The erythrocyte is one of the best characterized human cells. However, studies of the process whereby human reticulocytes mature to erythrocytes have been hampered by the difficulty of obtaining sufficient numbers of cells for analysis. In the present study, we describe an in vitro culture system producing milliliter quantities of functional mature human adult reticulocytes from peripheral blood CD34+ cells. We show that the final stage of reticulocyte maturation occurs by a previously undescribed mechanism in which large glycophorin A–containing vesicles forming at the cytosolic face of the plasma membrane are internalized and fuse with autophagosomes before expulsion of the autophagosomal contents by exocytosis. Early reticulocyte maturation is characterized by the selective elimination of unwanted plasma membrane proteins (CD71, CD98, and β1 integrin) through the endosome-exosome pathway. In contrast, late maturation is characterized by the generation of large glycophorin A–decorated vesicles of autophagic origin.
Introduction
The human erythrocyte is one of the best characterized mammalian cells, yet the process through which the enucleated erythroblast (reticulocyte) is converted to an erythrocyte remains poorly understood. Two distinct stages in reticulocyte maturation are evident from microscopic studies in animals.1 Reticulocytes formed immediately after enucleation (denoted R1) are motile, multilobular, and normally confined to the BM. Mature (R2) reticulocytes are nonmotile and much more mechanically stable than their multilobular predecessors and are released from the BM into the peripheral circulation.1,2 From the moment of enucleation until formation of the erythrocyte, the reticulocyte must lose approximately 20% of its surface area, reduce its volume, and degrade or eliminate residual cytosolic organelles. Current opinion is that the loss of surface area and degradation or elimination of residual organelles is achieved through 2 separate mechanisms. Plasma membrane loss is assumed to occur through the multivesicular endosome-exosome pathway in which small plasma membrane vesicles are endocytosed and incorporated into multivesicular endosomal bodies that subsequently fuse with the plasma membrane, releasing unwanted material as exosomes.3,4 Degradation and elimination of organelles is effected by autophagy, a process whereby unwanted materials are enclosed in a double membrane to form autophagosomes that are delivered to lysosomes and expelled from the cell.4 Substantial evidence suggests the endocytic and autophagic pathways converge.5 In addition, fusion of multivesicular bodies (which are derived from endosomes) and autophagosomes has been described in the erythroleukemic cell line K562.6 Early (R1) and late (R2) reticulocytes have different morphological and mechanical properties, but few studies have considered that different processes might be operative in the 2 cell types.
A clearer understanding of this final step in the maturation of human erythroid cells would facilitate the study of inherited and acquired anemias exhibiting reticulocytosis,7 inform attempts to elucidate the process whereby Plasmodium vivax invades human reticulocytes,8 and provide a means of monitoring the maturation state of therapeutic erythrocyte products generated ex vivo from diverse stem cell sources.9,–11
In the present study, we developed and characterized a robust ex vivo culture system that replicates human erythropoiesis using peripheral blood CD34+ cells isolated from the mononuclear cell fraction discarded from blood donations during routine processing. Substantial quantities of mature reticulocytes can be obtained from culture by passage through standard leukocyte filters without the need for the complex purification procedures required to isolate reticulocytes from the BM or peripheral blood.
Our results show that maturation of human (R2) reticulocytes uses a previously undescribed mechanism that involves the plasma membrane being taken into the cytosol of the maturing reticulocyte by endocytosis and the subsequent fusion of these endocytic intermediates with large, preexisting vesicles of autophagic origin. These hybrid vesicles are primed for fusion with the plasma membrane, and their contents are subsequently released from the maturing cell by exocytosis. This event constitutes the final step in the removal of redundant organelles in the maturing reticulocyte.
Methods
Erythroid cultures
A more than 95% pure population of CD34+, hemopoietic progenitor cells was isolated from human blood donor mononuclear cells by magnetic bead separation according to the manufacturer's protocol (Miltenyi Biotec). Blood donor mononuclear cells, a waste fraction from a donation of platelets by apheresis, were donated with informed consent in accordance with the Declaration of Helsinki (reviewed by the National Health Service National Research Ethics Service, Review of Ethics Committee reference number 08/H0102/26). Erythroid cells were cultured from CD34+ hemopoietic progenitor cells, at 37°C in a humid atmosphere of 5% CO2 in air in IMDM according to either protocol A or B (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CD34+ cells were seeded into stationary plastic tissue culture flasks at a density of 2 × 105 cells/mL and maintained in the range of 2-10 × 105 cells/mL by division and the addition of medium until the total volume reached 500 mL. For further expansion and terminal erythroid differentiation, the cells were transferred to 2-L glass vessels, stirred (15 rpm), and maintained at cell densities in the range of 1-6 × 106 cells/mL.
Patient samples
Samples were obtained from patients with normal erythropoiesis who had previously undergone splenectomy at least 6 months previously. These samples were anonymized for assay validation and control from excess material after diagnostic assay.
Removal of free nuclei and nucleated precursors
Cells were filtered at days 18-24 using a PALL WBF leukocyte filter. An empty 50-mL syringe was attached to the tubing upstream of the filter cassette to allow washing and loading of the cells. The filter was equilibrated with 50 mL of HBSS (Sigma-Aldrich) and up to 1010 cells in 100 mL of HBSS were loaded. The filter was washed with 500 mL of HBSS and the flow-through collected. Reticulocytes were pelleted from the flow-through by centrifugation at 400g for 5 minutes.
Examination of the expression of surface markers on reticulocytes by serology
Filtered reticulocytes were tested for the expression of blood group–active and other cell-surface antigens using standard serological techniques12 or a commercially available gel test (DiaMed). Names and sources of the monoclonal and polyclonal Abs and plant lectins used are provided in supplemental Table 1.
Preparation of cells for scanning electron microscopy
Approximately 5 to 10 × 105 cells before or after filtration were seeded onto poly-L-lysine (Sigma-Aldrich)–coated 13-mm cover slips. Cells were fixed with 1% (wt/wt) glutaraldehyde (Sigma-Aldrich) in 0.1M phosphate buffer (pH 7.4) for 1 hour and washed 3 times for 5 minutes with 0.1M phosphate buffer (pH 7.4). PBS was replaced with 1% (vol/vol) osmium tetroxide (Fluka) in 0.1M phosphate buffer (pH 7.4) for 1 hour. The cells were washed 3 times for 5 minutes in 0.1M PBS followed by one wash in dist. H2O. The cells were dehydrated using a graded ethanol series consisting of 15%, 25%, 50%, 70%, and 2 times 100% (vol/vol) EtOH for 10 minutes each. The coverslips were dried with hexamethyldisilazane (Sigma-Aldrich) for 3 minutes before mounting onto 0.5-inch aluminium specimen stubs (G301; Agar Scientific) using carbon double-sided sticky tables (Agar Scientific). The specimens were sputter coated using an EMITECH K575X Sputter Coater (Quorum Technologies) and imaged using a Quanta 400 scanning electron microscope (Fei) with xT microscope server software.
High-pressure freezing and TEM
Filtered reticulocytes were fixed by high-pressure freezing using a Leica EMPACT2 Plus RTS high-pressure freezer. Freeze substitution was performed in a Leica AFS2 apparatus using 1.1% osmium/0.1% uranyl acetate (5 hours at −90°C, followed by an increase of 5°C/h until reaching 0°C). Samples were dehydrated with acetone and infiltrated with increasing amounts of resin (25%, 50%, and 75%) Epon-acetone for 1 hour each, followed by 2 hours in 100% Epon resin before embedding in fresh Epon and being left to harden for 48 hours at 60°C. For transmission electron microscopy (TEM), thin sections (70 nm) were obtained using a diamond knife, counterstained, and then observed using a Tecnai 12-FEI 120kV BioTwin Spirit transmission electron microscope. Images of randomly selected cells were obtained using an FEI Eagle 4k × 4k CCD camera. For analysis, cells and organelles were outlined and measured (surface area and perimeter) using MetaMorph Version 6.3r7 software. The numbers of endocytic pits and exocytic events were rationalized to 100 μm of plasma membrane and the volume fractions of autophagosomes and amphisomes (area of organelles/area of cell) were expressed as a percentage.
Hemox analysis
The oxygen-carrying potential of the filtered reticulocytes, cord blood, and adult blood was determined using a hemox analyser (TCS Scientific) according to the manufacturer's instructions at 37°C. Twenty microliters of pelleted filtered reticulocytes, human cord blood, or adult human peripheral blood drawn by lancet were diluted into 5 mL of HEMOX solution (TCS) containing 20 μL of additive A (BSA-20; TCS) and 10 μL of antifoaming agent (AFA-25; TCS). The samples were oxygenated with air supplied from an external tank and deoxygenated with nitrogen gas. Changes in oxygen tension were measured using a Clark oxygen electrode. Increases in the oxyhemoglobin fraction were simultaneously monitored by dual-wavelength spectrophotometry at 560 and 576 nm. The data were analyzed using Hemox 2.00.13 Analytical Software (TCS).
Deformability and membrane stability
Reticulocyte elongation index, the measure of deformability, was measured over a range of shear stresses (0.3-30 Pa) by ektacytometry using a laser-assisted optical rotational cell analyzer (LORCA; Mechatronics) as described previously.13 The elongation index was defined as the ratio of the difference between the axes of the ellipsoid diffraction pattern and the sum of these 2 axes. Membrane stability was measured by ektacytometry using a cell analyzer, in which cells were deformed by a continuous high shear stress of 57.5 Pa for 1 hour.
Confocal microscopy
Cells were seeded on 0.01% (wt/vol) poly-L-lysine (Sigma-Aldrich)–coated coverslips and incubated for 30 minutes at 37°C in 5% CO2. Washes and dilutions were performed in buffer A (PBS containing 1 mg/mL of BSA; Park Scientific) and 2 mg/mL of glucose (Sigma-Aldrich). Cells were fixed in 1% (wt/vol) paraformaldehyde (TAAB; Aldermaston) and permeabilized in 0.05% (wt/vol) saponin (Sigma-Aldrich). For peripheral blood samples, 0.0075% glutaraldehyde (Sigma-Aldrich) was added to the fixation buffer. After permeabilization, all subsequent washes and Ab dilutions were carried out in buffer A plus 0.005% (wt/vol) saponin. A list of the primary Abs used is provided in supplemental Table 1. Secondary Abs used were goat anti–mouse Alexa Fluor 488– or goat anti–rabbit Alexa Fluor 546–conjugated Abs (Invitrogen) diluted in 4% (wt/vol) normal goat serum. Alexa Fluor 546 phalloidin was used to directly label F-actin and MitoTracker Deep Red FM was used as a mitochondrial-selective probe (both Invitrogen). Coverslips were mounted on Vectashield Mounting Medium (Vector Laboratories). Samples were imaged at 22°C using 40× oil immersion lenses (magnification 101.97 μm at zoom 3.8, 1.25 NA) on a Leica DMI 6000 inverted microscope with phase contrast connected to a Leica TCS SP5 confocal imaging system. Images were obtained using Leica LAS AF software and subsequently processed using Adobe Photoshop (Adobe). For live-cell imaging, reticulocytes were incubated with directly conjugated glycophorin A (GPA) Abs (BRIC256-FITC; IBGRL) at 4°C and then transferred to an IbiTreat μ-slide 8-well microscopy chamber (Ibidi). The slides were imaged on the Leica SP5 confocal system using the environmental chamber for temperature control at 37°C and CO2 enrichment.
For internalization assays, cells were seeded on poly-L-lysine–coated coverslips and incubated at 10°C for 10 minutes, followed by a further 20-minute 10°C incubation in the presence of anti-GPA Abs. Coverslips were transferred to 37°C for 0, 10, 20, 40, and 60 minutes before fixation. After 3 washes, cells were incubated with Fab fragment rabbit anti–mouse IgG (Jackson ImmunoResearch Laboratories) for 30 minutes. The cells were permeabilized with 0.05% (wt/vol) saponin and incubated with secondary Abs (see preceding paragraph). For LC-3 dual staining, cells were permeabilized directly after fixation and incubated with the anti–LC-3 Ab and subsequently with secondary Abs (see preceding paragraph). Control experiments were performed on fixed and permeabilized cells.
Analysis of vesicles released by filtered reticulocytes
Filtered reticulocytes (2.5 × 109) in final stage medium (5 × 107 cells/mL) were incubated at 37°C 5% CO2 for 72 hours. Reticulocytes were recovered by centrifugation (400g for 5 minutes) and plasma membranes prepared.14 Vesicles were obtained from the culture supernatant by centrifugation at 2000g for 10 minutes, followed by a further centrifugation at 100 000g for 45 minutes. The 100 000g hemoglobinated pellet was resuspended in PBS with 1mM PMSF. Reticulocyte membranes (5 μg of protein) and vesicles (7.5 μg of protein) were separated on a reduced 10% SDS-PAGE gel, transferred to nitrocellulose membrane, and probed for various erythrocyte proteins.
Results
Production of normal human reticulocytes
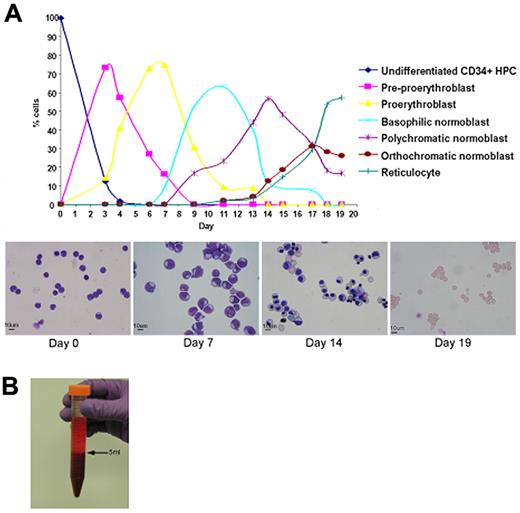
Adult peripheral blood CD34+ cells were cultured in IMDM containing SCF, IL3, and Epo according to protocol A or B (see supplemental Methods). The morphology of erythroid cells at each time point was identical using either protocol, but increased rates of proliferation and enucleation were obtained using protocol B (Figure 1A). Maximum proliferation occurred at the proerythroblast stage of differentiation. Overall expansion of cell numbers was ≥ 104-fold and enucleation ranged from 55%-95%. Leukocyte filtration was used to remove free nuclei, early R1 reticulocytes, and normoblasts. A 24-L culture yielded approximately 3 × 1010 cells after filtration (Figure 1B), which was composed of a mixture of mature R2 reticulocytes and erythrocytes (Figure 2).
Maturation, proliferation, and enucleation of CD34+ cells from adult peripheral blood. (A) Differential counts and cytomicrographs show the morphology of the cells at different stages in 2 representative cultures using protocol B. (B) Packed pellet of filtered reticulocytes.
Maturation, proliferation, and enucleation of CD34+ cells from adult peripheral blood. (A) Differential counts and cytomicrographs show the morphology of the cells at different stages in 2 representative cultures using protocol B. (B) Packed pellet of filtered reticulocytes.
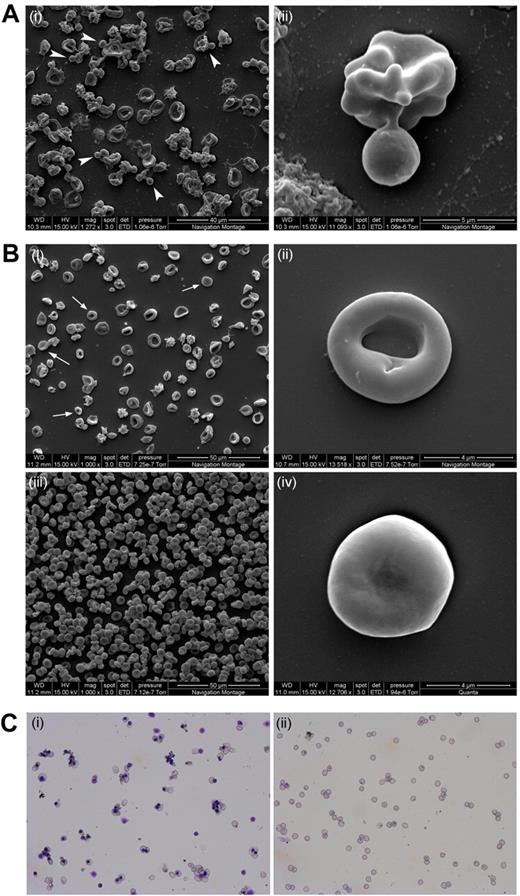
Scanning EM and cytospins of nascent reticulocytes before and after filtration. All cells had been cultured following protocol A. (A) Scanning electron microscopy image of day 18 reticulocytes pre-filtration. The arrowheads indicate free nuclei and/or enucleating cells (i). The panel on the right shows an enucleating reticulocyte (ii). (B) Scanning electron microscopy images of leukocyte filtered reticulocytes (i,ii,iv) and adult peripheral blood (iii). Arrows indicate some of the more mature reticulocytes. Panels ii and iv depict an R2 reticulocyte and an almost mature RBC, respectively. (C) Cytospin image of unfiltered (i) and leukocyte-filtered (ii) day 20 reticulocytes. Scale bar indicates 20 μm.
Scanning EM and cytospins of nascent reticulocytes before and after filtration. All cells had been cultured following protocol A. (A) Scanning electron microscopy image of day 18 reticulocytes pre-filtration. The arrowheads indicate free nuclei and/or enucleating cells (i). The panel on the right shows an enucleating reticulocyte (ii). (B) Scanning electron microscopy images of leukocyte filtered reticulocytes (i,ii,iv) and adult peripheral blood (iii). Arrows indicate some of the more mature reticulocytes. Panels ii and iv depict an R2 reticulocyte and an almost mature RBC, respectively. (C) Cytospin image of unfiltered (i) and leukocyte-filtered (ii) day 20 reticulocytes. Scale bar indicates 20 μm.
Characterization of reticulocytes
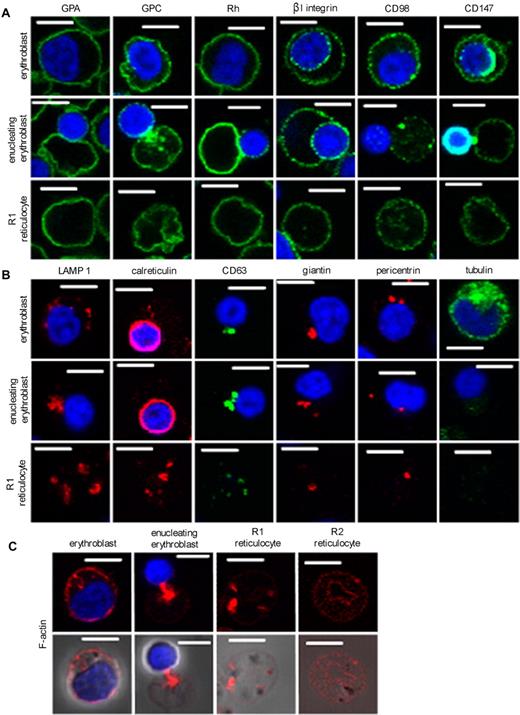
Cells at the final stages of maturation were examined by confocal microscopy using Abs specific for surface proteins known to be expressed on mature erythrocytes (GPA, GPC, and Rh polypeptides) and surface proteins lost or reduced in expression during reticulocyte maturation (β1, CD98, and CD147; Figure 3A). A substantial subpopulation of β1 and CD147 reactivity partitioned to the nucleus during enucleation.
Confocal analysis of erythroblasts, enucleating erythroblasts, and reticulocytes with Abs to organelle and plasma membrane marker proteins. All cells were cultured following protocol A. Cells were fixed in 1% (wt/vol) paraformaldehyde and permeabilized with 0.05% (wt/vol) saponin. (A) Cells were harvested on day 12 and stained for the presence of the plasma membrane markers glycophorin A, glycophorin C, Rh polypeptides, β1 integrin, CD98, and CD147. (B) Cells were stained for the presence of the organelle and cytosolic markers LAMP1 (lysosomes), calreticulin (ER), CD63 (endosomes), giantin (Golgi), pericentrin (centrioles), and tubulin. (C) Cells were stained for the presence of filamentous F-actin. Scale bars indicate 5 μm.
Confocal analysis of erythroblasts, enucleating erythroblasts, and reticulocytes with Abs to organelle and plasma membrane marker proteins. All cells were cultured following protocol A. Cells were fixed in 1% (wt/vol) paraformaldehyde and permeabilized with 0.05% (wt/vol) saponin. (A) Cells were harvested on day 12 and stained for the presence of the plasma membrane markers glycophorin A, glycophorin C, Rh polypeptides, β1 integrin, CD98, and CD147. (B) Cells were stained for the presence of the organelle and cytosolic markers LAMP1 (lysosomes), calreticulin (ER), CD63 (endosomes), giantin (Golgi), pericentrin (centrioles), and tubulin. (C) Cells were stained for the presence of filamentous F-actin. Scale bars indicate 5 μm.
Reticulocytes retained substantial lysosomal activity immediately after enucleation, as evidenced by staining for LAMP1. The endoplasmic reticulum (ER) marker calreticulin was prominent after enucleation despite a substantial proportion partitioning to the nucleus. The cultured reticulocytes also stained for the endosomal marker CD63, the Golgi marker giantin, and the centriole marker pericentrin (Figure 3B). Tubulin expression was reduced dramatically in reticulocytes compared with erythroblasts. Cytoplasmic levels of F-actin were high immediately after enucleation but largely absent in the cytoplasm of the more mature reticulocytes isolated from cultures by passage through leukocyte filters (Figure 3C).
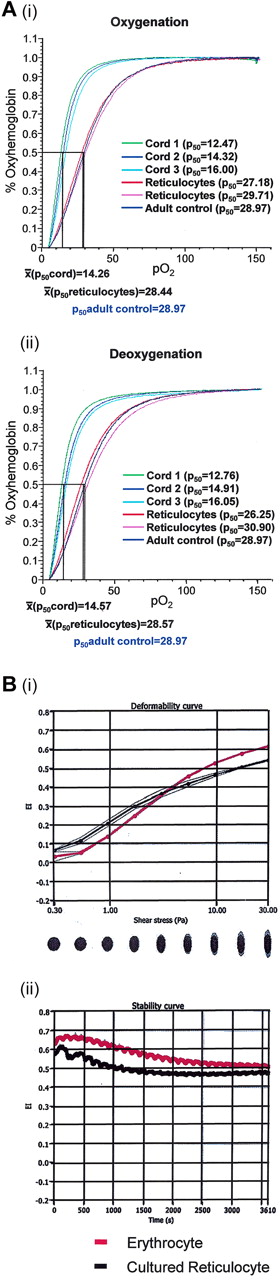
The total cell population passing through leukocyte filters had a normal adult erythrocyte N-glycosylation profile, as evidenced by normal expression of ABH and I antigens, and normal O-glycosylation, as evidenced by reactivity with the sialic acid–dependent anti-glycophorin R1.3 and by the absence of T and Tn antigen expression. Expression of P and P1 glycolipid antigens was also normal, but the cells lacked Le antigens. The latter was to be expected because these glycolipid antigens on circulating erythrocytes are acquired from plasma. Expression of blood group–active proteins was comparable with normal erythrocytes in all cases (supplemental Table 1). The filtered cells had weak expression of CD98 and CD36 but lacked CD71 and α4β1 when analyzed using serological methods. CD98 and CD36 were not detected on erythrocytes (supplemental Table 1). The cells expressed adult hemoglobin (supplemental Figure 1) and bound and released oxygen in a manner comparable with normal adult peripheral blood erythrocytes (Figure 4A). Postfiltration cells from a typical culture had a mean cell volume (n = 4) of 131 fL (normal range for erythrocytes, 80-100 fL) and a mean cell hemoglobin of 37.7 pg (normal range for erythrocytes, 26-34 pg). The membrane stability and deformability of the same cell preparation indicated that the cultured cells had reduced membrane stability and deformability compared with peripheral blood erythrocytes (Figure 4B).
Oxygen binding, deformability, membrane stability of nascent reticulocytes. (A) The oxygen association (left panel) and dissociation (right panel) curves are plotted for 20 μL of cord blood (mean p50 oxy = 14.26, deoxy = 14.57), cultured (following protocol B) and filtered reticulocytes (mean p50 oxy = 28.44, deoxy = 28.57), and adult peripheral blood (p50 adult oxy = 28.97, deoxy = 28.97). (B) Deformability curve of elongation index (EI) vs. shear stress for erythrocytes and cultured reticulocytes (i). Membrane stability curve of elongation index versus time for mature adult erythrocytes (red) and cultured reticulocytes (black) subjected to a continuous high shear stress of 57.5Pa (ii).
Oxygen binding, deformability, membrane stability of nascent reticulocytes. (A) The oxygen association (left panel) and dissociation (right panel) curves are plotted for 20 μL of cord blood (mean p50 oxy = 14.26, deoxy = 14.57), cultured (following protocol B) and filtered reticulocytes (mean p50 oxy = 28.44, deoxy = 28.57), and adult peripheral blood (p50 adult oxy = 28.97, deoxy = 28.97). (B) Deformability curve of elongation index (EI) vs. shear stress for erythrocytes and cultured reticulocytes (i). Membrane stability curve of elongation index versus time for mature adult erythrocytes (red) and cultured reticulocytes (black) subjected to a continuous high shear stress of 57.5Pa (ii).
Mature reticulocytes contain large, glycophorin A–positive vesicles that costain with markers of autophagosomes
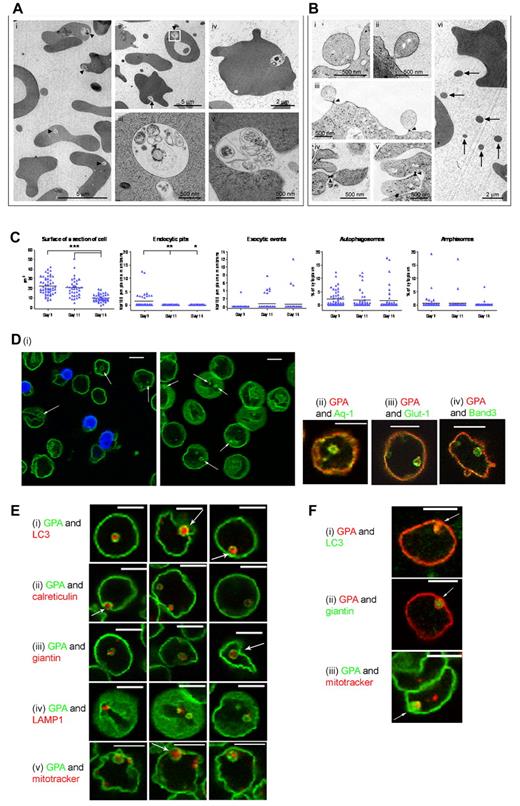
Mature reticulocytes/erythrocytes recovered after filtration were examined by TEM (Figure 5). The results revealed frequent cytoplasmic inclusions in mature reticulocytes, many containing membranous structures that were reminiscent of defunct organelles, which is suggestive of the involvement of the macroautophagy pathway for organelle sequestration. In some cases, these inclusions had been fixed in the act of fusion with the plasma membrane, thereby providing evidence for a route for exocytosis of the membrane bodies contained within (Figure 5A). Membrane fragments observed in reticulocyte preparations were of a size consistent with derivation from maturing reticulocytes after exocytosis and membrane blebbing (Figure 5B). Reticulocytes recovered by filtration showed a progressive reduction in size and a shift from endocytic to exocytic activity for 7 days after filtration (Figure 5C). When mature, permeabilized reticulocytes were examined by confocal microscopy and stained with anti-GPA, the presence of 1 or 2 large cytoplasmic vesicles (approximately 1.3 μm in diameter) was recorded per reticulocyte. These vesicles also contained other integral membrane proteins, including aquaporin-1 (AQP1), glucose transporter-1 (GLUT 1), and Band 3 (Figure 5Dii-iv). In general, the vesicles were comparable in size to the membrane-containing vesicles seen by TEM, and in a typical culture, 64.15% (± 9.37%) cells had GPA-positive vesicles (n = 313). GPA-staining vesicles were present in cultured reticulocytes before filtration and therefore were not an artifactual consequence of filtration (Figure 5Di).
TEM of cytoplasmic and membrane remodeling in erythroblasts and reticulocytes and detection of GPA-positive vesicles by confocal microscopy. (A) Representative TEM images of day 23 filtered reticulocytes containing large autophagic compartments (autophagosomes and amphisomes). (i-ii) Fields of R2 reticulocytes, some retaining large autophagic vacuoles (arrowheads) containing poorly degraded organelles; identical compartments were also observed undergoing exocytosis (arrow). (iii) Higher magnification image of the autophagic vacuole outlined in panel ii. (iv-v) Examples of exocytic events in reticulocytes. (B) Evidence for plasma membrane blebbing during human in vitro erythropoiesis. (i-ii) Plasma membrane blebs on the surface of erythroblasts. (iii-v) Plasma membrane blebs showing electron-dense constrictions at their bases (arrowheads). (vi) Example field of a reticulocyte culture containing cellular fragments (arrows) of comparable size to the plasma membrane blebs observed in erythroblasts. (C) Analysis of reticulocytes isolated after 7 days in final stage medium and analyzed at days 7, 11, and 14 (numbers of cells analyzed: 54 at day 7, 37 at day 11, and 43 at day 14). Black bars represent the mean. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (D) GPA-stained cells before and after filtration (left and right panels, respectively) show the presence of vesicles (arrows; i). (ii-iv) GPA (red) dual-stained aquaporin-1 (Aq-1), Glut-1, and band 3 (all green), respectively. (E) Filtered reticulocytes were dual stained with GPA (green) and Abs to organelle markers (red) for autophagy (LC3; i), ER (calreticulin; ii), Golgi (giantin; iii), lysosomes (LAMP1; iv), and mitochondria (MitoTracker; v). Arrows highlight vesicles fusing with the plasma membrane. All cells were cultured following protocol A. (F) Presence of GPA-positive vesicles in vivo. GPA-positive vesicles (red) containing organelle markers (green) for autophagy (LC3; i) and Golgi (giantin; ii) and GPA-positive vesicles (green) with mitochondrial probe MitoTracker (red) found in cells from peripheral blood (iii). Arrows highlight colocalization. Scale bars indicate 5 μm.
TEM of cytoplasmic and membrane remodeling in erythroblasts and reticulocytes and detection of GPA-positive vesicles by confocal microscopy. (A) Representative TEM images of day 23 filtered reticulocytes containing large autophagic compartments (autophagosomes and amphisomes). (i-ii) Fields of R2 reticulocytes, some retaining large autophagic vacuoles (arrowheads) containing poorly degraded organelles; identical compartments were also observed undergoing exocytosis (arrow). (iii) Higher magnification image of the autophagic vacuole outlined in panel ii. (iv-v) Examples of exocytic events in reticulocytes. (B) Evidence for plasma membrane blebbing during human in vitro erythropoiesis. (i-ii) Plasma membrane blebs on the surface of erythroblasts. (iii-v) Plasma membrane blebs showing electron-dense constrictions at their bases (arrowheads). (vi) Example field of a reticulocyte culture containing cellular fragments (arrows) of comparable size to the plasma membrane blebs observed in erythroblasts. (C) Analysis of reticulocytes isolated after 7 days in final stage medium and analyzed at days 7, 11, and 14 (numbers of cells analyzed: 54 at day 7, 37 at day 11, and 43 at day 14). Black bars represent the mean. *P ≤ .05; **P ≤ .01; ***P ≤ .001. (D) GPA-stained cells before and after filtration (left and right panels, respectively) show the presence of vesicles (arrows; i). (ii-iv) GPA (red) dual-stained aquaporin-1 (Aq-1), Glut-1, and band 3 (all green), respectively. (E) Filtered reticulocytes were dual stained with GPA (green) and Abs to organelle markers (red) for autophagy (LC3; i), ER (calreticulin; ii), Golgi (giantin; iii), lysosomes (LAMP1; iv), and mitochondria (MitoTracker; v). Arrows highlight vesicles fusing with the plasma membrane. All cells were cultured following protocol A. (F) Presence of GPA-positive vesicles in vivo. GPA-positive vesicles (red) containing organelle markers (green) for autophagy (LC3; i) and Golgi (giantin; ii) and GPA-positive vesicles (green) with mitochondrial probe MitoTracker (red) found in cells from peripheral blood (iii). Arrows highlight colocalization. Scale bars indicate 5 μm.
