required for aPC-dependent apoptosis inhibition in human podocytes. (A-B) Representative immunoblot images showing dissociation of PAR-2 and PAR-3 from Cav-1 and reorganization of Cav-1, PAR-2, and PAR-3 after treatment with aPC (20nM) in the presence or absence of MβCD (10mM). (C) Bar graph summarizing the frequency of apoptosis in PAN-treated Cav-1 knockdown human podocytes (Cav-1KD, left) and representative image showing shRNA-mediated knockdown of Cav-1 in human podocytes (semiquantitative RT-PCR, right). aPC and activating peptides (AP) for PAR-2 or PAR-3 fail to prevent PAN-induced apoptosis in Cav-1–deficient podocytes. (D) Representative immunoblots showing levels of Phospho-Cav-1 (pCav-1 [Tyr-14]) after treatment with aPC (20nM) at different time points in control (wild-type, wt) and PAR-3KD podocytes (top panel) and bar graphs summarizing the results (bottom panel). APC induces dephosphorylation of Cav-1 only in the presence of PAR-3. (E) Bar graph summarizing the frequency of apoptosis in PAN-stressed Cav-1KD human podocytes infected with adenoviral expression constructs, wild-type (Ad-Cav-1wt), or a Cav-1 mutant (Ad-Cav-1Y14A) and treated with aPC. aPC fails to protect against PAN-induced apoptosis in podocytes infected with phosphorylation-deficient Cav-1 mutant (Ad-Cav-1Y14A). (F) Representative immunoblots showing expression of Cav-1 in Cav-1KD podocytes transfected with adenoviral expression constructs (Ad-Cav-1wt, Ad-Cav-1Y14A). Data are mean ± SEM of at least 3 independent experiments. XP < .05 vs control. *P < .01 vs control (ANOVA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/3/10.1182_blood-2011-07-365973/5/m_zh89991184810005.jpeg?Expires=1769081969&Signature=3vwiHX3QSTrmK6IU8bWW438QoRYRDspOELsYVJ9jDwkpEcjdefk5o~LiT~O~PH4LubJL9-9z22mW9oPOfvtHx3HGMlac1sao8Kj6dLOqT43afFbZzGK2xNOs82wwb2qa96FxhNURmkGMgAsBpdcX4cqSPnD-iv~Gd7-DIS8q1rGtKhQ2fsgAa4CDUxLjM4REp~vRHC70UqZu5jf1ZYmReyMH2S6MX1vGg9bawBx1NnXlJYmBd3VJwEKr83HKOs1ID7XmISXvoYHv1UJhiAQzUG2hyM4I7VIQpwM0U2W9rWBk7fXS6YiBWq8t046TTftb7Zth5Ci-IRTzVUQwVMBGAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal