Abstract
Antiphospholipid Abs (APLAs) are associated with thrombosis and recurrent fetal loss. These Abs are primarily directed against phospholipid-binding proteins, particularly β2GPI, and activate endothelial cells (ECs) in a β2GPI-dependent manner after binding of β2GPI to EC annexin A2. Because annexin A2 is not a transmembrane protein, the mechanisms of APLA/anti-β2GPI Ab–mediated EC activation are uncertain, although a role for a TLR4/myeloid differentiation factor 88–dependent pathway leading to activation of NF-κB has been proposed. In the present study, we confirm a critical role for TLR4 in anti-β2GPI Ab–mediated EC activation and demonstrate that signaling through TLR4 is mediated through the assembly of a multiprotein signaling complex on the EC surface that includes annexin A2, TLR4, calreticulin, and nucleolin. An essential role for each of these proteins in cell activation is suggested by the fact that inhibiting the expression of each using specific siRNAs blocked EC activation mediated by APLAs/anti-β2GPI Abs. These results provide new evidence for novel protein-protein interactions on ECs that may contribute to EC activation and the pathogenesis of APLA/anti-β2GPI–associated thrombosis and suggest potential new targets for therapeutic intervention in antiphospholipid syndrome.
Introduction
Antiphospholipid syndrome (APS) is characterized by thrombosis and recurrent fetal loss in patients with circulating antiphospholipid Abs (APLAs) and is the most important cause of acquired thrombophilia.1-3 Prospective studies have demonstrated that patients with APS experience significant morbidity and mortality despite recommendations for indefinite anticoagulation.4 The term “antiphospholipid” is actually a misnomer, because the majority of APLAs are directed against phospholipid-binding proteins, of which β2-glycoprotein I (β2GPI) is the most common.5,6 The clinical importance of anti-β2GPI Abs has been demonstrated in several previous reports,7 and recent studies have shown that affinity-purified human anti-β2GPI Abs induce thrombosis in mice.8 Despite the clinical importance of APS, however, its pathogenesis has not been well defined.1,3,9
One mechanism by which APLAs/anti-β2GPI Abs may promote thrombosis is through β2GPI-dependent activation of endothelial cells (ECs).10-12 ECs play a critical role in the maintenance of blood fluidity through expression of anticoagulant proteins on their luminal surface and the elaboration of antithrombotic substances.13 However, EC activation leads to loss of these anticoagulant properties and transformation to a pro-adhesive, procoagulant phenotype.13 APLAs/anti-β2GPI Abs induce EC activation in vitro and in vivo, as determined by their ability to increase the expression of adhesion molecules (E-selectin, ICAM-1, VCAM-1), and tissue factor (TF) and to enhance the expression, synthesis, and/or secretion of pro-inflammatory cytokines and chemokines.3,10-12 These effects may account for the ability of APLAs/anti-β2GPI Abs to promote thrombosis in mice.14-17
We reported previously that anti-β2GPI Abs activate ECs through cross-linking of annexin A2–bound β2GPI11,18 ; others have demonstrated that activation occurs through a TLR4/myeloid differentiation factor 88 (MyD88)–dependent pathway culminating in NFκB activation.19 However, annexin A2 is not a transmembrane protein, so its role in anti-β2GPI Ab–induced EC activation is uncertain. To address this issue, we assessed whether annexin A2 associates with TLR4 and/or other cell-surface proteins to generate a signaling complex on ECs. Our studies suggest the existence of a novel multiprotein signaling complex that consists of annexin A2, TLR4, calreticulin, and nucleolin. Each component of this complex is essential for EC activation by APLAs/anti-β2GPI Abs.
Methods
Materials
Medium 199 was obtained from Cellgro and FBS from Thermo Scientific HyClone. Gelatin, white 96-well flat-bottom plates, and HRP-conjugated goat anti–rabbit Abs were from Fisher Scientific. Endothelial growth supplement was from Biomedical Technologies. Standard 96-well microplates were from Nunc, and 6-well tissue-culture Costar plates were from Corning. Purified β2GPI was purchased from Haematologic Technologies. Turbo-TMB and sulfo-succinimidyl 6-(biotinamido) hexanoate were from Pierce. CNBr-activated Sepharose 4B was from GE Healthcare. Electrophoresis gels, TRIzol RNA extraction reagent, DNAse, Maloney murine leukemia virus reverse transcriptase, Dynabeads Protein G, and the OneStepPlus quantitative PCR (qPCR) system were purchased from Invitrogen–Applied Biosystems. Oligo-dT primers were from IDT, and custom-designed and negative control random-sequence heteroduplex siRNAs were from either Dharmacon (Fisher Scientific) or Sigma-Aldrich. EC transfections were performed using X-tremeGENE. Luciferase activity due to activation of NF-κB–dependent transcription was measured using an NF-κB promoter construct kindly provided by Dr Nywana Sizemore (National Institutes of Health, Bethesda, MD) and a luciferase assay system (Promega); negative control DNA for these studies was the P214/PRL-TK DNA random sequence from Stratagene. A MyD88-inhibitory homodimerization peptide (and control peptide) was from Imgenex. All other reagents and protease inhibitors were from Sigma-Aldrich.
Abs
Goat anti–human anti-β2GPI Abs used for most of the studies assessing EC activation were from Bethyl Laboratories. Monoclonal goat anti–human E-selectin and biotinylated goat anti-calreticulin Abs were from R&D Systems. Anti–human TLR4 mAbs and HRP-conjugated goat anti–mouse and donkey anti–goat Abs were from Santa Cruz Biotechnology. Control goat, rabbit, and mouse IgGs were from Sigma-Aldrich. Murine mAbs against human nucleolin and β-actin and rabbit anti-apoER2′ Abs were from Abcam. Rabbit anti–human TLR2 mAb, anti–NF-κB p65 polyclonal Ab, and anti–NF-κB phospho-p65 (S536) Abs were from Cell Signaling Technology. Human anti-β2GPI Abs were affinity-purified from 2 patients with lupus anticoagulants, high-levels of anti-β2GPI Abs (> 75 U/mL), and a history of thrombosis, using β2GPI conjugated to Affigel-HZ (Bio-Rad), as described previously.11 Control human IgG was isolated from the pooled plasma of 5 healthy individuals using protein G. Abs used for immunohistochemistry included rabbit anti-calreticulin (Lifespan Biosciences) and rabbit anti-nucleolin (Thermo-Fisher Scientific). Hybridoma cells expressing the murine anti-β2GPI mAb FC1 and a control Ab against influenza hemagglutinin (7D10) were a kind gift of Dr Marc Monestier (Temple University, Philadelphia, PA). mAb FC1 was derived from spontaneously autoimmune (not by immunization) NZW × BXSB F1 mice.20 FC1 reacts with domain 1 of murine and human β2GPI and causes thrombosis in mice.21 All Abs contained < 0.03 IU/mL of lipopolysaccharide (LPS) as determined using the Limulus Amebocyte Assay (Sigma-Aldrich).