To confirm whether GPA-positive internal vesicles corresponded to the inclusions observed by TEM, we stained cultured reticulocytes with Abs recognizing the autophagosomal marker LC3. Dual staining with anti-GPA and anti-LC3 confirmed that these structures were likely to be the same: the GPA-labeled vesicles always costained with anti-LC3, thereby linking the autophagy pathway with the generation of GPA-stained internal vesicles (Figure 5Ei). At the resolution afforded by the confocal microscope, GPA staining was always peripheral to LC3 staining on these structures (Figure 5Ei). This result indicates that the LC3-decorated structures are likely to be within the lumen of the vesicle and that LC3 was not present on the limiting membrane. The late stages of autophagosome maturation were characterized by the fusion of autophagosomes with the endosomal/lysosomal compartments, leading to the formation of amphisomes and autolysosomes, respectively. Fusion of autophagosomes with the degradative compartment in yeast requires the regulated removal of LC3 from the outer membrane by the actions of the endopeptidase Atg4.15 The lack of clear LC3 labeling on the vesicular limiting membrane in reticulocytes indicates that autophagosome remnants are likely to have been delivered into the lumen of an expanding amphisome, which subsequently acquires GPA by intersecting with GPA-labeled endosomes. The GPA-positive vacuoles could also be stained with Abs to the ER marker calreticulin, the Golgi marker giantin, and a mitochondrial marker (Figure 5Eii-iii,v). As with LC3, each of these markers labeled structures within GPA-positive vesicles. It is therefore tempting to speculate that mitochondria, ER, and Golgi membranes had been delivered to the expanding vesicle through the process of macroautophagy. In contrast to the ER and Golgi markers, Abs against the lysosomal marker Lamp1 decorated structures that were often adjacent to (but not within) the reticulocyte vesicles (Figure 5Eiv), suggesting that fusion with lysosomal remnants within the reticulocyte cytoplasm is a feature of vesicular maturation. Intriguingly, GPA-positive vesicles sometimes appeared to be in the process of fusing with the plasma membrane (Figure 5Di arrows), which is consistent with the observations made at the ultrastructural level (Figure 5A). Reticulocytes with GPA-containing cytosolic vesicles and internal contents stained with anti-LC3, anti-giantin, and MitoTracker were also present in the peripheral blood of 2 splenectomized patients (Figure 5F).
The GPA surrounding autophagosomes comes from the reticulocyte plasma membrane
To ascertain the origin of the GPA associated with autophagosomes, aliquots of unfiltered reticulocytes maintained at 10°C were incubated with anti-GPA, transferred to 37°C, and the appearance of cytosolic GPA-staining vesicles was monitored over time. The results revealed formation of GPA vesicles at the cytosolic surface of the plasma membrane during the first 20 minutes. Thereafter, the cytosolic vesicles became noticeably larger (Figure 6A). In most cells, only a single GPA-staining vesicle was observed. Control experiments performed on pre-fixed cells showed no evidence of Ab internalization (data not shown). When the reticulocyte preparations were incubated and stained with both anti-GPA and anti-LC3, all GPA-positive vesicles observed after 60 minutes of incubation at 37°C were also LC3-positive. After 20 minutes of incubation, some LC3-negative GPA-positive vesicles were present (Figure 6B). These results are consistent with a process in which GPA-positive plasma membrane–derived cytosolic vesicles are formed and subsequently fuse with the outer membrane of autophagosomes before fusion with the plasma membrane and release of contents by exocytosis.
Endocytosis and exocytosis of GPA-positive vesicles in maturing reticulocytes. (A) Unfiltered reticulocytes cultured using protocol B were labeled with BRIC256 at 10°C, followed by incubation at 37°C for 0, 10, 20, 40, and 60 minutes. Incubation with rabbit anti–mouse Fab before permeabilization ensured that any external GPA stained red (Alexa Fluor 546) and internal GPA stained green (Alexa Fluor 488). (B) After a BRIC256 (green) internalization assay for 20 and 60 minutes, reticulocytes were dual stained with anti-LC-3 (red). Scale bars indicate 5 μm. (C) Scanning electron microscopy of filtered reticulocytes showing membrane blebs. Scale bars indicate 5 μm. (D) Live-cell imaging of a reticulocyte showing membrane blebbing (left: phase contrast; right: labeled with anti–GPA-FITC). Scale bars indicate 5 μm. (E) Immunoblot of vesicles purified from filtered reticulocytes (i, iii, v, vii, ix, and xi) and from membranes made from these reticulocytes (ii,iv,vi,viii,x,xii) using rabbit anti-glycophorin A (i-ii), mouse monoclonal anti–band 3 (BRIC170; iii-iv), rabbit anti-GLUT1 (v-vi), mouse monoclonal anti–β actin (clone AC-15; vii-viii), rabbit anti-ankyrin (with cross-reactivity for both α and β spectrin; ix-x), and mouse monoclonal anti α spectrin (BRIC174; xi-xii).
Endocytosis and exocytosis of GPA-positive vesicles in maturing reticulocytes. (A) Unfiltered reticulocytes cultured using protocol B were labeled with BRIC256 at 10°C, followed by incubation at 37°C for 0, 10, 20, 40, and 60 minutes. Incubation with rabbit anti–mouse Fab before permeabilization ensured that any external GPA stained red (Alexa Fluor 546) and internal GPA stained green (Alexa Fluor 488). (B) After a BRIC256 (green) internalization assay for 20 and 60 minutes, reticulocytes were dual stained with anti-LC-3 (red). Scale bars indicate 5 μm. (C) Scanning electron microscopy of filtered reticulocytes showing membrane blebs. Scale bars indicate 5 μm. (D) Live-cell imaging of a reticulocyte showing membrane blebbing (left: phase contrast; right: labeled with anti–GPA-FITC). Scale bars indicate 5 μm. (E) Immunoblot of vesicles purified from filtered reticulocytes (i, iii, v, vii, ix, and xi) and from membranes made from these reticulocytes (ii,iv,vi,viii,x,xii) using rabbit anti-glycophorin A (i-ii), mouse monoclonal anti–band 3 (BRIC170; iii-iv), rabbit anti-GLUT1 (v-vi), mouse monoclonal anti–β actin (clone AC-15; vii-viii), rabbit anti-ankyrin (with cross-reactivity for both α and β spectrin; ix-x), and mouse monoclonal anti α spectrin (BRIC174; xi-xii).
Plasma membrane loss during reticulocyte maturation occurs by blebbing
Examination of mature reticulocytes using the scanning electron microscope revealed some cells with membrane blebs (Figure 6C). Membrane blebs were also seen in the peripheral blood of 2 splenectomized patients (supplemental Figure 2). Evidence that these blebs contain GPA was obtained using live-cell imaging with FITC/anti-GPA (Figure 6D). When vesicles derived from cultured reticulocytes were isolated from the culture supernatant after 72 hours of incubation and examined by immunoblotting, the released vesicles contained GPA and other integral membrane proteins, including band 3 and GLUT 1, but lacked the skeletal proteins spectrin, ankyrin, and actin (Figure 6E).
Discussion
We have developed a liquid culture system for the production of erythrocytes from adult peripheral blood CD34+ cells to characterize the process by which committed adult erythroid progenitors mature to erythrocytes. Our results show significant amounts of β1 integrin and CD147 partitioning to the nucleus at enucleation and reveal that β1 integrin and CD98 expression is grossly reduced during the maturation of reticulocytes from R1 to R2. The loss of integrin β1 from the plasma membrane during reticulocyte maturation (via the endosome-exosome pathway) is well known.16 CD98hc mediates β1 integrin binding, acting as a chaperone facilitating the transport and assembly of amino acid transporters in the plasma membrane.17 CD147 also interacts with β1 integrin18 and acts as a chaperone enabling membrane insertion of monocarboxylate transporters.19 Xu and Hemler showed that CD98hc interacts with CD147 and proposed that together they play a central organizing role, coordinating transport of lactate and amino acids.20 Our present data show that, as the human reticulocyte matures, there is a concomitant reduction in expression of all of these proteins that is consistent with a reduced requirement for cell adhesion mediated through the integrin complex. Arginine import through the CAT1 and CD98hc transport systems is required for proliferation and differentiation of erythroid cells.21,22 In contrast, some CD147 is retained in the mature erythrocyte, possibly to facilitate lactate transport through MCT1. We observe a gross reduction in expression of tubulin and loss of cytosolic actin as the human reticulocyte matured, as described previously in murine reticulocytes.7 These observations are consistent with a loss of adhesive and motile functions, arginine import consequent to enucleation, and the gradual remodeling of the reticulocyte for its specialized function in respiration.
In the present study, we focused on the final stage of maturation in which R2 reticulocytes undergo membrane remodeling to become erythrocytes. Our results demonstrate the feasibility of generating a mixture of mature reticulocytes and erythrocytes from CD34+ cells in liquid culture without the use of feeder layers or other cellular additions. The morphological features of the cells produced are entirely consistent with previous studies of R2 reticulocytes isolated from the peripheral blood of healthy donors.1,2
R2 reticulocytes contain very few internal organelles when examined in the transmission electron microscope, but are characterized by the presence of large autophagic vacuoles. In some cases, the vacuoles fuse with the membrane, creating an aperture that facilitates exocytosis (Figure 5). The presence of autophagic vacuoles in human reticulocytes was described by Kent et al, who noted that the number of vacuoles increased in splenectomized individuals.23 Holroyde and Gardener found 54.3% vacuolated cells in the circulation of 20 splenectomized patients compared with 2.1% in a control group of healthy adults.24 They also showed clearance of 51Cr-labeled vacuolated cells from a splenectomized patient in 48-72 hours in a healthy recipient. Holm et al reported that hypercholesterolemic mice lacking the high-density lipoprotein receptor SR-BI have erythrocytes with large autophagolysosomes.25 They also showed that these autophagolysosomes were expelled when the erythrocytes were transfused into wild-type animals and proposed that the release of autophagolysosomes is a normal part of erythrocyte maturation. These studies provide persuasive evidence that mature reticulocytes released from the BM circulate with autophagic vacuoles, and that the final maturation step involving the removal of these vacuoles occurs in the spleen during the first 24-72 hours after release from the BM. However, it is clear that maturation proceeds in the absence of splenic involvement, because mature erythrocytes are observed in the reticulocyte fraction after filtration (Figure 2). A progressive reduction in cell size over time occurs concomitant with a shift from endocytic to exocytic activity (Figure 5C).
Filtered cells comprise mature reticulocytes and erythrocytes. These cells contain adult hemoglobin (supplemental Figure 1), have a normal capacity to bind and release oxygen (Figure 4A), a normal glycosylation profile as evidenced by a normal ABO and I groups, and lack of exposed cryptantigens T and Tn, and a normal expression of cell surface proteins as evidenced by serological analysis with numerous human blood group antisera and mAbs (supplemental Table 1). Unlike erythrocytes, they have weak expression of CD98 and CD36. CD36 is an adhesion molecule the expression of which is known to decrease as erythroid cells mature.26 The mean cell volume is higher than that of peripheral blood erythrocytes, and membrane stability and deformability is less than that of peripheral blood erythrocytes (Figure 4B), as would be expected for a population of cells predominantly composed of reticulocytes. It is well known that reticulocytes are mechanically less stable than erythrocytes.2,27 Waugh et al found that the energy required to dissociate the lipid bilayer from the underlying skeleton increased 4-fold between BM reticulocytes and mature erythrocytes.27
Reticulocytes but not erythrocytes undergo endocytosis. Zweig and Singer examined endocytosis in rabbit reticulocytes using ferritin-labeled concanavalin A and found the number of internalized vesicles per cell section declined from 3 for young reticulocytes to 0.94 for old reticulocytes.28 Old reticulocytes not only had fewer vesicles, but those that were present were larger than those found in young reticulocytes. In the present study, our examination of cultured, mature, human R2 reticulocytes by confocal microscopy using anti-GPA revealed the presence of large cytosolic vesicles comparable in size (> 120 nm) to those reported previously.28 GPA has not been associated previously with cytosolic vesicles. The most likely explanation for this finding is that a subpopulation of plasma membrane GPA is incorporated into vesicles during the process of membrane remodeling from mature reticulocytes to erythrocytes, a conclusion supported by our demonstration of the concomitant presence of Band 3, GLUT 1, and AQP 1. In this context, it is interesting that the amount of GPA in murine reticulocyte plasma membranes was greater than that in the corresponding erythrocyte membranes.10 Mature reticulocytes released from BM undergo final remodeling after release, resulting in a 10%-14% membrane loss in the first 48 hours.29 Consistent with this, the circumference of cultured reticulocytes lacking staining for either LAMP1 or CD63 (30.92 ± 2.31 μm, n = 8) was 10% less than those staining positive for either marker (33.96 ± 2.23 μm, n = 9).
To determine the source of GPA in GPA-positive cytosolic vesicles, R2 reticulocytes were incubated at 37°C in the presence of anti-GPA, and the appearance of GPA-positive vesicles observed over time (Figure 6A). The results clearly showed that GPA derives from the plasma membrane. To further define the nature of the GPA-staining vesicles, we costained with MitoTracker and Abs to giantin and LAMP1. Staining for these proteins indicated that the vesicles contained remnants of mitochondria, Golgi, and lysosomes, respectively, suggesting that they are autophagolysosomes. This was confirmed by demonstration of staining with the autophagosome marker protein LC3. It was also apparent that GPA-positive vesicles fuse with autophagosomes over time. Similar GPA-containing vesicles were found in vivo. These data provide direct evidence that the plasma membrane is internalized and fuses with autophagosomes in human R2 reticulocytes. A study by Ravikumar et al showed that an analogous situation occurs in HeLa cells, in which plasma membrane endocytic vesicles are associated with autophagosome precursors.30 The investigators speculated that the plasma membrane serves as a membrane reservoir that is used during periods of intense autophagosome synthesis. This may also explain how final maturation of the enucleated reticulocyte is achieved. Reticulocytes contain substantial amounts of the ER chaperone calreticulin31 within GPA-positive vesicles (Figure 5Eii), raising the possibility it plays a role in autophagy. In this context, it is interesting that calreticulin binds the autophagy protein GABARAP with high affinity.32,33
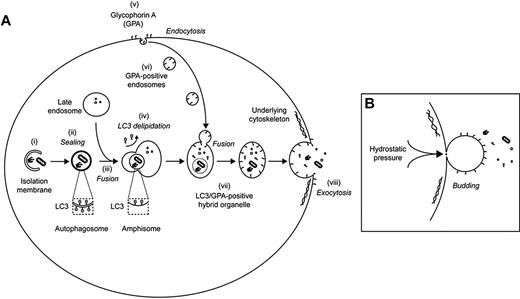
The results of the present study suggest a previously unrecognized mechanism for final maturation of reticulocytes in which autophagosomes are packaged within large, GPA-positive endocytic vesicles that fuse with the plasma membrane, releasing their contents by exocytosis. Loss of the GPA-containing vesicular membrane may then occur by blebbing (Figure 7). The occurrence of membrane blebs in cultured reticulocytes and in vivo (Figure 6C and supplemental Figure 2), and the presence of GPA in blebs observed by live-cell imaging (Figure 6D) are evidence of such a process. Gasko and Danon observed membrane blebbing from rabbit reticulocytes in semisolid agar.34 Extracellular vesicles present in reticulocyte preparations (Figure 5B), such as cytosolic vesicles, contain GPA, band 3, and GLUT1 (Figure 6E); however, they lack the cytoskeletal proteins actin, ankyrin, and spectrin, suggesting that they may derive from regions of the plasma membrane that are unlinked to the cytoskeleton and that they correspond to the blebs observed by microscopy. It is tempting to speculate that exocytosis and membrane blebbing are two aspects of a single process. Nevertheless, we cannot rule out the possibility that membrane blebbing and exocytosis occur independently at different sites on the maturing reticulocyte. Further work will be required to clarify this.
Model for the involvement of GPA-labeled endosomes in the exocytosis of autophagocytosed cytoplasmic content during reticulocyte maturation. (A) Autophagosomes derive from isolation membranes (i) that expand to engulf organelles and other cytoplasmic content before sealing (ii). Fusion of autophagosomes with late endosomes generates amphisomes (iii). As a prelude to fusion, autophagosomes lose LC3 from their outer membrane (a process known as delipidation), meaning that LC3 is found only on the inside of the resultant amphisome. During reticulocyte maturation, active endocytosis of GPA is observed (v), and these endosomes (vi) converge with LC3-positive amphisomes to generate an LC3/GPA–positive hybrid organelle (vii) that has the capacity to fuse with the plasma membrane (viii). Exocytosis may occur at sites of weakened underlying skeleton and is predicted to release cytoplasmic content concomitant with delivering GPA back on to the plasma membrane. (B) The above model predicts that plasma membrane surface area would increase as a result of internal vesicle maturation and exocytosis. We postulate, therefore, that the exocytic event is coupled with a process of plasma membrane blebbing, facilitated in vivo by passage through the spleen. By coupling these events, integral membrane proteins of the exocytic vesicle (including GPA) would be incorporated stochastically into the nascent bud, thereby effecting the shedding of redundant material that had previously been enriched in endosomes.
Model for the involvement of GPA-labeled endosomes in the exocytosis of autophagocytosed cytoplasmic content during reticulocyte maturation. (A) Autophagosomes derive from isolation membranes (i) that expand to engulf organelles and other cytoplasmic content before sealing (ii). Fusion of autophagosomes with late endosomes generates amphisomes (iii). As a prelude to fusion, autophagosomes lose LC3 from their outer membrane (a process known as delipidation), meaning that LC3 is found only on the inside of the resultant amphisome. During reticulocyte maturation, active endocytosis of GPA is observed (v), and these endosomes (vi) converge with LC3-positive amphisomes to generate an LC3/GPA–positive hybrid organelle (vii) that has the capacity to fuse with the plasma membrane (viii). Exocytosis may occur at sites of weakened underlying skeleton and is predicted to release cytoplasmic content concomitant with delivering GPA back on to the plasma membrane. (B) The above model predicts that plasma membrane surface area would increase as a result of internal vesicle maturation and exocytosis. We postulate, therefore, that the exocytic event is coupled with a process of plasma membrane blebbing, facilitated in vivo by passage through the spleen. By coupling these events, integral membrane proteins of the exocytic vesicle (including GPA) would be incorporated stochastically into the nascent bud, thereby effecting the shedding of redundant material that had previously been enriched in endosomes.
In a typical culture, approximately 60% of cells recovered after filtration contain cytosolic GPA-positive vesicles, indicating that membrane remodeling is incomplete in these cells. Final remodeling most likely occurs during passage through the spleen in vivo. Therefore, cells containing GPA-positive vesicles would be expected to circulate as normal peripheral blood reticulocytes when transfused, with final maturation occurring in the spleen of the recipient. Splenectomized patient control samples also demonstrated the presence of such vesicles, which is consistent with this hypothesis.
Our present results suggest that reticulocyte maturation occurs in two stages. An early phase occurs primarily in the BM, when plasma membrane components not required in the erythrocyte (CD71, CD98, and α4β1) are rapidly eliminated through the endosomal-exosome pathway. In a later phase, after release from the BM, large plasma membrane vesicles are internalized, fuse with autophagosomes, and the contents are mechanically expelled, most likely during passage through the spleen. The same process may also result in the loss of plasma membrane by blebbing, thereby providing the critical adjustment allowing optimal interaction between the lipid bilayer and skeleton to give the erythrocyte stability and deformability characteristics that sustain it through multiple passages through the peripheral circulation. The reticulocyte solves the problem of final maturation to an erythrocyte in the absence of a nucleus by combining endocytosis and autophagy in one continuous process.
Previous studies of human reticulocyte maturation have been hampered by the difficulty of obtaining reticulocyte preparations in sufficient quantities for detailed analysis. The availability of a well-characterized in vitro culture system overcomes these difficulties. The results of the present study demonstrate the potential for producing functional mature adult reticulocytes and erythrocytes from hematopoietic stem cells using a liquid-culture system free of feeder layers, demonstrating the feasibility of manufacturing products from stem cells that are suitable for transfusion therapy ex vivo.
Note added in proof: After this paper was submitted for publication, Giarratana et al reported that cultured human red cells are viable in vivo.35
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lee Bruce (Bristol Institute for Transfusion Sciences) for assistance with the Hemox analysis and Mike Wiltshire (Components Development Laboratory, National Health Service Blood and Transplant) for assistance with the LORCA analysis.
This work was funded by grants from The Wellcome Trust, National Health Service Blood and Transplant, and the Department of Health (United Kingdom).
Wellcome Trust
Authorship
Contribution: R.E.G., S.K., N.C., T.J.M., and V.B. designed and performed research, analyzed data, and wrote the manuscript; K.T. performed research; E.J.M. provided patient samples and clinical information; J.D.L. and S.F.P. designed research and wrote the manuscript; and D.J.A. designed research and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof David Anstee, BITS, NHSBT, North Bristol Park, Filton, Bristol BS34 7QH, United Kingdom; e-mail: david.anstee@nhsbt.nhs.uk; or Dr Jon Lane, Cell Biology Laboratories, Department of Biochemistry, University of Bristol, School of Medical and Veterinary Sciences, University Walk, Bristol BS8 1TD, United Kingdom; e-mail: jon.lane@bristol.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal