Paroxysmal nocturnal hemoglobinuria (PNH) is characterized by complement-mediated intravascular hemolysis because of the lack from erythrocyte surface of the complement regulators CD55 and CD59, with subsequent uncontrolled continuous spontaneous activation of the complement alternative pathway (CAP), and at times of the complement classic pathway. Here we investigate in an in vitro model the effect on PNH erythrocytes of a novel therapeutic strategy for membrane-targeted delivery of a CAP inhibitor. TT30 is a 65 kDa recombinant human fusion protein consisting of the iC3b/C3d-binding region of complement receptor 2 (CR2) and the inhibitory domain of the CAP regulator factor H (fH). TT30 completely inhibits in a dose-dependent manner hemolysis of PNH erythrocytes in a modified extended acidified serum assay, and also prevents C3 fragment deposition on surviving PNH erythrocytes. The efficacy of TT30 derives from its direct binding to PNH erythrocytes; if binding to the erythrocytes is disrupted, only partial inhibition of hemolysis is mediated by TT30 in solution, which is similar to that produced by the fH moiety of TT30 alone, or by intact human fH. TT30 is a membrane-targeted selective CAP inhibitor that may prevent both intravascular and C3-mediated extravascular hemolysis of PNH erythrocytes and warrants consideration for the treatment of PNH patients.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a blood disorder clinically characterized by intravascular hemolysis, thrombophilia, and bone marrow failure.1,–3 A unique feature of PNH is the presence of clonal populations of blood cells that are defective in glycosylphosphatidylinositol (GPI)–anchor biosynthesis,4 because they derive from stem cells harboring an acquired somatic mutation in the phosphatidylinositol glycan class A (PIG-A) gene.5,6 GPI-linked surface proteins include CD55 (also known as decay-accelerating factor [DAF])7,8 and CD59 (or membrane inhibitor of reactive lysis [MIRL]),9,10 2 major complement regulators on the cell surface. Because of the lack of these 2 regulators, PNH erythrocytes (red blood cells [RBCs]) are exquisitely vulnerable to complement activation. Indeed, the main mechanism of hemolysis in PNH is the intravascular destruction of CD59 deficient RBCs by the membrane attack complex (MAC; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).11 MAC formation in patients can be abrupt and massive when complement is triggered by specific conditions, as with an infection, explaining the paroxysms of hemoglobinuria that have given PNH its name. However, at a lower rate, hemolysis in PNH is continuous, and it is accounted for by the so-called tickover of the complement alternative pathway (CAP). Tickover occurs through spontaneous hydrolysis of C3, binding of factor B to this form of C3, and subsequent formation of a C3 cleavage and activating multiprotein complex designated a C3 convertase (supplemental Figure 1).12,13 As PNH RBCs are deficient in CD55, both the initial C3 convertase generated by tickover, as well as the amplification loop of complement (supplemental Figure 1) are not blocked, allowing unregulated complement activation on the PNH RBC surface.
Because MAC assembly is triggered by the production of C5b, a logical approach to preventing intravascular hemolysis in PNH is the C5 blockade: this has been achieved with the introduction of eculizumab, an anti-C5 monoclonal antibody (mAb; Soliris, Alexion Pharmaceuticals).14 The clinical results of this novel therapy have demonstrated that eculizumab is highly effective for PNH patients.15,,,–19 Although eculizumab protects PNH RBCs from hemolysis, it does not prevent the initial steps of complement activation, and consequently in patients treated with eculizumab a variable proportion (up to 80%) of the PNH RBCs become opsonized by C3d and probably upstream C3 activation fragments; and there is evidence that such RBCs may become susceptible to extravascular hemolysis.20,,–23 Consequently, targeting the early steps of complement activation rather than C5 in PNH might prevent both the MAC-mediated intravascular hemolysis and the extravascular hemolysis of C3 fragment opsonized RBCs. However, complete systemic C3 blockade in vivo may prove problematic, because genetic deficiency of C3 in man is associated not only with susceptibility to certain infections, but also with immune complex disease.24
TT30 is a novel recombinant protein that is designed to control both intravascular and extravascular hemolysis of PNH RBCs. TT30 is a 65 kDa fusion protein consisting of the iC3b/C3dg-binding domain of complement receptor 2 (CR2; ie, the short consensus repeats [SCRs] 1-4), and the CAP inhibitory domain of factor H (fH; ie, SCR1-5), a complement inhibitor present in human blood whose primary role is to control fluid phase tickover (supplemental Figure 2).25 fH selectively inhibits the activation of CAP and the CAP C3 convertase, without affecting complement activation and C3 convertase activity arising from the classic pathway (CCP) and mannose/lectin (CMP) pathway. fH both prevents formation and promotes the decay of the CAP C3 convertase and also prevents its formation by acting as a cofactor with factor I (fI) to cleave C3b into the inactive fragment iC3b (supplemental Figure 1). The CR2 domain of TT30 specifically targets the fusion protein to sites of complement activation, which are marked by deposition of C3 fragments including iC3b and C3d. Thus TT30 is designed to selectively inhibit CAP activation specifically at sites of complement activation, which in the case of PNH, is the surface of GPI-deficient RBCs.
In this paper, we show that TT30 is a highly effective inhibitor of complement-dependent hemolysis of PNH RBCs. Furthermore, we demonstrate that TT30 blocks CAP activation and prevents C3 fragment opsonization of PNH RBCs. These in vitro studies suggest that TT30 effectively prevents CAP C3 convertase formation and can be expected to effectively prevent both intravascular and extravascular hemolysis in patients with PNH.
Methods
Human samples
Peripheral blood was collected from 32 patients with proven diagnosis of PNH. Globally, blood samples were available from 21 patients who were not receiving any anticomplement treatment, and from 12 patients who were receiving the anti-C5 mAb eculizumab (1 patient was drawn both before and during eculizumab treatment). Eculizumab was administered according to the standard schedule15,,,–19 (900 mg every 14 ± 2 days, after a loading phase of 600 mg every 7 ± 1 days for 4 doses). Blood was collected in standard ethylenediaminetetraacetic acid (EDTA) and serum vacutainer tubes (BD Pharmingen; Becton Dickinson Italia) after venipuncture according to standard procedures, after informed consent in accordance with the Declaration of Helsinki as approved by the Federico II University institutional review board. Blood was usually collected at the time of venipuncture for standard laboratory testing, with the exception of some PNH patients on eculizumab, from whom blood was also drawn within 60 minutes of eculizumab administration (as source for serum containing eculizumab). In addition, blood was collected from 10 healthy individuals as a source for ABO-matched normal sera.
RBC and serum preparation
After collection of peripheral blood samples, RBCs were obtained from EDTA tubes after centrifugation (5′ at 350g) and 3 washings in saline, to prepare a final 50% RBC resuspension (approximately 5 × 106 cells/μL). For midterm storage, RBCs were resuspended at 50% in hypertonic saline-adenine-glucose-mannitol (SAG-M) solution (hemapheresis bags from Fresenius-Kabi Italy) and stored at 4°C up to 4 weeks. Fresh sera were obtained from serum tubes after immediate centrifugation (15′ at 1350g); sera were used within 1 hour, or stored at −80°C for subsequent use. In experiment with normal sera, a pool from at least 2 healthy subjects was used, to prevent biases because of inter-patient variability in complement activity.
The extended acidified serum assay
C3 tickover and CAP activation is accelerated by serum acidification, and it was exploited in our in vitro assay to investigate the effect of TT30 on complement pathways in PNH. Starting from the standard diagnostic acidified serum assay (Ham test), a modified assay was developed to provide a model that allows for in vitro investigation of both early CAP-directed activation as well as terminal complement cascade activation on the PNH RBC surface, which leads to C3 fragment deposition and MAC-mediated hemolysis, respectively. This assay provides a surrogate model for chronic, continuous, complement activation through the tickover mechanism, which leads to hemolysis of PNH cells. It also serves as a model for in vitro C3-deposition on PNH RBCs in the presence of terminal complement inhibition by eculizumab. For this purpose, RBCs from PNH patients were incubated at final hematocrits of 2% (or 16% and 33%, in selected experiments) with sera (either from ABO-matched healthy individual or PNH patients on eculizumab) supplemented by MgCl2 (at a final 1.5mM concentration). CAP activation was achieved by lowering the pH, through addition of HCl (1:20 of HCl 0.1N); the final pH ranged between 6.7 and 6.9. The complement inhibitor TT30 (or controls) were added to the tubes at different concentration before complement activation. Tubes were incubated at 37°C for 24 hours, with the RBC pellet analyzed for hemolysis and C3-deposition at specific time-points at 1 and 24 hours.
Assessment of hemolysis and C3 fragment deposition
Hemolysis of PNH RBCs and C3-deposition on the RBC surface were assessed by flow cytometry, as previously described.20,26,–28 In brief, 1 μL of the RBC pellet was resuspended in 1 mL of saline; the resulting suspension (approximately 104 RBCs/μL) was incubated with either a phycoerythrin (PE)–conjugated or an allophycocyanin (APC)–conjugated anti-CD59 (59PE or 59APC Valter Occhiena, used 1:10 and 1:25, respectively), and a fluorescein isothiocyanate (FITC)–conjugated anti-C3 polyclonal antibodies (Ab14396 Abcam; a 1:20 working solution from the original tube was used at a final dilution of 1:50). Samples were incubated at room temperature for 1 hour, and then analyzed with a FACScan cytometer (Becton Dickinson Italia), after dilution by adding 10 volumes of saline (or FACS flow, Becton Dickinson Italia).20 The percentage of type III PNH (CD59−) and of normal (N, CD59+) RBCs was derived from RBC pellets incubated in different conditions, as well as from baseline samples; hemolysis was measured by comparing baseline (pre) and postincubation (post) percentage of PNH RBCs. The proportion of surviving PNH RBCs (%PNH post/%PNH pre) was normalized, based on the determination of residual normal RBCs (dividing by %N post/%N pre); the rate of hemolysis was then calculated as the reciprocal of the percentage of survival. This finally leads to the formula previously published by Ferreira et al26 : %lysis = 100 − (%PNH post/%N post) × (%N pre/%PNH pre) × 100. C3-deposition was expressed as both percentage of C3+ PNH RBCs [(%C3/%PNH) × 100] at specific time-points and percentage C3-deposition from initial PNH RBCs, according to the following formula: %C3-deposition = (%C3/%PNH) × %survival (where survival = [%PNH post/%N post] × [%N pre/%PNH pre] × 100).27,28
Kinetics of distinct C3-fragment deposition and processing
The RBC pellet obtained as described in “RBC and serum preparation” was also incubated with different anti-C3 mAb, which have a known specificity for either C3b/iC3b or C3dg/C3d. They include the anti-C3dg/C3d mAbs 1H8 and 14H10, as well as the anti-C3b/iC3b mAbs 2C5, 3C11, 3E7, 7C12, and 8E11; the specificity of all these antibodies has been described elsewhere.29 Counter-staining was accomplished with either the APC-conjugated anti-CD59 mAb or, in selected experiments, with a PE-conjugated anti-glycophorin A (GPA) mAb (Becton Dickinson Italia; used 1:25); this latter was used to identify RBC ghosts. To investigate the concomitant presence of C3b/iC3b and C3dg/C3d fragments a biotinylated anti-C3d was also used (A702, Quidel); A702 was used 1:10 with RBC suspension, and detected by either PE or APC-conjugated streptavidin (Becton Dickinson Italia; used 1:20 after RBC washing). Samples were incubated at room temperature for 1 hour, and analyzed as described “Assessment of hemolysis and C3 fragment deposition.”
Complement inhibitors
TT30 was provided by Alexion (formerly Taligen Therapeutics) as frozen 160μM stocking solution. The vials were stored at −80°C, and held at room temperature 1 hour before the experiments. Working solutions of 120μM and further appropriate dilutions were obtained by adding adequate volumes of PBS. As a negative control, we used: (1) recombinant fH SCR1-5 (Alexion Pharmaceuticals); (2) CR2 SCR1-4 (Alexion Pharmaceuticals); and (3) human purified fH (A137 Complement Technology); they were used at concentration equimolar to TT30 (1μM). In addition, an anti-CR2 mAb (mAb 1048, Green Mountain Antibody), which specifically inhibits the binding of CR2 (and thus of TT30) to C3 fragments was used in blocking experiments.30 As an in vitro surrogate of eculizumab use, sera from patients on eculizumab were used. Such sera were collected within 60 minutes of eculizumab administration, at an expected peak concentration of at least 200 μg/mL.15 This eculizumab concentration is approximately 1 log higher than the minimal inhibitory concentration.15
TT30 binding
TT30 binding was assessed by flow cytometry using 2 different FITC-conjugated mAbs specific for either fH (Valter Occhiena) or CR2 (HB5, Santacruz Biotechnology; HB5 is known to not interfere with C3 fragment binding to CR2).31 These mAbs bind the 2 different portions of the fusion protein TT30. Both mAbs were used 1:10 with the RBC suspension (prepared by diluting the RBC pellet 1:1000 in saline), and incubated 1 hour at room temperature. A counter-staining with the anti-CD59 (either PE or APC-conjugated) and the biotinylated anti-C3d A702 (detected by either PE or APC-conjugated streptavidin, see above) was used to assess the colocalization of TT30 and C3frag on PNH RBCs.
Statistical analysis
Standard descriptive statistical measures were used to analyze the rate of hemolysis and C3 deposition.
Results
TT30 abrogates hemolysis of PNH RBCs in the extended acidified serum assay (EASA)
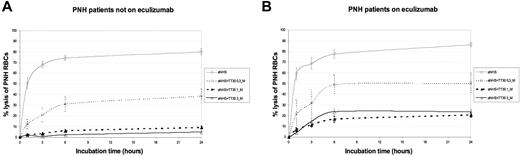
We have developed an experimental method to examine CAP-mediated early and terminal complement activation on PNH RBCs. In the EASA, RBCs obtained from PNH patients were incubated for 24 hours at 37°C in ABO-matched normal human serum (NHS) in which complement activation was promoted by lowering the pH. CAP activation results in progressive lysis of the majority of PNH RBCs, as assessed by flow cytometric analysis of the residual RBC pellet,26 regardless of whether the samples were from untreated or eculizumab-treated patients (80.2 ± 2.8 and 86.6 ± 2.1, respectively; Figure 1). This indicates that the system produces hemolysis whether or not RBCs have C3 on their membrane. With RBCs from untreated PNH patients, preincubation of NHS with TT30 resulted in a concentration-dependent reduction of hemolysis, which was almost complete (5 ± 1.1%) at a TT30 level of 3μM (Figure 1A). Similar results were obtained using RBCs from PNH patients on eculizumab (Figure 1B); in some cases we observed a minimal hemolysis, which was probably the result of a build up of C5 convertase which may have taken place in vivo as a consequence of C3b deposition.
Effect of TT30 on hemolysis of PNH RBCs from untreated and eculizumab-treated PNH patients in the EASA. (A) PNH patients not on eculizumab. Absolute percentage lysis of PNH RBCs in aNHS with and without TT30. Lines represent the mean values of 26 experiments (10 in duplicate, 1 in triplicate, and 3 in quadruplicate) on RBCs obtained from 14 untreated PNH patients (2 patients were drawn 4 times, 3 patients drawn 3 times, and the remaining 9 just once); error bars represent SEM. The continuous thin black line indicates aNHS; dashed thin black line, TT30 0.3μM; dashed bold black line, TT30 1μM; and continuous bold black lines, TT30 3μM. (B) PNH patients on eculizumab. Absolute percentage lysis of PNH RBCs in aNHS with and without TT30. Lines represent the mean values of 26 experiments (12 in duplicate, 1 in triplicate, and 3 in quadruplicate) on RBCs obtained from 6 PNH patients on eculizumab treatment (1 patient was drawn 14 times, 1 patient drawn 9 times, and the remaining 3 just once); error bars represent SEM. The continuous thin black line indicates aNHS; dashed thin black line, TT30 0.3μM; dashed bold black line, TT30 1μM; and continuous bold black lines, TT30 3μM.
Effect of TT30 on hemolysis of PNH RBCs from untreated and eculizumab-treated PNH patients in the EASA. (A) PNH patients not on eculizumab. Absolute percentage lysis of PNH RBCs in aNHS with and without TT30. Lines represent the mean values of 26 experiments (10 in duplicate, 1 in triplicate, and 3 in quadruplicate) on RBCs obtained from 14 untreated PNH patients (2 patients were drawn 4 times, 3 patients drawn 3 times, and the remaining 9 just once); error bars represent SEM. The continuous thin black line indicates aNHS; dashed thin black line, TT30 0.3μM; dashed bold black line, TT30 1μM; and continuous bold black lines, TT30 3μM. (B) PNH patients on eculizumab. Absolute percentage lysis of PNH RBCs in aNHS with and without TT30. Lines represent the mean values of 26 experiments (12 in duplicate, 1 in triplicate, and 3 in quadruplicate) on RBCs obtained from 6 PNH patients on eculizumab treatment (1 patient was drawn 14 times, 1 patient drawn 9 times, and the remaining 3 just once); error bars represent SEM. The continuous thin black line indicates aNHS; dashed thin black line, TT30 0.3μM; dashed bold black line, TT30 1μM; and continuous bold black lines, TT30 3μM.
Kinetics of C3 fragment deposition and processing in the EASA
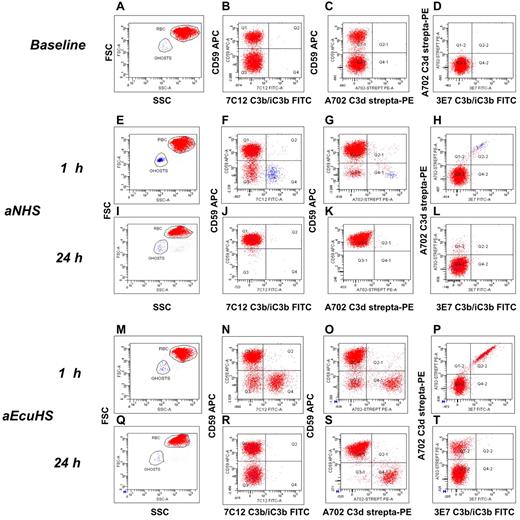
The early stages of CAP activation and hemolysis were investigated as a function of time by flow cytometric analysis of the pellet of unlysed RBCs. With RBCs from untreated PNH patients (Figure 2A-D), after 1 hour incubation in aNHS we found that: (1) the proportion of PNH RBCs is significantly reduced, consistent with selective lysis of PNH RBCs (Figure 2F); (2) Of the few surviving PNH RBCs, most have no C3b/iC3b or C3d deposition (Figure 2F-H); and (3) RBC ghosts (the intact membrane of lysed RBC, identified by physical parameters: Figure 2E and by glycophorin A staining, data not shown) are CD59 negative (PNH phenotype) and have bound both C3b/iC3b and C3d fragments (Figure 2F-H), consistent with complement-mediated lysis of the PNH RBCs. At 24 hours all PNH RBCs had been lysed (Figure 2I-L).
Kinetics of C3 fragment deposition and processing in the EASA. (A-D) Fresh PNH RBCs from untreated patient, baseline data. (A) Forward scatter (y-axis) versus side scatter (SSC). (B) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (C) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); (D) C3d biotinylated-streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels A-E). (E-L) aNHS. (E-H) C3b/iC3b and C3d deposition at 1 hour. (I-L) C3b/iC3b and C3d deposition at 24 hours. (E-I) forward scatter (y-axis) versus SSC. (F-J) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (G-K) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); (H-L) C3d biotinylated-streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels E-I). (M-T) aEcuHS. (M-P) C3b/iC3b and C3d deposition at 1 hour. (Q-T) C3b/iC3b and C3d deposition at 24 hours. (M-Q) forward scatter (y-axis) versus SSC. (N-R) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (O-S) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); P, T: C3d biotinylated-Streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels M-P).
Kinetics of C3 fragment deposition and processing in the EASA. (A-D) Fresh PNH RBCs from untreated patient, baseline data. (A) Forward scatter (y-axis) versus side scatter (SSC). (B) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (C) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); (D) C3d biotinylated-streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels A-E). (E-L) aNHS. (E-H) C3b/iC3b and C3d deposition at 1 hour. (I-L) C3b/iC3b and C3d deposition at 24 hours. (E-I) forward scatter (y-axis) versus SSC. (F-J) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (G-K) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); (H-L) C3d biotinylated-streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels E-I). (M-T) aEcuHS. (M-P) C3b/iC3b and C3d deposition at 1 hour. (Q-T) C3b/iC3b and C3d deposition at 24 hours. (M-Q) forward scatter (y-axis) versus SSC. (N-R) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (O-S) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); P, T: C3d biotinylated-Streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels M-P).
When similar experiments were carried out using fresh serum containing eculizumab (obtained from PNH patients on eculizumab, aEcuHS) we observed substantial inhibition of lysis: most PNH RBCs remain intact, and progressively accumulate C3 fragments on their surface. Initially both C3b/iC3b and C3d fragments were detected (Figure 2M-P); gradually, over a 24 hour interval, these were converted into C3d (Figure 2Q-T). This experiment recapitulates the observation of C3 fragment deposition in vivo on PNH RBC in patients on eculizumab.20 These findings provide further evidence supporting the role of CAP activation in the C3 fragment opsonization and lysis of PNH RBCs.
TT30 inhibits C3 fragment deposition on PNH RBCs
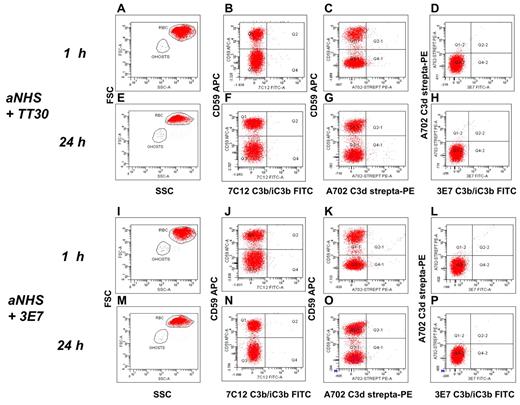
Having found that EASA enables us to analyze early CAP activation on PNH RBCs, we next investigated how this process is affected by TT30. We first made sure that TT30, even at a level of 30μM, did not interfere with the detection of either C3b/iC3b or C3d by the anti-C3 mAbs used in this study. In brief, PNH RBCs obtained from eculizumab-treated patients (with known proportion of C3+ RBCs) were preincubated 1 hour with TT30 30μM, and then stained with our set of anti-C3b/iC3b and anti-C3d mAb (as well as with the polyclonal anti-C3). The proportion of C3+ RBCs did not change after the preincubation with TT30, nor did the intensity of fluorescence decrease (supplemental Figure 3). Then we found that after 1 to 24 hours incubation in aNHS + 3μM TT30, PNH RBCs from untreated patients did not show C3 fragment deposition by flow cytometric analysis (Figure 3A-H). Notably, even the very early product of CAP activation, C3b/iC3b, was not detectable at any time point on PNH RBCs. Prevention of lysis and of C3 fragment deposition is consistent with a complete blockade of both CAP C3 and C5 convertase activity. Similar results were obtained by inhibiting the CAP C3 convertase with the anti-C3b mAb 3E7 (Figure 3I-P), which is known to completely prevent C3 activation on PNH RBCs.32
TT30 inhibits C3 fragment deposition on PNH RBCs in the EASA. (A-H) aNHS + TT30 3μM. (A-D) C3b/iC3b and C3d deposition at 1 hour. (E-H) C3b/iC3b and C3d deposition at 24 hours. (A-E) forward scatter (y-axis) versus side scatter (SSC). (B-F) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (C-G) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); (D-H) C3d biotinylated-streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels A-E). (I-P) aNHS +3E7 2μM. (I-L) C3b/iC3b and C3d deposition at 1 hour. (M-P) C3b/iC3b and C3d deposition at 24 hours. (I-M) forward scatter (y-axis) versus SSC. (J-N) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (K-O) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); (L-P) C3d biotinylated-streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels I-M).
TT30 inhibits C3 fragment deposition on PNH RBCs in the EASA. (A-H) aNHS + TT30 3μM. (A-D) C3b/iC3b and C3d deposition at 1 hour. (E-H) C3b/iC3b and C3d deposition at 24 hours. (A-E) forward scatter (y-axis) versus side scatter (SSC). (B-F) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (C-G) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); (D-H) C3d biotinylated-streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels A-E). (I-P) aNHS +3E7 2μM. (I-L) C3b/iC3b and C3d deposition at 1 hour. (M-P) C3b/iC3b and C3d deposition at 24 hours. (I-M) forward scatter (y-axis) versus SSC. (J-N) CD59 APC (y-axis) versus C3b/iC3b FITC (x-axis; mAb 7C12). (K-O) CD59 APC (y-axis) versus C3d biotinylated-streptavidin PE (x-axis; mAb A702); (L-P) C3d biotinylated-streptavidin PE (y-axis) versus C3b/iC3b FITC (x-axis; mAb 3E7). Red and blue dots represent intact RBCs and RBC ghosts, respectively, as gated based on physical parameters (panels I-M).
Membrane targeting of TT30 by the CR2 domain is required for full inhibition of hemolysis
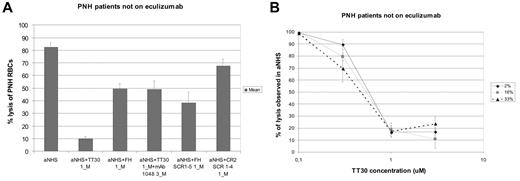
To verify that the CR2 moiety is crucial for the action of TT30, we used the anti-CR2 mAb 1048, which binds to the C3-binding domain of CR2 and is known to directly inhibit the binding of CR2 (and thus of TT30) to C3 fragments.33 Indeed, mAb 1048 at a molar concentration 3 times higher than TT30 markedly decreases the protection from hemolysis by TT30 (hemolysis of RBCs from untreated PNH patients was 49.4 ± 6.2%; Figure 4A). Similar results were obtained when RBCs from eculizumab-treated PNH patients were studied (hemolysis 56.1 ± 6.2%; supplemental Figure 4A). Interestingly, in the presence of excess mAb 1048, the residual effect of TT30 was quantitatively similar to that of an equimolar concentration of purified human fH (Figure 4A, supplemental Figure 4A). Furthermore, similar degree of inhibition was also observed using recombinant fH SCR1-5 (Figure 4A, supplemental Figure 4A), whereas CR2 SCR1-4 did not produce any inhibition of hemolysis (Figure 4A, supplemental Figure 4A). This result suggests that the residual partial inhibitory effect of TT30 was probably attributed to the fH moiety of TT30 acting in the fluid phase.
Abrogation of TT30 inhibition of hemolysis and dose-response curve at different hematocrits; PNH patients not on eculizumab. (A) Abrogation of TT30 effect by the blocking anti-CR2 mAb 1048 and comparison with human recombinant fH. Absolute percentage lysis of PNH RBCs in aNHS with and without TT30 ± the blocking anti-CR2 mAb 1048, or with the human recombinant fH (1μM), the human fH SCR1-5 (fH portion of TT30, 1μM) or the human CR2 SCR1-4 (CR2 portion of TT30, 1μM). Histograms represent the mean values of 6 experiments (4 in duplicate) on RBCs obtained from 4 untreated PNH patients; error bars represent SEM. (B) dose-response curve of TT30 on hemolysis at different hematocrits. Lysis (y-axis) of PNH RBCs according to TT30 concentration (x-axis); lysis is expressed as relative percentage of the lysis observed without any inhibitor (in each experiment, 100% represent the lysis observed in aNHS). Curves represent the mean of 14 experiments performed on samples obtained from 7 PNH patients (1 was drawn 4 times, 1 drawn 3 times, and 2 drawn twice), at different percentage of hematocrits. The continuous black line indicates hematocrit 2%; continuous gray line, hematocrit 16%; and dashed black line, hematocrit 33%. Error bars represent SEM.
Abrogation of TT30 inhibition of hemolysis and dose-response curve at different hematocrits; PNH patients not on eculizumab. (A) Abrogation of TT30 effect by the blocking anti-CR2 mAb 1048 and comparison with human recombinant fH. Absolute percentage lysis of PNH RBCs in aNHS with and without TT30 ± the blocking anti-CR2 mAb 1048, or with the human recombinant fH (1μM), the human fH SCR1-5 (fH portion of TT30, 1μM) or the human CR2 SCR1-4 (CR2 portion of TT30, 1μM). Histograms represent the mean values of 6 experiments (4 in duplicate) on RBCs obtained from 4 untreated PNH patients; error bars represent SEM. (B) dose-response curve of TT30 on hemolysis at different hematocrits. Lysis (y-axis) of PNH RBCs according to TT30 concentration (x-axis); lysis is expressed as relative percentage of the lysis observed without any inhibitor (in each experiment, 100% represent the lysis observed in aNHS). Curves represent the mean of 14 experiments performed on samples obtained from 7 PNH patients (1 was drawn 4 times, 1 drawn 3 times, and 2 drawn twice), at different percentage of hematocrits. The continuous black line indicates hematocrit 2%; continuous gray line, hematocrit 16%; and dashed black line, hematocrit 33%. Error bars represent SEM.
Dose-response curve of TT30 on hemolysis at different hematocrits
Given the unique mechanism of action of TT30, its effects in vivo might depend not only on its level in the plasma but also on the mass of RBCs that are the actual target of TT30. Therefore it was important to test whether the effect of TT30 varies as a function of the hematocrit value. We found that the dose-response curves were essentially the same at hematocrits of 2%, 16%, or of 33%, which is rather near a normal hematocrit in vivo, with an IC50 of approximately 0.5μM (Figure 4B). Similar results were obtained with RBC from eculizumab-treated patients (supplemental Figure 4B).
TT30 binds to PNH RBCs in the EASA
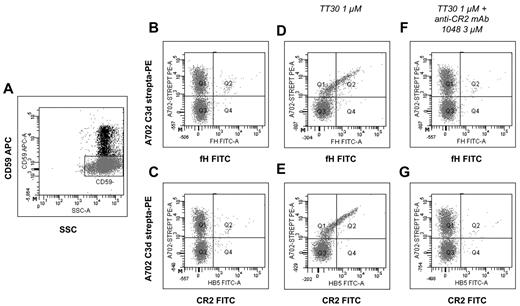
To further confirm that TT30 exerts a local regulatory effect on the RBC membrane, we examined TT30 binding to PNH RBCs using 2 mAbs: (1) an anti-fH that recognizes the CAP-inhibitory domain of TT30; and (2) an anti-CR2 that recognizes the C3 fragment-binding domain of TT30 (and was previously shown not to interfere with C3-CR2 binding).30 Because we know that TT30 prevents C3 deposition in vitro, we used in these experiments RBCs freshly obtained from PNH patients on eculizumab, a substantial fraction of which are covalently tagged with C3 fragments in vivo (see Figure 2). After 1 hour incubation in aNHS with 1 to 3μM TT30 we found that a similar proportion of RBCs had become positive for the anti-fH and the anti-CR2 mAbs: we infer that these antibodies were recognizing TT30 on the RBC surface (Figure 5D-E). By counter-staining for CD59, we were able to prove that TT30 binding was restricted to PNH (CD59−) RBCs (Figure 5D-E). Consistent with a C3 fragment targeting mechanism, the extent of TT30 binding correlated with the amount of C3d on PNH RBC surface (Figure 5D-E). In addition, preincubation with anti-CR2 mAb 1048, which blocks CR2 binding to iC3b/C3d but does not interfere with the detecting anti-CR2 mAb (data not shown), completely prevented TT30 binding, confirming that the TT30 binding is mediated by its CR2 moiety recognizing C3 fragments on the surface of PNH RBC (Figure 5F-G).
Detection of membrane-bound TT30 on PNH RBCs from an eculizumab-treated PNH patient. RBCs from a PNH patients on eculizumab were incubated 1 hour in aNHS with or without TT30 (± the anti-CR2 blocking mAb 1048); TT30 was detected by using either an anti-fH mAb (panels B,D,F,H) or an anti-CR2 mAb (C,E,G,I), both FITC-conjugated. The biotinylated anti-C3d mAb A702 was used to detect C3d (with APC-streptavidin); an APC-conjugated anti-CD59 was used to identify PNH RBCs. (A) Gating strategy on PNH RBCs. RBCs from an eculizumab-treated patient were used for this experiment; CD59 APC (y-axis) versus side scatter (SSC). PNH RBCs were identified by CD59 expression, and gated accordingly for further analysis of TT30 binding. (B-C) TT30 binding on fresh RBCs. Fresh PNH RBCs have a substantial C3d deposition, but do not show any cross-binding with the anti-fH or anti-CR2 mAb (B-C, respectively). C3d biotinylated-Streptavidin PE (y-axis; mAb A702) versus either fH or CR2-FITC (x-axis; B-C, respectively); analysis on CD59− RBCs (gate as shown in panel A). (D-E) TT30 binding after exposure in aNHS + TT30. After in vitro exposure to aNHS + TT30 3μM, TT30 can be detected on PNH (CD59−) RBCs by both the anti-fH (D) and the anti-CR2 (E). The saber-like pattern indicates the colocalization of C3d and TT30 on PNH RBC surface, with the most pronounced bound-TT30 detected on RBCs with the most abundant C3d deposition. C3d biotinylated-Streptavidin PE (y-axis; mAb A702) versus either fH or CR2-FITC (x-axis; D-E, respectively); analysis on CD59− RBCs (gate as shown in panel A). (F-G) TT30 binding after blocking by the anti-CR2 mAb 1048. Preincubation of TT30 with a 3-fold excess of the anti-CR2 mAb 1048 (TT30 1μM and mAb 1048 3μM) abrogates TT30 binding on PNH RBCs. C3d biotinylated-Streptavidin PE (y-axis; mAb A702) versus either fH or CR2-FITC (x-axis; F-G, respectively); analysis on CD59− RBCs (gate as shown in panel A).
Detection of membrane-bound TT30 on PNH RBCs from an eculizumab-treated PNH patient. RBCs from a PNH patients on eculizumab were incubated 1 hour in aNHS with or without TT30 (± the anti-CR2 blocking mAb 1048); TT30 was detected by using either an anti-fH mAb (panels B,D,F,H) or an anti-CR2 mAb (C,E,G,I), both FITC-conjugated. The biotinylated anti-C3d mAb A702 was used to detect C3d (with APC-streptavidin); an APC-conjugated anti-CD59 was used to identify PNH RBCs. (A) Gating strategy on PNH RBCs. RBCs from an eculizumab-treated patient were used for this experiment; CD59 APC (y-axis) versus side scatter (SSC). PNH RBCs were identified by CD59 expression, and gated accordingly for further analysis of TT30 binding. (B-C) TT30 binding on fresh RBCs. Fresh PNH RBCs have a substantial C3d deposition, but do not show any cross-binding with the anti-fH or anti-CR2 mAb (B-C, respectively). C3d biotinylated-Streptavidin PE (y-axis; mAb A702) versus either fH or CR2-FITC (x-axis; B-C, respectively); analysis on CD59− RBCs (gate as shown in panel A). (D-E) TT30 binding after exposure in aNHS + TT30. After in vitro exposure to aNHS + TT30 3μM, TT30 can be detected on PNH (CD59−) RBCs by both the anti-fH (D) and the anti-CR2 (E). The saber-like pattern indicates the colocalization of C3d and TT30 on PNH RBC surface, with the most pronounced bound-TT30 detected on RBCs with the most abundant C3d deposition. C3d biotinylated-Streptavidin PE (y-axis; mAb A702) versus either fH or CR2-FITC (x-axis; D-E, respectively); analysis on CD59− RBCs (gate as shown in panel A). (F-G) TT30 binding after blocking by the anti-CR2 mAb 1048. Preincubation of TT30 with a 3-fold excess of the anti-CR2 mAb 1048 (TT30 1μM and mAb 1048 3μM) abrogates TT30 binding on PNH RBCs. C3d biotinylated-Streptavidin PE (y-axis; mAb A702) versus either fH or CR2-FITC (x-axis; F-G, respectively); analysis on CD59− RBCs (gate as shown in panel A).
Discussion
TT30 is a 65 kDa fusion protein (supplemental Figure 2)34 consisting of (1) the iC3b/C3dg-binding domain of the C3 fragment receptor CR2 (SCR1-4), which will bind to iC3b and to C3d on red cells; and (2) the complement inhibitory domain of fH (SCR1-5),35,36 an inhibitor of CAP activation. The fH moiety of TT30, like fH itself, can both accelerate the decay of the CAP C3 convertase by displacing the Bb peptide from C3b, and act as a cofactor for the inactivation of C3b by the serine protease factor I (fI).
In this study, we tested extensively the efficacy of TT30 in preventing CAP activation and subsequent hemolysis of PNH RBCs. We modeled in vitro 2 important steps of what appears to occur in PNH patients in vivo: first, initial complement activation, resulting in C3 fragment opsonization of PNH RBCs, which may be a surrogate for C3-mediated extravascular hemolysis; second, terminal effector complement activity, resulting in hemolysis in vitro, as a surrogate for MAC-mediated intravascular hemolysis. In vivo, C3 fragment deposition becomes evident during eculizumab treatment when intravascular hemolysis is blocked.20,–22,37 We reproduced this phenomenon in an in vitro system using sera from PNH patients treated with eculizumab. Eculizumab resulted in progressively increasing deposition of C3b/iC3b and eventually conversion to C3d on all PNH RBCs (paralleling our previous in vivo observations).20 In vitro TT30 produced dose-dependent inhibition of both C3 fragment deposition and hemolysis with an IC50 of 0.5μM (32 μg/mL).
Reproducing in vitro what we have already found in vivo was an essential step for the experimental study of the mechanism of action of TT30. To pinpoint the site of TT30 action, we tested specifically for a membrane-bound effect versus a fluid phase effect. An anti-CR2 mAb, which interferes with the binding of the CR2 domain of TT30 to the iC3b/C3d target on RBCs, substantially reduced its protective effect on lysis of PNH RBCs. After TT30 binding to PNH RBCs is prevented by this antibody, it still has a residual capacity to inhibit hemolysis: this capability is similar to that of an equimolar concentration of human purified fH. Moreover, the fH moiety on its own has a similar effect; and we noted that the concentration that we have used is approximately 5 times lower than the physiologic concentration of fH in normal plasma (500-800 μg/mL)38 ; the concentration of fH may be somewhat higher in PNH patients.39 TT30 was designed deliberately to target selectively the cell membrane as the site of complement activation,34 and our data indeed suggest that TT30 targeting to the cell membrane is required for full inhibitory effect.
We demonstrated that TT30 is physically present on the RBC surface by taking advantage of the fact that a substantial proportion of PNH RBCs in eculizumab-treated patients are covalently tagged with C3d. In addition, we demonstrated that TT30 binds to these C3 fragments through the CR2 domain, as mAb 1048, which is known to disrupt CR2-C3d binding, also abrogates TT30 binding to C3d-coated RBC. TT30 binding was not demonstrated on RBCs of patients who were not on eculizumab and in whom C3d is not detectable on PNH RBCs. We presume that in blood samples from these patients there is a steady state whereby very few molecules of TT30, undetectable by flow cytometry, are sufficient to prevent the formation of C3 convertase and therefore sufficient to prevent complement activation that is ongoing.
TT30, by supplying the function of fH, is able to act as a cofactor for fI to convert nascent C3b into iC3b, thus generating more of its own target and disabling the CAP amplification loop. At the same time, on PNH RBCs TT30 serves a surrogate for the function of the CD55 that is missing; thus, it prevents formation and promotes decay of the CAP C3 convertase by displacing factor B and factor Bb, respectively, from the C3b-bound state. This result is in keeping with the previous finding that competitive inhibition of fH binding through the use of a decoy peptide markedly increases the in vitro lysis of PNH red cells.26 Taken together, these data support the notion that, whether C3 is activated in vitro by lowering the pH, or in vivo through the CAP C3 tickover, very few TT30 molecules per RBC are sufficient to prevent further significant activation of C3 (Figure 6). Because, in vivo, the number of PNH RBCs that need to be protected is huge, it is important in terms of clinical translation, that we have found that TT30 is effective at the same concentration even with a hematocrit of 33%. It was recently shown in a cynomolgus monkey model that TT30 is bioavailable by both intravenous (IV) injection and subcutaneous injection, leading to plasma levels sufficiently high to prevent CAP-mediated (but not CCP-mediated) MAC formation in sera drawn from treated animals.34 Effective plasma levels were sustained for 8 hours after IV injection and 24 hours after subcutaneous injection; the half-life was not formally established, and it may not be informative because patients will have an individually variable biodistribution because of increased (C3+) cells and tissue targets. From our current data it is hard to predict what plasma levels in vivo in PNH patients will be needed for a complete prevention of hemolysis, because our in vitro hemolytic assay employs supraphysiologic complement activation. Thus, pharmacokinetic and pharmacodynamic data will have to be assessed in humans, especially in the context of systemic or tissue-specific dysregulated complement activation, such as in PNH or other CAP-mediated diseases.
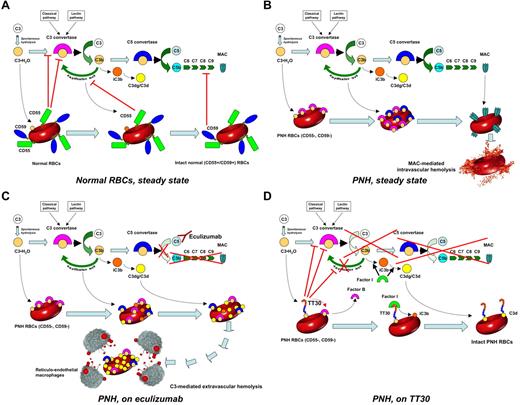
Complement cascade modulation on normal and PNH RBCs, with or without complement inhibitors. (A) Normal RBCs, steady state. Normal RBCs (CD55+, CD59+) are protected from complement activation by CD55, which down-regulate the C3-convertase, and by CD59, which inhibits the MAC assembly. Thus, in steady state normal RBCs can withstand the challenge of complement activation. (B) PNH RBCs, steady state. PNH RBCs (CD55−, CD59−) suffer from impaired complement regulation at the level of both C3-activation (because of the lack of CD55) and MAC assembly (because of the lack of CD59). As a result, in steady state, PNH RBCs eventually undergo intravascular hemolysis because of complement activation. (C) PNH RBCs, on eculizumab. On eculizumab treatment, PNH RBCs are protected from lysis because of the blockade of terminal effector complement; however, because of the impaired C3-convertase regulation, they suffer from continuous complement activation and subsequent membrane deposition of C3 fragments. As a result, on eculizumab, PNH RBCs may become susceptible to extravascular hemolysis secondary to removal of C3-opsonized RBCs by reticuloendothelial system (RES) macrophages, resulting in possible reduced life-span PNH RBCs. (D) PNH RBCs, on TT30. As soon as complement activation is initiated, the CR2-domain of TT30 delivers the complement-modulatory domain of fH to the RBC surface. Then, TT30 disables the C3-convertase, and, as cofactor of factor I, it promotes the conversion of active C3b into iC3b and then C3dg/C3d, preventing all downstream events because of the complement cascade (as well as additional complement activation on the red cell membrane). As a result, irrespective of the blockade of C5, TT30 may confer to PNH RBCs a normal survival even in presence of complement activation.
Complement cascade modulation on normal and PNH RBCs, with or without complement inhibitors. (A) Normal RBCs, steady state. Normal RBCs (CD55+, CD59+) are protected from complement activation by CD55, which down-regulate the C3-convertase, and by CD59, which inhibits the MAC assembly. Thus, in steady state normal RBCs can withstand the challenge of complement activation. (B) PNH RBCs, steady state. PNH RBCs (CD55−, CD59−) suffer from impaired complement regulation at the level of both C3-activation (because of the lack of CD55) and MAC assembly (because of the lack of CD59). As a result, in steady state, PNH RBCs eventually undergo intravascular hemolysis because of complement activation. (C) PNH RBCs, on eculizumab. On eculizumab treatment, PNH RBCs are protected from lysis because of the blockade of terminal effector complement; however, because of the impaired C3-convertase regulation, they suffer from continuous complement activation and subsequent membrane deposition of C3 fragments. As a result, on eculizumab, PNH RBCs may become susceptible to extravascular hemolysis secondary to removal of C3-opsonized RBCs by reticuloendothelial system (RES) macrophages, resulting in possible reduced life-span PNH RBCs. (D) PNH RBCs, on TT30. As soon as complement activation is initiated, the CR2-domain of TT30 delivers the complement-modulatory domain of fH to the RBC surface. Then, TT30 disables the C3-convertase, and, as cofactor of factor I, it promotes the conversion of active C3b into iC3b and then C3dg/C3d, preventing all downstream events because of the complement cascade (as well as additional complement activation on the red cell membrane). As a result, irrespective of the blockade of C5, TT30 may confer to PNH RBCs a normal survival even in presence of complement activation.
Our view of complement modulation on PNH RBCs by different complement inhibitors is somewhat in contrast with previous (but limited) observations on RBCs from patients with isolated CD55 (the so-called Inab phenotype)40 or CD59 deficiency.41 Indeed, on PNH RBCs use of eculizumab results in a functional replacement of CD59, but C3 deposition and subsequent extravascular hemolysis have not been observed in Inab patients in vivo.42 However, it has been reported that C3 opsonization in vitro is increased on Inab RBCs once CD59 is specifically inhibited by a blocking mAbs,43 suggesting a possible additional direct or indirect role for CD59-mediated MAC inhibition in the “upstream” regulation of the C3 convertase (in addition to its classic role in defending the membrane from the MAC). Our previous20 and current findings support this hypothesis. On the other hand, although in our in vitro model functional replacement of CD55 by TT30 is sufficient to prevent hemolysis, isolated deficiency of CD59 resulted in vivo in a clinical phenotype that overlaps with PNH.41 Therefore one may argue that in modulating C3 convertase activity TT30 is more efficient than CD55, as the former seems to keep the rate of formation of C5b low enough to control MAC generation even when CD59 is lacking. Taken together, these observations highlight the fact that even in the presence of specific anticomplement agents the phenotype of PNH remains unique: it is not a mimic of any known inherited condition.
Any attempt to interfere with the complement cascade for therapeutic purposes must come to terms with the potential risks that a selective blockade of 1 or more elements of the complement system might entail.44,–46 Inherited defects of complement proteins are informative in this regard.47 For example, an inherited deficiency of C3 in humans has a much more severe clinical phenotype than an inherited deficiency of C5, including not only severe infection (from capsulated or pyogenic organisms) but also immune complex diseases.24 Furthermore, inactivating mutation of factor B have been associated with glomerulonephritis in a few heterozygous patients,48 even if factor B knockout mice have not yet shown a specific disease phenotype.47,49 Thus, at the clinical level we are faced with a dilemma: systemic blockade of C5 is effective in preventing MAC formation, but not in preventing proximal CAP activation, which may lead to C3 fragment deposition on PNH RBC and extravascular hemolysis; on the other hand, systemic blockade of C3, which would prevent C3 fragment opsonization of PNH RBCs, may carry a risk of other complications. An anti-C3 mAb was recently developed that blocks the CAP and is specific for C3 fragments (C3b/iC3b), as opposed to intact C3.32 Although this approach has proven successful in an in vitro PNH model, it may be problematic in that it may predispose to immune complex disease, and may lead to further opsonization through the membrane-bound anti-C3 fragment mAb, and thus fail to overcome the problem of extravascular hemolysis. In contrast, TT30 is designed to selectively inhibit the CAP only, and in a targeted fashion, that is, on the surface of PNH RBC rather than systemically: this may improve its safety profile. Inherited polymorphisms or mutations in fH have been associated with a variety of complement-mediated diseases,50 such as atypical hemolytic-uremic syndrome,51 age-related macular degeneration,52 and dense deposit disease.53 However, all of these conditions result from loss-of-function mutations.50 On account of its structure TT30 may be regarded rather as a gain-of-function mutation (none of which are known): therefore we argue that there is not a priori reason why it ought to predispose to any complement-mediated disease. A murine analog of a CR2/fH and other CR2-targeted fusion proteins have proven effective in various models of complement-mediated diseases,54,–56 with little toxicity (in particular, without any increase in mortality in a model of acute septic peritonitis).47 In humans, we may anticipate that TT30, because of its CAP-selective and membrane-preferential action, may preserve physiologic immune-complex clearance and microorganism elimination, while fully abrogating hemolysis of PNH RBCs.
We must also recognize that a possible activation of the complement pathway through the CCP or the CMP can limit the clinical efficacy of TT30 in certain situations, especially in consideration of the build up of PNH RBCs. For instance, during infectious-related paroxysms CCP or CMP may contribute to hemolysis, in addition to the direct CAP activation on PNH RBCs by bacterial lipopolysaccharides. However, we must recall that CAP is required to amplify any initial complement activation, eventually contributing for optimal operation also of the other 2 complement pathways.46,57 Therefore, hemolysis of PNH RBCs requires a functional CAP, and the risk of catastrophic hemolytic crises during TT30 treatment seems low; however, it remains a potential limitation which has to be addressed in future in vivo studies.
In conclusion, we report a novel strategy to deliver specific inhibition of the CAP at the cell membrane level, which appears promising for PNH and may be relevant also for other complement-mediated diseases.58 Our experiments support the notion that TT30 targets C3 fragments on PNH RBCs to deliver surface-based fH activity. Thus, by blocking the CAP and its amplification loop, the CR2/fH fusion protein TT30 inhibits both intravascular hemolysis and C3 fragment opsonization of PNH RBCs. Our preclinical data provide a strong rationale for testing TT30 in PNH patients; indeed, TT30 is now under investigation in a phase 1 clinical trial enrolling untreated PNH patients (NCT01335165).59
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Taligen Therapeutics (now Alexion Pharmaceuticals) for supporting this study with an unrestricted research grant and providing the TT30 used in this study. They also thank the Aplastic Anemia/Myelodysplastic Syndrome Foundation, for supporting the research activity on PNH with a research grant to A.M.R. Finally, they thank the Italian Leukemia Association (Associazione Italiana Leucemie [AIL] section of Naples “Bruno Rotoli”) for contributing to the salary of A.M.R.
This project was conceived together with Professor Bruno Rotoli, who reviewed with great enthusiasm some of these results before he passed away on May 19, 2009. Bruno Rotoli was a dear friend and an unforgettable, inspiring colleague and mentor for some of the authors. They dedicate this work to Bruno's dearest memory, in honor of his life-long contributions to PNH and to medicine.
Authorship
Contribution: A.M.R. conceived the study together with V.M.H., and performed the experimental work with the help of C.P., R.N., and M.S.; A.M.R. discussed and interpreted the data with R.N., L.d.V., C.J.H., M.F.-H., V.M.H, C.S., M.A.L., R.P.T., and L.L; the paper was written by A.M.R., R.N., R.P.T., L.L., and V.M.H.; and all the authors critically revised the paper and contributed to the preparation of its final version.
Conflict-of-interest disclosure: A.M.R. received a research grant from Taligen Therapeutics (now Alexion Pharmaceuticals); he also served as a consultant for the same company. A.M.R. and R.N. have received lecture fees from Alexion Pharmaceuticals. V.M.H. serves as a consultant and has received research support from Taligen and Alexion Pharmaceuticals: he also received royalty payments for patents held by the University of Colorado. M.F.-H. and C.J.H. are Alexion employees. The remaining authors declare no competing financial interests.
Correspondence: Antonio M. Risitano, Hematology, Department of Biochemistry and Medical Biotechnologies, Federico II University of Naples, Via Pansini 5, 80131 Naples, Italy; e-mail: amrisita@unina.it.