ECs
Human umbilical ECs (HUVECs) were isolated as described previously,18 and maintained in Medium 199 containing 10% FBS, 1% penicillin-streptomycin, and EC growth supplement. All experiments were performed using cells of passage 3 or lower.
Assessment of EC activation
EC activation was measured as described previously.11,22 Confluent ECs in 96-well microplates were incubated for 3 hours in serum-free medium before incubation for 5 hours with 60nM human β2GPI and 180nM anti-β2GPI IgG Abs. Cells were washed and then fixed by exposure to 2% paraformaldehyde for 15 minutes. After blocking nonspecific binding, cells were incubated with 1 μg/mL of goat anti–human E-selectin Abs for 16 hours at 4°C. Subsequently, cells were washed with TBST and bound Abs were detected using HRP-conjugated donkey anti–goat IgG followed by the chromogenic substrate Turbo-TMB. Relative amounts of EC-bound E-selectin Abs were determined by measuring A450.
Isolation of EC surface annexin A2–binding proteins
To determine whether annexin A2 associates with other EC-surface proteins, biotinylated EC-surface proteins were affinity purified using annexin A2 immobilized on CNBr-conjugated Sepharose 4B. Cell-surface proteins were biotinylated using sulfosuccinimidyl-6-[biotinamido]-6-hexanamidohexanoate. Cell extracts were prepared by scraping cells into 0.1M Tris-HCl, pH 8.0, containing 1% Triton X-100, 8% glycerol, 0.1M NaCl, 0.005M EDTA, 2.5mM β-glycerophosphate, 10 μg/mL of PMSF, 50 μg/mL of antipain dihydrochloride, 10 μg/mL of aprotinin, 10 μg/mL of benzamidine, 10 μg/mL of leupeptin, 0.35 ng/mL of Pefabloc, 10 μg/mL of TLCK, 10 μg/mL of chymostatin, 10 μg/mL of pepstatin, and 50 μg/mL of TPCK (lysis buffer). Lysates were sonicated, then centrifuged for 10 minutes at 10 000g. Annexin A2–binding proteins were purified using a 1-mL column containing immobilized annexin A2 (5 mg of annexin A2/mL gel) or a control column containing immobilized BSA. Bound proteins were eluted using 0.1M glycine-HCl, pH 2.7. Fractions of 0.5 mL were collected and proteins were separated using 10% SDS-PAGE and then transferred to PVDF and detected using streptavidin-peroxidase and chemiluminescence. Alternatively, in selected experiments, PVDF membranes were immunoblotted using anti-TLR4 Abs.
The identity of proteins identified through this approach was pursued through larger-scale affinity purification. Detergent extracts from 2.5 × 108 ECs were subjected to affinity chromatography using a 5-mL column of immobilized annexin A2. After washing until the A280 of the effluent reached 0, bound proteins were eluted using 0.1M glycine-HCl, pH 2.7; 0.5 mL fractions were collected, separated using 10% SDS-PAGE, and stained using Coomassie Brilliant Blue. Stained proteins were excised and analyzed by mass spectrometry using a Finnigan LCQ-Deca ion trap mass spectrometer with a Protana microelectrospray ion source, interfaced to a 10-cm × 75-μm internal diameter Phenomenex Jupiter C18 reverse-phase column. All collected CID spectra were analyzed through a search on the National Center for Biotechnology Information nonredundant database using TurboSEQUEST software bundled into Bioworks Browser 3.1. Inter-pretation was assisted by additional searches using Mascot Version 1.9 and Fasta software as needed using The University of Virginia Web site (http://fasta.block.virginia.edu/fasta_www/fasta_list2shtml).
qPCR
Real-time qPCR was performed according to established protocols. Primer sequences used are listed in Table 1. Total RNA was isolated using TRIzol, and reverse transcription was performed using 0.3 units/μL of Maloney murine leukemia virus. Real-time qPCR was conducted using the StepOnePlus PCR System (Invitrogen). Reactions were performed in 96-well plates in a volume of 10 μL using the following parameters: denaturation at 94°C for 10 minutes, 40 cycles at 95°C for 15 seconds each, and a final cycle at 62°C for 60 seconds. Amplification of GAPDH cDNA was used as an internal control. The fold induction was calculated after normalization to GAPDH using the δ δ CT method.
Primers used for qPCR analyses
| Gene . | Forward . | Reverse . |
|---|---|---|
| Annexin A2 | 5′CTCTACACCCCCAAGTGCAT3′ | 5′TCAGTGCTGATGCAAGTTCC3′ |
| E-selectin | 5′TGCGATGCTGCCTACTTGTG3′ | 5′AGAGAGTGCCACTACCAAGGGA3′ |
| TLR4 | 5′AGACCTGTCCCTGAACCCTAT3′ | 5′TTCTAAACCAGCCAGACCTTG3′ |
| ICAM-1 | 5′CGTGGGGAGAAGGAGCTGAA3′ | 5′CAGTGCGGCACGAGAAATTG3′ |
| VCAM-1 | 5′TGGGCTGTGAATCCCCATCT3′ | 5′GGGTCAGCGCGTGGAATTGGTC3′ |
| MyD88 | 5′CTCCTCCACATCCCTTCC3′ | 5′CCGCACGTTCAAGAACAGAGA3′ |
| MD2 | 5′TTCCACCCTGTTTTCTTCCA3′ | 5′AATCGTCATCAGATCCTCGG3′ |
| S100A10 | 5′GAGTTCCCTGGATTTTGGAA3′ | 5′CACTGGTCCAGGTCCTTCAT3′ |
| GAPDH | 5′GCCATCAATGACCCCTTCATT3′ | 5′TCTCGCTCCTGG AAGATGG3′ |
| Gene . | Forward . | Reverse . |
|---|---|---|
| Annexin A2 | 5′CTCTACACCCCCAAGTGCAT3′ | 5′TCAGTGCTGATGCAAGTTCC3′ |
| E-selectin | 5′TGCGATGCTGCCTACTTGTG3′ | 5′AGAGAGTGCCACTACCAAGGGA3′ |
| TLR4 | 5′AGACCTGTCCCTGAACCCTAT3′ | 5′TTCTAAACCAGCCAGACCTTG3′ |
| ICAM-1 | 5′CGTGGGGAGAAGGAGCTGAA3′ | 5′CAGTGCGGCACGAGAAATTG3′ |
| VCAM-1 | 5′TGGGCTGTGAATCCCCATCT3′ | 5′GGGTCAGCGCGTGGAATTGGTC3′ |
| MyD88 | 5′CTCCTCCACATCCCTTCC3′ | 5′CCGCACGTTCAAGAACAGAGA3′ |
| MD2 | 5′TTCCACCCTGTTTTCTTCCA3′ | 5′AATCGTCATCAGATCCTCGG3′ |
| S100A10 | 5′GAGTTCCCTGGATTTTGGAA3′ | 5′CACTGGTCCAGGTCCTTCAT3′ |
| GAPDH | 5′GCCATCAATGACCCCTTCATT3′ | 5′TCTCGCTCCTGG AAGATGG3′ |
RNA interference and luciferase reporter assays
Heteroduplex siRNAs were custom designed as pools containing 4 different sequences specifically targeting annexin A2, TLR2, TLR4, calreticulin, nucleolin, or apoER2 (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article); control RNAs were of identical nucleotide composition as siRNA but in random sequence. Transfection was performed using X-tremeGENE transfection reagent per the manufacturer's instructions. Briefly, 1 × 105 ECs in a 12-well plate were incubated with 200nM siRNA in 50 μL of Opti-MEM and 5 μL of X-tremeGENE for 24 hours. Cells were replated and cultured in complete medium for 24 hours, and then either harvested and assessed for expression of the target protein or assessed for E-selectin expression in response to β2GPI and anti-β2GPI Abs.
To determine whether inhibition of AnxA2, TLR4, calreticulin, or nucleolin expression affected NF-κB activity, we used a construct containing the NF-κB promoter linked to the luciferase gene. Two micrograms of plasmid was transfected into ECs in the presence of specific or random sequence siRNA. Transfected cells were seeded into 12-well plates, and 48 hours later were activated by incubation with β2GPI and anti-β2GPI Abs. Cells were then lysed and 180 μL of luciferase activation reagent 2 was added to 20 μL of cell lysate in a white 96-well flat-bottom plate. Luminescence was measured using a Perkin-Elmer Victor3 Multilabel Counter luminometer. Luciferase activity was normalized to total protein.
Immunoprecipitation and immunoblotting
Immunoprecipitation experiments were performed to further confirm the interaction among annexin A2, TLR4, calreticulin, and nucleolin. ECs were pretreated with 60nM human β2GPI and 180nM control IgG or anti-β2GPI Abs for 5 hours, and detergent extracts were prepared. Extracts were subjected to immunoprecipitation using Dynabeads Protein A/G and 4-6 μg of nucleolin, TLR4, or annexin A2 Abs, or an equal amount of isotype-control IgG. Immunoprecipitates were separated using 4%-12% SDS-PAGE and proteins were transferred to PVDF membranes. Membranes were blocked with 3% BSA in TBST before incubation with primary Abs. Bound Abs were detected using HRP-conjugated secondary Abs and SuperFemto Western blot reagent. Because of cross-reactivity of secondary Abs with the IgG used for immunoprecipitation, calreticulin, which has similar molecular mass as the IgG heavy chain, was detected using a biotinylated calreticulin Ab followed by streptavidin-HRP.
Immunohistochemistry
Staining of formalin-fixed, paraffin-embedded tissue sections was performed on a Ventana Benchmark automated immunostainer (Ventana Medical Systems) using a Ventana iVIEW DAB Detection kit. Primary Abs included rabbit anti-nucleolin and rabbit anti-calreticulin diluted at 1:100 and 1:500, respectively. Goat anti–rabbit biotinylated secondary Abs (Vector Laboratories) were used at a 1:200 dilution. Images were collected using a light microscope with a 63× objective (Leica).
Statistics
Data are expressed as means ± SD. All experimental points were determined in triplicate and all assays were repeated at least 3 times. Differences between control and experimental conditions were assessed using the Student 2-tailed t test for unpaired samples or 1-way ANOVA. P < .05 was considered significant.
Human subjects
All plasma samples were obtained after signed informed consent in accordance with the Declaration of Helsinki and with approval of the Cleveland Clinic Foundation Institutional Review Board.
Results
siRNA-mediated inhibition of TLR4 expression inhibits APLA/anti-β2GPI–mediated EC activation
Although APLAs/anti-β2GPI Abs have been reported to activate ECs through a TLR4/MyD88–dependent pathway,19 we have not reconciled this observation with our previous work demonstrating binding of β2GPI to annexin A2.11 Moreover, a role for additional receptors such as TLR223,24 and apoER221,25,26 in anti-β2GPI–mediated EC activation and/or thrombosis has been reported.
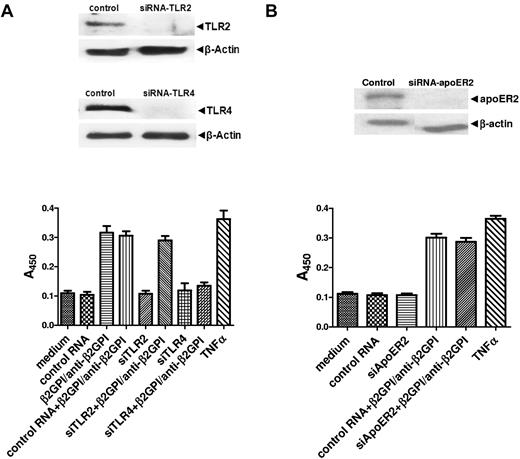
To address the roles of these receptors in the activation of ECs by anti-β2GPI Abs, we first transfected cells with siRNA specific for TLR4 or TLR2 or random sequence RNA of identical composition (control); each of the specific siRNAs inhibited the expression of target proteins, whereas the control RNA had no effect. After incubation with β2GPI and anti-β2GPI Abs, cells treated with control RNA or TLR2 siRNA increased their expression of cell-surface E-selectin, which is used as a marker of activation, to a similar extent. In contrast, TLR4 siRNA caused marked inhibition of EC activation (Figure 1A). Identical results were observed using affinity-purified human anti-β2GPI Abs and the mAb FC1. These studies demonstrate an essential role for TLR4 in anti-β2GPI Ab–mediated EC activation.
APLA/anti-β2GPI–mediated EC activation is mediated by TLR4. HUVECs were incubated in medium alone, treated with control RNA of random sequence but identical nucleotide composition as specific siRNA, or with specific siRNA against TLR2, TLR4, or apoER2. Twenty-four hours later, cells were replated in 6- or 96-well plates, incubated for an additional 24 hours, then harvested and analyzed for expression of the targeted protein or assessed for cell-surface E-selectin expression in response to β2GPI and anti-β2GPI Abs or TNFα. (A) Cells treated with siRNA to TLR2 or TLR4. (B) Cells treated with siRNA to apoER2. Error bars represent the mean ± SD of triplicate points. P = .0016 by ANOVA for cells treated with TLR4 siRNA versus control or TLR2 siRNA. P = .48 for cells treated with apoER2 siRNA versus control RNA. The results shown are from 1 representative experiment of 3.
APLA/anti-β2GPI–mediated EC activation is mediated by TLR4. HUVECs were incubated in medium alone, treated with control RNA of random sequence but identical nucleotide composition as specific siRNA, or with specific siRNA against TLR2, TLR4, or apoER2. Twenty-four hours later, cells were replated in 6- or 96-well plates, incubated for an additional 24 hours, then harvested and analyzed for expression of the targeted protein or assessed for cell-surface E-selectin expression in response to β2GPI and anti-β2GPI Abs or TNFα. (A) Cells treated with siRNA to TLR2 or TLR4. (B) Cells treated with siRNA to apoER2. Error bars represent the mean ± SD of triplicate points. P = .0016 by ANOVA for cells treated with TLR4 siRNA versus control or TLR2 siRNA. P = .48 for cells treated with apoER2 siRNA versus control RNA. The results shown are from 1 representative experiment of 3.
In additional studies, we did not observe inhibition of HUVEC activation when cells were pretreated with apoER2 siRNA (Figure 1B). However, results from Ramesh et al suggest that inhibition of activation may be caused by siRNA-mediated knockdown of apoER2 in human aortic ECs and bovine aortic ECs.21
Annexin A2 associates with other EC-surface proteins
The results of the studies depicted in Figure 1 demonstrate a critical role for TLR4 in anti-β2GPI–mediated EC activation, which, together with the results of our previous studies, suggest that TLR4 and annexin A2 might interact on the cell surface. To address this hypothesis, we isolated EC-surface proteins that bound to immobilized annexin A2. Annexin A2 affinity chromatography of extracts from ECs on which cell surface proteins were biotinylated yielded protein bands of approximately 95, 85, 62, and 36 kDa; no bands were observed in eluates from a BSA-Sepharose column (supplemental Figure 1A). In addition to development using streptavidin peroxidase, the β2GPI eluate was immunoblotted using anti-TLR4 Abs; these studies identified the highest molecular weight band as TLR4 (supplemental Figure 1B).
To determine the identity of the other annexin A2–binding proteins, extracts from 2.5 × 108 ECs were affinity purified using immobilized annexin A2 or BSA. Annexin A2 eluates stained with Coomassie Brilliant Blue revealed 4 proteins of similar size as those isolated from cell-surface biotinylated ECs (supplemental Figure 1C). The 3 unidentified bands were analyzed by mass spectrometry after tryptic digestion (supplemental Figures 2-4; supplemental Table 2). These studies identified annexin A2 (approximately 36 kDa; 7 peptides, 19% coverage), calreticulin (approximately 62 kDa; 20 peptides, 52% coverage), and nucleolin (approximately 95 kDa; 12 peptides, 16% coverage), and demonstrated that TLR4, calreticulin, and nucleolin bound to immobilized annexin A2, although we could not determine whether these interactions were direct.
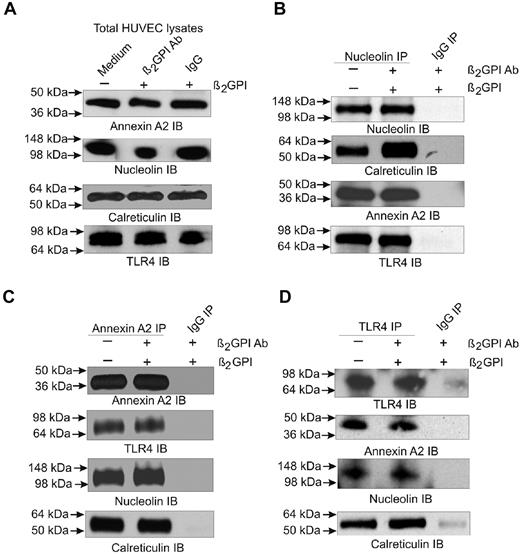
Given the known roles of calreticulin and nucleolin in EC function and signaling,27-31 we further examined interactions among annexin A2, TLR4, calreticulin, and nucleolin using immunoprecipitation. Cell extracts prepared from unstimulated ECs or cells incubated with β2GPI and anti-β2GPI Abs were subjected to immunoprecipitation using Abs against annexin A2, TLR4, or nucleolin. Immunoprecipitated proteins were separated by SDS-PAGE and analyzed by immunoblotting. We observed annexin A2, nucleolin, calreticulin, and TLR4 in whole EC extracts (Figure 2A). The nucleolin immunoprecipitate also contained calreticulin, annexin A2, and TLR4 (Figure 2B), whereas the annexin A2 immunoprecipitate contained TLR4, nucleolin, and calreticulin (Figure 2C). The TLR4 immunoprecipitate contained the same 3 proteins (Figure 2D). In some cases, increased amounts of protein appeared to coimmunoprecipitate from extracts of cells pretreated with β2GPI and anti-β2GPI Abs, but these experiments were not quantitative. Control IgG did not immunoprecipitate any proteins. These results provide definitive evidence for interactions among annexin A2, calreticulin, nucleolin, and TLR4, and suggest that a complex of these proteins exists on ECs even before exposure to β2GPI and anti-β2GPI Abs.
Detection of annexin A2 and TLR4-associated proteins by immunoprecipitation. ECs were either untreated or incubated with β2GPI and anti-β2GPI Abs for 5 hours. Detergent extracts were prepared and analyzed by 4%-12% SDS-PAGE before (A) or after immunoprecipitation using Abs against nucleolin (B), annexin A2 (C), or TLR4 (D). Whole extracts and each of the immunoprecipitates were then immunoblotted using Abs against annexin A2, nucleolin, calreticulin, or TLR4. Extracts were also immunoprecipitated using control IgG, which did not precipitate any detectable protein. The results shown are from 1 representative experiment of 3.
Detection of annexin A2 and TLR4-associated proteins by immunoprecipitation. ECs were either untreated or incubated with β2GPI and anti-β2GPI Abs for 5 hours. Detergent extracts were prepared and analyzed by 4%-12% SDS-PAGE before (A) or after immunoprecipitation using Abs against nucleolin (B), annexin A2 (C), or TLR4 (D). Whole extracts and each of the immunoprecipitates were then immunoblotted using Abs against annexin A2, nucleolin, calreticulin, or TLR4. Extracts were also immunoprecipitated using control IgG, which did not precipitate any detectable protein. The results shown are from 1 representative experiment of 3.
Each member of the multiprotein complex contributes to APLA/anti-β2GPI–induced EC activation
To assess whether the proteins associated with annexin A2 or TLR4 were required for anti-β2GPI–induced EC activation, we used specific siRNAs to inhibit their expression. Decreased expression of each protein significantly inhibited EC activation in response to β2GPI and anti-β2GPI Abs (Figure 3A-B). Inhibition of protein expression by multiple siRNAs used simultaneously was not additive, suggesting that the proteins function in a single pathway. Identical results were obtained using goat anti–human β2GPI Abs, affinity-purified human anti-β2GPI Abs, and mAb FC1.
Annexin A2, TLR4, calreticulin, and nucleolin are necessary for activation of ECs by β2GPI and anti-β2GPI Abs. ECs were either not pretreated or were pretreated with control RNA of random sequence but identical composition as specific siRNAs or with siRNAs specific for annexin A2, TLR4, calreticulin, or nucleolin. Twenty-four hours later, cells were replated in 6- or 96-well plates, incubated for an additional 24 hours, and then harvested and analyzed for protein expression (A) or assessed for cell-surface E-selectin expression in response to β2GPI and either control IgG or anti-β2GPI Abs (B). Error bars represent the means ± SD of quadruplicate points. Decreases in E-selectin expression in cells pretreated with control versus specific siRNA before incubation with β2GPI and anti-β2GPI Abs were highly significant (P < .0001 by ANOVA). The results shown are from 1 representative experiment of 3.
Annexin A2, TLR4, calreticulin, and nucleolin are necessary for activation of ECs by β2GPI and anti-β2GPI Abs. ECs were either not pretreated or were pretreated with control RNA of random sequence but identical composition as specific siRNAs or with siRNAs specific for annexin A2, TLR4, calreticulin, or nucleolin. Twenty-four hours later, cells were replated in 6- or 96-well plates, incubated for an additional 24 hours, and then harvested and analyzed for protein expression (A) or assessed for cell-surface E-selectin expression in response to β2GPI and either control IgG or anti-β2GPI Abs (B). Error bars represent the means ± SD of quadruplicate points. Decreases in E-selectin expression in cells pretreated with control versus specific siRNA before incubation with β2GPI and anti-β2GPI Abs were highly significant (P < .0001 by ANOVA). The results shown are from 1 representative experiment of 3.
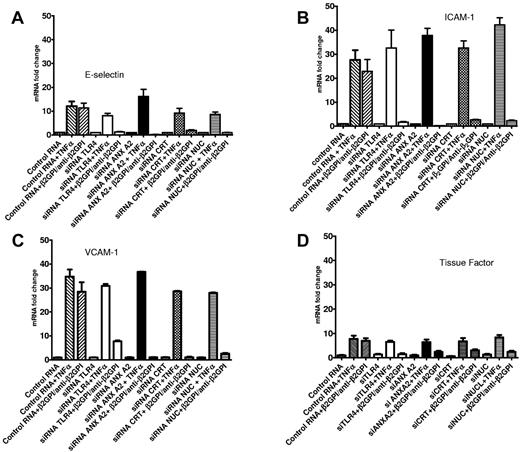
To assess whether these inhibitory effects were mediated at the mRNA level and to confirm effects on EC activation using additional markers, we determined whether siRNA directed to annexin A2, TLR4, calreticulin, or nucleolin decreased the level of mRNA that encoded several cell-surface adhesion molecules for which expression was stimulated in response to anti-β2GPI Abs. Increases in the expression of mRNAs encoding E-selectin, ICAM-1, and VCAM-1 mRNA that occurred in the presence of β2GPI and anti-β2GPI Abs were blocked by these siRNAs (Figure 4A-C). Similar results were observed when levels of TF mRNA were assessed (Figure 4D). These findings likely reflect a reduction in transcriptional activity, but additional studies will be required to confirm this hypothesis. The specificity of these findings is highlighted by the fact that TNF-α, which does not signal through TLR4, caused similar increases in E-selectin, ICAM-1, VCAM-1, and TF mRNAs that were not prevented by siRNA-mediated knock-down of AnxA2, TLR4, calreticulin, or nucleolin (Figure 4A-D).
Annexin A2, TLR4, calreticulin, and nucleolin promote EC activation in response to β2GPI and anti-β2GPI Abs through regulation of mRNA levels. ECs were pretreated with either control RNA of random sequence but identical composition as specific siRNA or with specific siRNA against annexin A2, TLR4, calreticulin, or nucleolin. Twenty-four hours later, cells were replated in 6-well microplates, incubated for an additional 24 hours, and then either harvested and analyzed for expression of the targeted protein (not shown) or incubated with β2GPI and anti-β2GPI Abs or TNFα. The content of specific mRNAs was then analyzed by qPCR. Graphs depict the levels of E-selectin (A), ICAM-1 (B), VCAM-1 (C), or TF (D) mRNA. In all cases, specific siRNAs significantly reduced the levels of mRNA for each of the cell-adhesion molecules in response to β2GPI and anti-β2GPI Abs, but not in response to TNFα. Error bars represent the means ± SD of quadruplicate points. The results shown are from 1 representative experiment of 3.
Annexin A2, TLR4, calreticulin, and nucleolin promote EC activation in response to β2GPI and anti-β2GPI Abs through regulation of mRNA levels. ECs were pretreated with either control RNA of random sequence but identical composition as specific siRNA or with specific siRNA against annexin A2, TLR4, calreticulin, or nucleolin. Twenty-four hours later, cells were replated in 6-well microplates, incubated for an additional 24 hours, and then either harvested and analyzed for expression of the targeted protein (not shown) or incubated with β2GPI and anti-β2GPI Abs or TNFα. The content of specific mRNAs was then analyzed by qPCR. Graphs depict the levels of E-selectin (A), ICAM-1 (B), VCAM-1 (C), or TF (D) mRNA. In all cases, specific siRNAs significantly reduced the levels of mRNA for each of the cell-adhesion molecules in response to β2GPI and anti-β2GPI Abs, but not in response to TNFα. Error bars represent the means ± SD of quadruplicate points. The results shown are from 1 representative experiment of 3.
The multiprotein signaling complex mediates activation of NF-κB
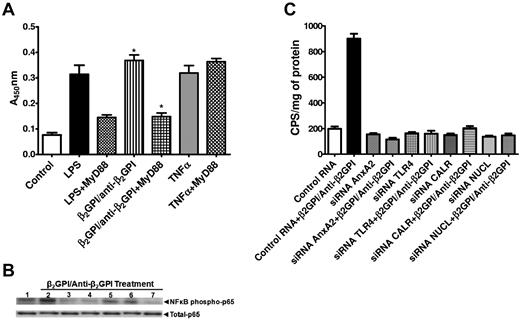
Activation of NF-κB occurs via a TLR4/MyD88–dependent signaling pathway and mediates the activation of ECs by anti-β2GPI Abs.19 Inhibition of NF-κB also attenuates the prothrombotic effects of APLAs/anti-β2GPI Abs in mice.32 We confirmed the importance of a TLR4/MyD88–dependent pathway in the activation of ECs by anti-β2GPI Abs, observing that a peptide that prevents MyD88 homodimerization (DRQIKIWFQNRRMKWKKRDVLPGT) blocked EC activation by approximately 70%, as determined by measurement of EC-surface E-selectin (Figure 5A).
Activation of the TLR4/MyD88/NF-κB signaling pathway is dependent on assembly of the multiprotein signaling complex. (A) ECs were plated in 96-well plates and either not pretreated or pretreated with a cell-permeable peptide that contains a sequence from the MyD88/TIR homodimerization domain that blocks MyD88-dependent TLR4 signaling. Cells were then exposed to LPS, β2GPI and anti-β2GPI Abs, or TNFα. The peptide inhibited EC activation in response to LPS and anti-β2GPI Abs, but not TNFα, demonstrating the dependence of activation on a TLR4/MyD88 pathway.19 Error bars represent the means ± SD of triplicate points. *P < .0001 for cells treated with β2GPI/anti-β2GPI Abs alone versus β2GPI/anti-β2GPI in the presence of the homodimerization domain peptide. (B) ECs were pretreated with control RNA of random sequence but identical composition as specific siRNA (lanes 1 and 2), TLR4 siRNA (lane 3), annexin A2 siRNA (lane 4), calreticulin siRNA (lane 5), nucleolin siRNA (lane 6), or all 4 siRNAs simultaneously (lane 7). Cells were then incubated with β2GPI and anti-β2GPI Abs, and cell lysates prepared and assessed for phosphorylated NF-κB p65 (S536) and total NF-κB content by immunoblotting. (C). ECs were transfected with a construct containing an NF-κB promoter sequence linked to a luciferase reporter gene. Cells were then treated with control RNA or siRNA directed against annexin A2, TLR4, nucleolin, or calreticulin. After incubation with β2GPI and anti-β2GPI Abs, cell lysates were prepared and luciferase activity determined. Pretreatment of cells with each of the siRNAs caused significant reductions in luciferase activity compared with cells pretreated with control RNA (P < .0001 by ANOVA). Error bars represent the means ± SD of triplicate points. The results shown are from 1 representative experiment of 3.
Activation of the TLR4/MyD88/NF-κB signaling pathway is dependent on assembly of the multiprotein signaling complex. (A) ECs were plated in 96-well plates and either not pretreated or pretreated with a cell-permeable peptide that contains a sequence from the MyD88/TIR homodimerization domain that blocks MyD88-dependent TLR4 signaling. Cells were then exposed to LPS, β2GPI and anti-β2GPI Abs, or TNFα. The peptide inhibited EC activation in response to LPS and anti-β2GPI Abs, but not TNFα, demonstrating the dependence of activation on a TLR4/MyD88 pathway.19 Error bars represent the means ± SD of triplicate points. *P < .0001 for cells treated with β2GPI/anti-β2GPI Abs alone versus β2GPI/anti-β2GPI in the presence of the homodimerization domain peptide. (B) ECs were pretreated with control RNA of random sequence but identical composition as specific siRNA (lanes 1 and 2), TLR4 siRNA (lane 3), annexin A2 siRNA (lane 4), calreticulin siRNA (lane 5), nucleolin siRNA (lane 6), or all 4 siRNAs simultaneously (lane 7). Cells were then incubated with β2GPI and anti-β2GPI Abs, and cell lysates prepared and assessed for phosphorylated NF-κB p65 (S536) and total NF-κB content by immunoblotting. (C). ECs were transfected with a construct containing an NF-κB promoter sequence linked to a luciferase reporter gene. Cells were then treated with control RNA or siRNA directed against annexin A2, TLR4, nucleolin, or calreticulin. After incubation with β2GPI and anti-β2GPI Abs, cell lysates were prepared and luciferase activity determined. Pretreatment of cells with each of the siRNAs caused significant reductions in luciferase activity compared with cells pretreated with control RNA (P < .0001 by ANOVA). Error bars represent the means ± SD of triplicate points. The results shown are from 1 representative experiment of 3.
We also examined the effect of inhibiting the expression of components of the annexin A2–dependent multiprotein signaling complex on the activation of NF-κB by anti-β2GPI Abs. NF-κB activation was assessed by measuring phosphorylation of Ser536 of the p65 subunit of NF-κB, which is required for p65 nuclear translocation,33 and by assessing NF-κB transcriptional activity in ECs transfected with a vector containing the NF-κB promoter and a luciferase reporter. In response to anti-β2GPI Abs, we observed phosphorylation of p65 in cells pretreated with control RNA, but not in cells pretreated with siRNA against individual or combinations of proteins comprising the multiprotein complex (Figure 5B). Similarly, whereas NF-κB transcriptional activity was stimulated by anti-β2GPI Abs in cells transfected with control RNA, suppression of annexin A2, TLR4, nucleolin, or calreticulin inhibited NF-κB transcriptional activity in response to these Abs (Figure 5C). Identical results were obtained using human anti-β2GPI Abs and the mAb FC1.
These results demonstrate the importance of each of the proteins of the annexin A2–dependent multiprotein signaling complex in the activation of NF-κB, a central mediator of EC activation in response to APLAs/anti-β2GPI Abs.
Expression of members of the multiprotein signaling complex is increased during APLA/anti-β2GPI–mediated EC activation
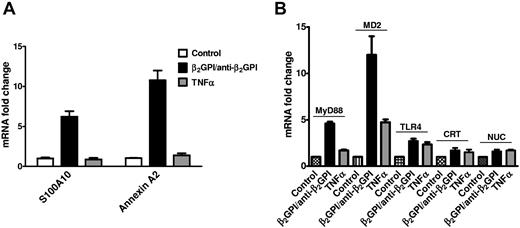
Although our studies suggest that a multiprotein signaling complex mediates the activation of ECs in response to anti-β2GPI Abs, the stoichiometry of this complex and how each of the members associate with one another remain uncertain. To begin to address these issues, we assessed whether anti-β2GPI Abs stimulate the expression of individual components of the signaling complex, as well as accessory proteins such as S100A10 or MD2, which might modulate the activity of the complex by enhancing cell-surface expression and/or signaling activity.
We first determined whether the expression of annexin A2 and S100A10 (p11), an essential component of the annexin A2 heterotetramer on the EC surface,34,35 was increased by β2GPI and anti-β2GPI Abs, observing that the expression of mRNA encoding each was significantly increased in response to these Abs, but not to TNF-α (Figure 6A).
Activation of ECs by anti-β2GPI Abs induces increased expression of components of the multiprotein signaling complex and accessory proteins. (A) ECs were incubated with β2GPI and anti-β2GPI Abs or TNFα to induce activation. Total RNA was then isolated, reverse-transcribed, and analyzed for content of S100A10 and annexin A2 mRNA using qPCR. Significant increases in both mRNA species occurred in response to β2GPI and anti-β2GPI Abs, but not TNFα. (B) ECs were activated as described in panel A, and RNA isolated, reverse transcribed, and analyzed for content of MyD88, MD2, TLR4, calreticulin, and nucleolin mRNA using qPCR. Significant increases in mRNA species encoding MyD88 and MD2, with lesser increases in mRNA encoding TLR4, calreticulin, and nucleolin, were observed. These exceeded those observed in response to TNFα. Error bars represent the means ± SD of triplicate points.
Activation of ECs by anti-β2GPI Abs induces increased expression of components of the multiprotein signaling complex and accessory proteins. (A) ECs were incubated with β2GPI and anti-β2GPI Abs or TNFα to induce activation. Total RNA was then isolated, reverse-transcribed, and analyzed for content of S100A10 and annexin A2 mRNA using qPCR. Significant increases in both mRNA species occurred in response to β2GPI and anti-β2GPI Abs, but not TNFα. (B) ECs were activated as described in panel A, and RNA isolated, reverse transcribed, and analyzed for content of MyD88, MD2, TLR4, calreticulin, and nucleolin mRNA using qPCR. Significant increases in mRNA species encoding MyD88 and MD2, with lesser increases in mRNA encoding TLR4, calreticulin, and nucleolin, were observed. These exceeded those observed in response to TNFα. Error bars represent the means ± SD of triplicate points.
We next assessed the expression of additional components of the annexin A2–dependent multiprotein signaling complex and the TLR4-signaling pathway in response to β2GPI and anti-β2GPI Abs (Figure 6B). Expression of mRNAs encoding MD2 and MyD88, central components of the TLR4/MyD88-signaling pathway,36,37 was markedly stimulated.
These findings suggest that EC activation in response to APLAs/anti-β2GPI Abs might be enhanced by a concurrent increase in the expression of several proteins involved in signal transduction through the annexin A2-TLR4–dependent pathway.
Role of lipid rafts in signaling through the annexin A2–dependent multiprotein complex
Lipid rafts facilitate the lateral assembly of proteins within the cell membrane and enhance signal transduction.38 It has been suggested that anti-β2GPI Abs may interact with β2GPI, in association with annexin A2 and TLR4, in lipid rafts within the monocyte plasma membrane.39 We hypothesized that the complex consisting of annexin A2, TLR4, calreticulin, and nucleolin might assemble in EC lipid rafts. To address this hypothesis, we pretreated ECs with 650μM methyl-β-cyclodextrin, a concentration chosen to avoid toxicity (supplemental Figure 5A), before incubation with β2GPI and anti-β2GPI Abs. Methyl-β-cyclodextrin pretreatment inhibited EC activation, as determined by cell-surface E-selectin expression, by approximately 50% (supplemental Figure 5B).
These findings are consistent with a role for lipid rafts in enhancing the formation of the multiprotein signaling complex that mediates EC activation in response to APLAs/anti-β2GPI Abs.
Expression of nucleolin and calreticulin on the cell surface
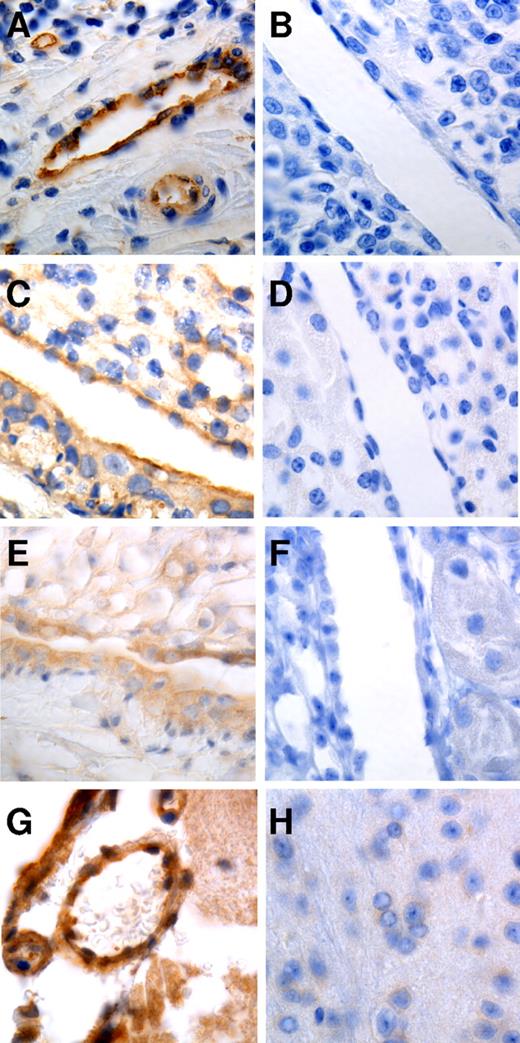
Although nucleolin and calreticulin are generally considered to be intracellular proteins, several studies have demonstrated that they mediate important activities on the cell surface.27-31 To examine whether these proteins occur on the surface of ECs in vivo, we stained tissues for nucleolin and calreticulin. We observed robust staining for nucleolin in vascular endothelium in human tonsil and murine kidney (Figure 7A-D), and in numerous other tissues, including aorta, vasa vasorum, and spleen. Calreticulin was also observed on ECs in the murine kidney, brain, and vasa vasorum, among other sites (Figure 7E-H).
Staining of human and murine tissues for nucleolin and calreticulin. Formalin-fixed, paraffin-embedded tissue was stained with Abs to nucleolin (A,C) calreticulin (E,G), or equal concentrations of control IgG (B,D,F,H). Strong endothelial staining for nucleolin can be observed in human tonsil (A) and mouse kidney (C). Endothelial staining for calreticulin can be observed in mouse kidney (E) and brain (G). Photomicrographs were taken at 63×.
Staining of human and murine tissues for nucleolin and calreticulin. Formalin-fixed, paraffin-embedded tissue was stained with Abs to nucleolin (A,C) calreticulin (E,G), or equal concentrations of control IgG (B,D,F,H). Strong endothelial staining for nucleolin can be observed in human tonsil (A) and mouse kidney (C). Endothelial staining for calreticulin can be observed in mouse kidney (E) and brain (G). Photomicrographs were taken at 63×.
Discussion
In the present study, we describe a complex consisting of annexin A2, TLR4, calreticulin, and nucleolin that is present on the EC surface and mediates the activation of ECs in response to β2GPI and anti-β2GPI Abs. We affinity purified TLR4, calreticulin, and nucleolin using immobilized annexin A2, and coimmunoprecipitated all members of the complex using Abs against annexin A2, TLR4, or nucleolin. This complex is present on the surface of unstimulated ECs, but immunoprecipitation studies suggest that its assembly may be increased in the presence of β2GPI and anti-β2GPI Abs. Whereas previous studies have demonstrated an association of annexin A2 and TLR4,40 our present findings are the first to suggest that this association may occur in the context of a multiprotein complex. The expression of critical components of the complex, including annexin A2, S100A10, and TLR4, was increased during EC activation by β2GPI/anti-β2GPI Abs.
The importance of this multiprotein complex in the activation of ECs by anti-β2GPI Abs is illustrated by the fact that specific siRNAs to each of the components blocks EC activation in response to these Abs, as determined by measurement of cell-surface E-selectin expression.11,22 Inhibiting the expression of these components also prevents increases in E-selectin, ICAM-1, VCAM-1, and TF mRNA, all of which encode proteins of importance in the pathogenesis of anti-β2GPI–mediated thrombosis. An intact multiprotein complex was also found to be essential for the activation of NF-κB.19 The fact that blocking the expression of multiple proteins at once did not cause additional inhibition of EC activation, which was nearly complete even when the expression of a single protein was blocked, suggests that the proteins comprising this complex function as members of a single pathway.
These results provide new insight into the mechanisms by which anti-β2GPI Abs activate ECs, in particular the nature of the interaction between annexin A2 and TLR4. Previous studies from our laboratory demonstrated that annexin A2 provided a high-affinity binding site for β2GPI on ECs18 and that oligomerization of annexin A2–bound β2GPI by anti-β2GPI Abs mediated EC activation.11 However, the importance of these observations has been questioned, because annexin A2 is not a transmembrane protein and thus may not be capable of transmembrane signal transduction. It has been difficult to reconcile these observations with reports demonstrating that EC activation by anti-β2GPI Abs is mediated through activation of NF-κB occurring through a TLR4/MyD88–dependent pathway.19 The findings in the current study reconcile these observations by demonstrating assembly of a complex that includes annexin A2 and TLR4, as well as calreticulin and nucleolin. Defining the physical nature of this complex and how it assembles will require additional work including direct assessment of protein-protein interactions, because whether annexin A2 and TLR4 interact directly or require nucleolin and calreticulin for “bridging” has not yet been determined.
Further studies will also be required to define the molecular mechanism by which this complex mediates cell activation. We hypothesize that a subfraction of the component proteins exist on unstimulated ECs as part of the multiprotein complex. After binding of β2GPI to annexin A2, anti-β2GPI Abs recognize the bound ligand and induce annexin A2 oligomerization,11 which leads to oligomerization of the multiprotein complex, including TLR4. The transmembrane and intracellular domains of TLR4 have an innate propensity to oligomerize, which is resisted in part by regions within the extracellular domain, particularly the juxtamembrane region.41,42 However, we propose that the recruitment of TLR4 to discrete membrane regions increases its propensity to oligomerize, resulting in ligand-independent TLR4 activation.41,43 Although studies to investigate this hypothesis are in progress, our present results nevertheless extend previous findings regarding the interactions of anti-β2GPI Abs with ECs and the mechanism by which TLR4 signaling is activated. Previous work in this area includes that by Sorice et al, who suggested a model in which β2GPI interacts with annexin A2 and TLR4 within lipid rafts in monocytes.39 A similar mechanism might mediate cell activation by pathologic anti–annexin A2 Abs.44
In addition to annexin A2 and TLR4, a role for TLR2 has been suggested in mediating the activation of ECs prestimulated with TNF-α by APLAs.24 However, we observed that siRNA-mediated inhibition of TLR2 expression did not affect the activation of unstimulated ECs by anti-β2GPI Abs, suggesting that TLR2 may play a limited role in the activation of unperturbed cells. Consistent with this conclusion, TLR2 is expressed at very low levels by nonactivated ECs in vivo.45
A role for apoER2 in thrombosis induced by APLAs/anti-β2GPI Abs has also been demonstrated.21,26 A β2GPI-FXI apple domain 4 fusion protein that dimerizes spontaneously stimulates the adhesion of platelets to collagen under flow in a manner inhibited by apoER2 and glycoprotein 1b, which has been proposed as another binding site for β2GPI on platelets.46 Moreover, Ramesh et al reported that inhibition of apoER2 expression by siRNA in bovine aortic ECs inhibited activation by anti-β2GPI Abs.21 Whereas we were not able to demonstrate inhibition of HUVEC activation by apoER2 siRNA, our preliminary studies suggest mild inhibition of activation of bovine and human aortic ECs by anti-β2GPI Abs, suggesting that the role of apoER2 may be cell-type specific. Additional work will be required to compare the role of an apoER2 versus an annexin A2-TLR4–mediated signaling pathway, keeping in mind that redundancy in activation mechanisms may exist.
Like annexin A2, nucleolin is generally not considered to be an extracellular protein. However, there is evidence supporting a functional role for nucleolin on the EC surface. Christian et al reported that anti-nucleolin Abs injected in vivo localize to the surface of angiogenic endothelium,27 and recent studies have demonstrated that EC-surface nucleolin may function as a receptor for endostatin.28 Nucleolin has also been shown to be necessary for increased expression of Kruppel-like factor 2 in response to shear stress.29 We have confirmed EC expression of nucleolin in human and murine tissues, with a staining pattern consistent with surface localization. Likewise, calreticulin, despite the lack of a classic secretion peptide or transmembrane domain, is present in human plasma, extracellular matrix, and body fluids, and may be found at increased concentrations in inflammatory disorders such as rheumatoid arthritis.30 Localization to the EC surface has been associated with several functions attributed to calreticulin, including the ability of thrombospondin to mediate focal adhesion disassembly47 and adhesion of ECs to C1q.48 Calreticulin localizes to the neutrophil surface in vivo, where it is anchored via interactions with CD59.31
Activation of ECs by anti-β2GPI Abs increased the expression of annexin A2 and S100A10 mRNA.34,35 Although we demonstrated previously that annexin A2 binds β2GPI directly, without a requirement for S100A10, S100A10 is required for transport of annexin A2 to the cell surface and stabilization in the heterotetrameric form.35 Therefore, increased expression of annexin A2 and S100A10 might enhance binding of β2GPI to cells, increasing their susceptibility to activation. We also observed increased expression of TLR4, MD2 (a TLR4 coreceptor that promotes oligomerization and signaling37 ), and MyD88 during anti-β2GPI–induced EC activation. These proteins are critical components of the TLR4/MyD88-signaling pathway, the activity of which might be enhanced through increased expression.49 These findings suggest that anti-β2GPI Abs might induce responses in ECs that reciprocally enhance susceptibility to activation. Moreover, secreted MD2 might sensitize neighboring ECs and/or monocytes to activation by these Abs.37
In summary, the results of the present study demonstrate the existence of a signaling complex consisting of annexin A2, TLR4, calreticulin, and nucleolin that mediates NF-κB–dependent activation of ECs by anti-β2GPI Abs. These may not be the only components of the complex. For example, we also detected vimentin in our mass spectrometric analysis, and vimentin-cardiolipin complexes have been implicated in EC activation by anti-β2GPI Abs.50 Additional studies focused on defining the interactions between the component proteins of this complex and the mechanisms by which they assemble may provide further insight into anti-β2GPI Ab–induced thrombosis and opportunities for therapeutic intervention in APS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Marc Monestier (Temple University, Philadelphia, PA) for the kind gift of hybridoma cells elaborating mAbs FC1 and 7D10.
K.R.M. is principal investigator on project 4. K.L.A. and F.V.F. were supported by National Institutes of Health grant 5T32HL007147 to K.R.M.
National Institutes of Health
Authorship
Contribution: K.L.A., F.V.F., V.B., and J.Z. performed the experiments; B.W. performed the proteomic analyses; and K.R.M. conceived the idea for this project, supervised the work, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Keith R. McCrae, MD, Taussig Cancer Institute and Department of Cell Biology, R4-018, Cleveland Clinic, 9500 Euclid Ave, NC10, Cleveland, OH 44195; e-mail: mccraek@ccf.org.
References
Author notes
K.L.A. and F.V.F. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal