Abstract
Natural killer (NK) cells are potent anti-viral and antitumor “first responders” endowed with natural cytotoxicity and cytokine production capabilities. To date, attempts to translate these promising biologic functions through the adoptive transfer of NK cells for the treatment of cancer have been of limited benefit. Here we trace the fate of adoptively transferred murine NK cells and make the surprising observation that NK cells traffic to tumor sites yet fail to control tumor growth or improve survival. This dysfunction is related to a rapid down-regulation of activating receptor expression and loss of important effector functions. Loss of interferon (IFN)γ production occurs early after transfer, whereas loss of cytotoxicity progresses with homeostatic proliferation and tumor exposure. The dysfunctional phenotype is accompanied by down-regulation of the transcription factors Eomesodermin and T-bet, and can be partially reversed by the forced overexpression of Eomesodermin. These results provide the first demonstration of NK-cell exhaustion and suggest that the NK-cell first-response capability is intrinsically limited. Further, novel approaches may be required to circumvent the described dysfunctional phenotype.
Introduction
Natural cytotoxicity and rapid cytokine production make natural killer (NK) cells an attractive cell population to study for the treatment of patients with malignancies. Several groups have attempted to harness this biologic activity through the adoptive transfer of mature allogeneic, autologous, or syngeneic (in the mouse) NK cells with or without hematopoietic cell transplantation (HCT). Clinical results have demonstrated the feasibility and safety of infusing up to 1 × 108 NK cells/kg/dose into patients.1 Although some responses were noted in patients with high-risk acute myeloid leukemia (AML), all published trials have been single-arm studies where NK-cell infusion is accompanied by chemotherapy, irradiation, or a nonmyeloablative HCT, thus precluding definitive assessment of the role of NK cells in the reported responses.2-4 Furthermore, long-lasting responses are rare. Where functional assessment of reisolated NK cells was reported, these assays were usually performed after several days of in vitro activation and hence the reported cytotoxicity results may not reflect the actual functional capacity of NK cells circulating in the host or infiltrating the tumor.3 Notably, older literature in which patients were randomized to lymphokine-activated killer (LAK) cells or IL-2 alone did not show additional benefit of the LAK cells.5
Although prolongation of survival after adoptive NK therapy has been shown to occur in several mouse models, long-term disease-free survival is rare despite experimental conditions including the administration of higher doses of NK cells than are clinically feasible, colocalized injection of tumor with NK cells, depletion of regulatory T cells with additional immunomodulatory therapy, or genetic modification of the NK cells.6-9
To delineate the barriers to successful NK immunotherapy, we traced the fate of freshly isolated adoptively transferred NK cells using several murine tumor models. We found that NK cells rapidly home to and accumulate within tumor sites, yet fail to reject the tumor because of a rapid down-regulation of activating receptors and deactivation of effector functions, such as cytotoxicity and cytokine production. This dysfunction depended on NK-cell proliferation induced during homeostatic expansion after adoptive transfer as well as during tumor exposure. This phenomenon is reminiscent of CD8+ T cell exhaustion upon chronic antigen exposure, is accompanied by down-modulation of the canonical transcription factors Eomesodermin (Eomes) and T-bet and is partially reversed by overexpression of Eomes. Collectively, these preclinical results urge caution in the application of NK cells as an adoptive cellular therapy platform and suggest that a deeper understanding is required of their fate and function after transfer into patients.
Methods
Mice
C57BL/6 (H-2b, CD45.2), Balb/c (H-2d), and FVB (H-2q) mice were purchased from Charles River Laboratories. C57BL/6 CD45.1 and B6(Cg)–Tyrc−2J/J (albino C57BL/6) animals were from The Jackson Laboratory, and CB.17 severe combined immunodeficiency (SCID) mice were from Harlan Laboratories. Luciferase-expressing (luc+) FVB/N L2G85 (H-2q) and C57BL/6 (H-2b, CD45.1) were previously described.10,11 Rag2−/−γc−/− mice on the Balb/c or C57BL/6 backgrounds were a gift of Dr I. Weissman (Stanford University). NKG2D deficient mice on the C57BL/6 background were a gift of Dr D. Raulet (University of California, Berkeley). Bone marrow (BM) cells from DTR-FOXP3-eGFP mice on the C57BL/6 background were a gift of Dr M. Smyth (Peter MacCallum Cancer Center, Melbourne, Australia). Recipients were aged 8 to 10 weeks and donors were aged 8 to 16 weeks. Experiments were performed in sex-matched donor-recipient pairs. All animal protocols were approved by the University Committee on Use and Care of Laboratory Animals at Stanford University.
Cell isolation
BM cells were harvested and processed as previously described.12 NK cells were isolated from splenocytes as previously described.12 In brief, splenocytes were harvested and processed in phosphate-buffered saline (PBS; Invitrogen) with 2% fetal calf serum (FCS; Invitrogen) in a wire mesh to obtain a single cell suspension. After red cell lysis, cells were positively selected using DX5 magnetic beads (Miltenyi Biotec) on a magnetic column. The enriched DX5+ population was then sorted for CD3−DX5+ or CD3−NK1.1+ using a Becton Dickinson Aria or Aria II cell sorter to > 98% purity. When NK cells were expanded in vitro, 1 × 106 purified NK cells were plated/mL in RPMI supplemented with 10% FCS, 2mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (all from Invitrogen), and 5 μg/mL 2-mercaptoethanol (Sigma-Aldrich) supplemented with 1500 units/mL of recombinant human IL-2 (Chiron). Human NK cells were isolated from buffy coat preparations, CD3 depleted using Miltenyi MACS beads, and expanded using rhIL-15 25 ng/mL (CellGenix) in LifeCell Flasks (Baxter Healthcare) for 17 days.
Tumor models
Parental A20 B-cell lymphoma (H-2d) was from ATCC. The A20 luc+yfp cell line was created as described.13 RMA and RMA-S cell lines were a gift of Dr J. Sunwoo (Stanford University). The Hoxa9-Meis1 primary murine AML was created as previously described14 according to a protocol provided by Dr G. Nolan (Stanford University; http://www.stanford.edu/group/nolan/protocols/pro_helper_dep.html).
The following in vivo tumor models were used. Model 1: Balb/c mice were injected intravenously with 1 × 104 to 1 × 106 parental A20 or A20-luc followed 1 week later by lethal irradiation (800 rad in divided doses) and T-cell depleted BM (TCD)–BM with 0.5 to 1.0 × 106 NK cells (from Balb/c, C57BL/6, or FVB donors, as indicated). Model 2: Balb/c mice were lethally irradiated and injected with 1 × 104 A20 cells and 1 × 106 allogeneic NK cells along with TCD-BM. Model 3: recipient C57BL/6 mice were lethally irradiated (960 rad in divided doses) then received 0.5 × 106 C57BL/6 BM along with 0.5 to 1 × 106 sorted NK cells, at the same time as 1 × 103 to 1 × 106Hoxa9-Meis1 leukemia, as indicated. Model 4: recipient Balb/c mice were injected with 1 to 3 × 106 tumor cells subcutaneously into the right flank, followed 10 to 14 days later (at a tumor size of ∼ 1cm2) by lethal irradiation (800 rad in divided doses 4 hours apart) and injection of TCD-BM (0.5 × 106 for syngeneic transplants, 5 × 106 for allogeneic transplants). Some mice also received 0.5 to 1 × 106 sorted NK cells intravenously. All intravenous injections were via the tail vein, unless otherwise specified. Tumor size was measured using manual callipers. Unless otherwise stated, no exogenous IL-2 was administered. Model 5: recipient CB.17 SCID mice received 200 rad single dose on day −2, then injected with 1 × 106 Raji cells on day 0 and 1 to 2 × 107 human NK cells on day +2 with or without rituximab or isotype control 100 ug; mice then received a second dose of rituximab 100 ug on day +7; mice in this model were treated with IL-2 5 × 104 units every second day for 7 doses. Depletion of regulatory T cells (Tregs) was performed as previously described.15
Cytotoxicity assays
Target cell lines (1 × 106) were labeled with 300 μCi 51Cr (PerkinElmer) for 4 hours at 37°C at 5% CO2 and cytotoxicity was determined as previously described16 after incubation with NK cells for 4 to 18 hours, as indicated. Caspase 6 activation as a measure of target cell apoptosis was performed using the Cytoxilux Plus G2D2 kit according to the manufacturer's instructions (Oncoimmunin).
In vivo BLI
Bioluminescent imaging (BLI) was performed as previously described with an IVIS spectrum charge-coupled device imaging system (Xenogen)12 and images were analyzed with Living Image 3.0 software (Xenogen).
Flow cytometry reagents
Cells were stained using the following reagents after blockade of Fc receptors (clone indicated in parentheses): CD45.1 (A20), H-2Kb (AF6-88.5.5.3), IFNγ (XMG1.2), T-bet (4B10), Eomesodermin (Dan11mag), FOXP3 (FJK-16s), Rat immunoglobulin IgG1κ, Rat IgG2a, and FOXP3 fixation and permeabilization buffer, were from eBioscience. H-2Dd (34-5-8S), NKG2A/C/E (20d5), Ly49C/I (5E6), Ly49D (4E5), Ly49A (A1), Ly49G2 (4D11), and CD107a (1D4B) were obtained from BD Pharmingen. CD3 (145-2C11), CD49b (DX5), NK1.1 (PK136), NKp46 (29A1.4), KLRG1 (2F1), CD4 (GK1.5), CD25 (PC61), FR4 (TH6), NKG2D (CX5), CD27 (LG.3A10), CD11b (M1/70), were obtained from Biolegend. Live Dead Fixable Aqua was from Invitrogen. Events were collected on a 4-laser LSRII (Becton Dickinson) and analyzed using FlowJo Version 8.8.6 software (TreeStar).
Ex vivo stimulation and intracellular staining for cytokines
NK cells were harvested from animals or from culture, resuspended at up to 1 × 107/mL in 200 to 400 μL RPMI with 10% FCS, and stimulated with phorbol myristate acetate 40 ng/mL and ionomycin 2μM (both from Sigma-Aldrich) for 5 hours at 37°C. Brefeldin 1X (Biolegend) was added 1 hour into the incubation. Unstimulated controls were used for setting electronic flow cytometric gates.
CFSE labeling
NK cells magnetically enriched for CD49b were labeled with 2.5μM Vybrant carboxyfluorescein diacetate succinimidyl ester (CFSE) Tracer Kit (Molecular Probes) in PBS for 5 minutes at 37°C. The reaction was then quenched by the addition of cold RPMI with 10% FCS and cells were washed 3 times to remove excess CFSE. NK cells were then sorted to high purity.
NK-cell transduction
Donor mice were injected with low molecular weight (LMW) poly:IC 80 ug (Invivogen) on day −2 to stimulate NK-cell proliferation. On day 0, NK cells were harvested and sorted, followed by suspension of 3 × 106 cells in retroviral supernatant (Eomes; T-bet or empty MIG retrovirus) along with polybrene 8 ug/mL and spun for 90 minutes at room temperature according to a protocol modified from Kao et al.17 Eomes, T-bet, and control plasmids were kindly provided by Dr J. Wherry (University of Pennsylvania).
Statistical analysis
Animal survival was analyzed using the log-rank test. Differences in cytotoxicity, cytokine production, receptor expression, and median fluorescence intensity were analyzed using a 2-tailed Student t test or a 1-way ANOVA with the Dunnett multiple comparison test (analyses performed using GraphPad Prism Version 5 software).
Results
Adoptive NK-cell immunotherapy fails to control the growth of NK-sensitive tumor cells
To examine the ability of adoptively transferred NK cells to eradicate established malignancy, we selected several well-characterized tumor cell line models and 1 novel primary AML model. We first confirmed that the B-cell lymphoma line A20 and the T-cell lymphoma lines EG7 and RMA/S were sensitive to NK cell–mediated killing by standard chromium release assays (Figure 1A and data not shown) as previously reported. The Hoxa9-Meis1 primary AML model was previously described14 and was generated in our laboratory by retroviral transduction of mouse BM cells. Blasts were found to be sensitive to NK cytotoxicity (Figure 1B). Next we established clinically relevant in vivo tumor models by engrafting mice with disseminated lymphoma followed 7 days later by total body irradiation (TBI) and infusion of TCD-BM with or without highly purified NK cells. Tumor burden was initially reduced in both treatment groups after irradiation, but surprisingly NK cells failed to impact tumor burden (Figure 1C) or survival of tumor-bearing mice (Figure 1D). Coadministration of up to 100:1 NK:A20 cells or 1000:1 NK:Hoxa9-Meis1 AML cells failed to eradicate tumor or impact survival (Figure 1E-F; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Adoptive transfer of NK cells fails to control the growth of tumor targets shown to be NK-sensitive in vitro. (A) Specific lysis of chromium labeled A20 tumor cells by IL-2–stimulated C57BL/6 NK after a 4-hour incubation. Median ± error is shown. (B) Primary AML cells (C57BL/6, H-2b) did not uptake chromium and apoptosis was detected by flow cytometric caspase activation assay after a 2-hour incubation with IL-2 stimulated Balb/c NK cells. Inset shows an example of caspase activity flow cytometry plots. Median ± error is shown. (C) Tumor burden by BLI of Balb/c mice receiving 1 × 106 luciferase-expressing A20 tumor cells on day −7, followed on day 0 by TBI, BM rescue and a concurrent infusion of 0.5 × 106 C57BL/6 NK cells (bottom panel) or PBS (top panel). One representative mouse from each group is shown (left), and summary statistics with n = 4 per group (right). (D) Survival of mice bearing disseminated A20 lymphoma treated with TBI and BM rescue with or without NK cells; P = .62. (E) Tumor burden by flow cytometry of BM from C57BL/6 mice 26 days after injection of 1 × 103gfp+Hoxa9-Meis1 leukemia with or without 1 × 106 NK cells (allogeneic FVB, H-2q; syngeneic C57BL/6, H-2b) after TBI and BM rescue. Gated on total live cells. (F) Survival of mice receiving allogeneic (FVB H-2q) or syngeneic (C57BL6 H-2b) NK cells along with Hoxa9-Meis1 leukemia; P = .93. Data are representative of at least 3 experiments with at least 4 mice per group (A,C,E), or are a composite of 2 experiments with n = 13 to 14 per group (D,F).
Adoptive transfer of NK cells fails to control the growth of tumor targets shown to be NK-sensitive in vitro. (A) Specific lysis of chromium labeled A20 tumor cells by IL-2–stimulated C57BL/6 NK after a 4-hour incubation. Median ± error is shown. (B) Primary AML cells (C57BL/6, H-2b) did not uptake chromium and apoptosis was detected by flow cytometric caspase activation assay after a 2-hour incubation with IL-2 stimulated Balb/c NK cells. Inset shows an example of caspase activity flow cytometry plots. Median ± error is shown. (C) Tumor burden by BLI of Balb/c mice receiving 1 × 106 luciferase-expressing A20 tumor cells on day −7, followed on day 0 by TBI, BM rescue and a concurrent infusion of 0.5 × 106 C57BL/6 NK cells (bottom panel) or PBS (top panel). One representative mouse from each group is shown (left), and summary statistics with n = 4 per group (right). (D) Survival of mice bearing disseminated A20 lymphoma treated with TBI and BM rescue with or without NK cells; P = .62. (E) Tumor burden by flow cytometry of BM from C57BL/6 mice 26 days after injection of 1 × 103gfp+Hoxa9-Meis1 leukemia with or without 1 × 106 NK cells (allogeneic FVB, H-2q; syngeneic C57BL/6, H-2b) after TBI and BM rescue. Gated on total live cells. (F) Survival of mice receiving allogeneic (FVB H-2q) or syngeneic (C57BL6 H-2b) NK cells along with Hoxa9-Meis1 leukemia; P = .93. Data are representative of at least 3 experiments with at least 4 mice per group (A,C,E), or are a composite of 2 experiments with n = 13 to 14 per group (D,F).
NK cells traffic to and accumulate within tumor sites
One possible explanation for the inability of NK cells to impact in vivo tumor growth despite clear in vitro cytotoxicity may be their failure to localize to tumor. Using NK cells derived from luciferase-transgenic animals (luc+ NK) we previously showed that after adoptive transfer into nontumor-bearing hosts, NK cells traffic to lymphoid organs as early as day 3, achieve a maximal signal at day 10 to 12 after transfer and remain detectable for ∼ 4 weeks.18 The disseminated tumor models used here grow in the spleen and BM and hence should be accessible to NK cells. However, to definitively demonstrate that NK cells can home to tumor sites, we implanted tumor subcutaneously and injected luc+ NK cells intravenously 10 to 14 days later. Whereas NK cells transferred into nontumor-bearing hosts home to lymph nodes as previously described,18 NK cells transferred into tumor-bearing hosts trafficked through lymph nodes sites and accumulated within the tumor (Figure 2A). Strikingly, NK-cell accumulation correlated with increasing tumor size, suggesting that NK cells fail to eradicate tumor despite successful recognition and homing (Figure 2B). As was found in the disseminated tumor models, survival of tumor-bearing mice was not enhanced by the addition of NK cells (data not shown). Furthermore, the addition of exogenous IL-2 increased the overall NK-cell signal without enhancing tumor rejection or survival (Figure 2A-C). Similar findings were noted with higher doses of NK cells, higher doses of IL-2, with intratumoral inoculation of NK cells (Figure 2C), with syngeneic as well as allogeneic (MHC-mismatched) NK cells and using the EG7, RMA, and RMA/S cell lines (data not shown).
NK cells traffic to and accumulate within tumor sites. (A) Trafficking of C57BL/6 luc+ NK cells to A20 tumor implanted subcutaneously in Balb/c recipients. Red circles indicate tumor site and size; control groups received NK cells without tumor implantation. ROI quantification indicates photons/s/area and correlates with the number of NK cells within the site. Mice received PBS (left) or recombinant human IL-2 5 × 104 units intraperitoneally every second day (right). One mouse representative of each group is shown. (B) Quantification of NK-cell accumulation by BLI in the group receiving no IL-2. NK-cells accumulate in proportion to tumor growth. Each line represents an individual mouse. Results are representative of 5 individual experiments with up to 5 mice per group. (C) NK-cell homing and accumulation on day 14 within tumor-bearing Balb/c mice receiving standard-dose (1 × 106) freshly isolated C57BL/6 NK; standard-dose NK in combination with high-dose IL-2 (5 × 105 units daily for 14 days); high-dose NK cells (6 × 106) stimulated ex vivo in IL-2 for 5 days; or intratumoral standard-dose NK. Unless stated otherwise all groups received standard-dose IL-2 5 × 104 units every second day. NK cells were derived from luciferase-transgenic animals. Three mice from each group are shown (left panel) and BLI is quantified (right panel). ANOVA P = .18 for differences in BLI between the different groups. (D). Survival of animals in (C); P = .88. Results are representative of 2 experiments containing 5 mice in each group.
NK cells traffic to and accumulate within tumor sites. (A) Trafficking of C57BL/6 luc+ NK cells to A20 tumor implanted subcutaneously in Balb/c recipients. Red circles indicate tumor site and size; control groups received NK cells without tumor implantation. ROI quantification indicates photons/s/area and correlates with the number of NK cells within the site. Mice received PBS (left) or recombinant human IL-2 5 × 104 units intraperitoneally every second day (right). One mouse representative of each group is shown. (B) Quantification of NK-cell accumulation by BLI in the group receiving no IL-2. NK-cells accumulate in proportion to tumor growth. Each line represents an individual mouse. Results are representative of 5 individual experiments with up to 5 mice per group. (C) NK-cell homing and accumulation on day 14 within tumor-bearing Balb/c mice receiving standard-dose (1 × 106) freshly isolated C57BL/6 NK; standard-dose NK in combination with high-dose IL-2 (5 × 105 units daily for 14 days); high-dose NK cells (6 × 106) stimulated ex vivo in IL-2 for 5 days; or intratumoral standard-dose NK. Unless stated otherwise all groups received standard-dose IL-2 5 × 104 units every second day. NK cells were derived from luciferase-transgenic animals. Three mice from each group are shown (left panel) and BLI is quantified (right panel). ANOVA P = .18 for differences in BLI between the different groups. (D). Survival of animals in (C); P = .88. Results are representative of 2 experiments containing 5 mice in each group.
NK-cell dysfunction is not rescued by depletion of regulatory T cells or recruitment of ADCC
The suppressive impact of the tumor microenvironment on immune effector cells is well recognized. Tregs in particular have been shown to inhibit NK-cell function,19,20 and in the setting of adoptive T-cell immunotherapy the depletion of tumor-associated Tregs in murine models can enhance antitumor immunity.21 We therefore sought to deplete intratumoral Tregs by reconstituting wild-type (WT) mice with diphtheria toxin receptor-FOXP3-eGFP (DEREG) BM at the time of tumor inoculation, followed by treatment with diphtheria toxin (DT) at the time of NK-cell immunotherapy. Using this model, most (but not all) tumor-infiltrating Tregs were derived from the donor BM and hence were susceptible to genetic depletion (data not shown). In control animals, 50%-60% of CD4+ cells within the tumor were CD25+FOXP3+ Tregs, and there was no difference in Treg frequency between mice receiving NK cells or not (Figure 3A). Treatment with DT led to a significant but incomplete reduction in tumoral Tregs (Figure 3A-B); this result was expected given the incomplete donor-recipient chimerism in the tumor. Despite achieving a reduction in tumoral Tregs, there was no difference in the rate of tumor growth between mice with intact versus depleted Treg compartments (Figure 3C). Similar results were obtained using antibody-mediated depletion with the anti-FR4 antibody22 (Figure 3D). Together these results suggest that Tregs may not have an important role in controlling the NK-cell antitumor activity in this model.
NK-cell dysfunction is not rescued by depletion of regulatory T cells or recruitment of ADCC. (A-B) CD4+CD25+FoxP3+ Tregs infiltrate the tumor microenvironment. Effective depletion of Tregs can be achieved after injection of DT every second day into C57BL/6 reconstituted with DEREG or WT control BM; P = .006 for WT mice versus DEREG mice in the NK groups (2-tailed unpaired Student t test). (C) Genetic depletion of Tregs has no effect on tumor growth after NK-cell infusion; ANOVA P = .68. (D) Antibody-mediated depletion of Tregs using the anti-FR4 antibody TH6 does not impact tumor growth after NK-cell infusion. TH6 (anti-FR4) or isotype control were injected intravenously every 3 days beginning day 0 for 2 weeks; ANOVA P = .80. (E) Treatment of SCID mice bearing the human B-cell lymphoma Raji with in vitro expanded human NK cells and rituximab. Mice received tumor with isotype antibody (n = 18) or with rituximab 100 μg (n = 19), NK cells 1 × 107 (n = 14), high-dose NK cells 2 × 107 (n = 5), or NK 1 × 107+ rituximab (n = 18). Results are representative of 2 experiments with 4 to 5 mice per group (A-D) or are a composite of 4 experiments (E).
NK-cell dysfunction is not rescued by depletion of regulatory T cells or recruitment of ADCC. (A-B) CD4+CD25+FoxP3+ Tregs infiltrate the tumor microenvironment. Effective depletion of Tregs can be achieved after injection of DT every second day into C57BL/6 reconstituted with DEREG or WT control BM; P = .006 for WT mice versus DEREG mice in the NK groups (2-tailed unpaired Student t test). (C) Genetic depletion of Tregs has no effect on tumor growth after NK-cell infusion; ANOVA P = .68. (D) Antibody-mediated depletion of Tregs using the anti-FR4 antibody TH6 does not impact tumor growth after NK-cell infusion. TH6 (anti-FR4) or isotype control were injected intravenously every 3 days beginning day 0 for 2 weeks; ANOVA P = .80. (E) Treatment of SCID mice bearing the human B-cell lymphoma Raji with in vitro expanded human NK cells and rituximab. Mice received tumor with isotype antibody (n = 18) or with rituximab 100 μg (n = 19), NK cells 1 × 107 (n = 14), high-dose NK cells 2 × 107 (n = 5), or NK 1 × 107+ rituximab (n = 18). Results are representative of 2 experiments with 4 to 5 mice per group (A-D) or are a composite of 4 experiments (E).
NK recognition of tumors can be enhanced by antibody-dependent cellular cytotoxicity (ADCC) and is thought to be an important mechanism of action of several clinically available monoclonal antibodies including the anti-CD20 antibody rituximab.23 To evaluate whether the presence of a monoclonal antibody would enhance the ability of NK cells to reject tumor, we injected immunodeficient mice with a human B-cell lymphoma (Raji) and treated them with ex vivo expanded human NK cells along with rituximab. The addition of rituximab clearly enhanced survival of tumor-bearing mice. However, as with the murine experiments human NK cells failed to reject tumor and did not demonstrate an additive effect in the presence of rituximab (Figure 3E).
Tumor-infiltrating NK cells down-regulate activating receptors and show impaired effector functions
To recognize and become activated by their targets, NK cells of C57BL/6 origin use several activating receptors including NK1.1 and NKG2D, and are inhibited by several receptors including NKG2A/C/E and the Ly49 family members Ly49C and Ly49I, among others.24 Having shown that NK cells are unable to reject tumor despite robust homing and accumulation, we next characterized the cell surface expression of activating and inhibitory receptors on CD45.1+ NK cells reisolated 2 weeks after transfer into CD45.2+ tumor-bearing recipients. The phenotype of naive NK cells on D0 before transfer is shown in supplemental Figure 2A. Transferred NK cells showed marked down-regulation of NKG2D and NK1.1 as well as of the integrin CD49b (DX5) in comparison to splenic-derived activated NK cells. Receptor down-regulation occurred in NK cells reisolated from the tumor as well as the spleen, but was most pronounced in tumor-infiltrating NK cells (Figure 4A-B). There were minor but nonsignificant differences in expression patterns of the receptors NKG2A/C/E (inhibitory in the presence of the atypical MHC class I molecule Qa-1), Ly49C/I (inhibitory in the presence of the C57BL/6 MHC class I molecule H-2b, and hence expected to be licensed and noninhibitory when transferred from C57BL/6 donors to Balb/c recipients), Ly49D (activating in the presence of CHO cells), and Ly49A/G2 (inhibitory in the presence of the Balb/c MHC class I molecule H-2d) among splenic, tumoral, and control NK cells (supplemental Figure 2B). The rescue BM in this model was derived from CD45.2+ mice, allowing us to compare the receptor expression pattern on transferred mature CD45.1+ NK cells with that of BM derived CD45.2+ NK cells. The majority of NKG2D+, NKp46+, and DX5+ cells were BM derived (supplemental Figure 2C). Reisolated NK cells were then tested for functional capacity. As naive splenic NK cells require stimulation to demonstrate cytokine production and cytolysis, NK cells cultured in vitro with IL-2 were used as controls. IFNγ production was markedly reduced in splenic NK cells and completely lost in tumor-infiltrating NK cells (Figure 4C-D). Although we could not demonstrate a difference between the cytolytic capacity of splenic-derived and tumor-derived reisolated NK cells, both these populations were markedly deficient compared with NK cells derived from control animals (Figure 4E).
Tumor-infiltrating NK cells down-regulate activating receptors and show impaired effector functions. (A) Purified CD3−DX5+ or CD3−NK1.1+ NK cells from CD45.1+ congenic donors were transferred into CD45.2+ recipients. All analyses are gated on live singlet lymphocytes. Within the spleen and tumor of tumor-bearing mice (rows 2 and 3), transferred CD45.1+ NK cells down-regulated NK1.1, NKG2D, and DX5 compared with ex vivo expanded CD45.2+ NK cells (top row). (B) Statistical comparison of events in panel A, showing marked loss of activating receptors on intratumoral NK cells compared with cultured and splenic NK cells; ANOVA with the Dunnett multiple comparison test. (C) IFNγ production is diminished within reisolated NK cells. Gating is based on nonstimulated controls (not shown). (D) Statistical comparison of events in panel C, showing loss of cytokine production on intratumoral NK cells compared with cultured or splenic NK cells; ANOVA with Dunnett multiple comparison test. (E) CD45.1+ cells were sorted from spleen or tumor and assessed for cytotoxicity against chromium-labeled A20 cells. Analyses are gated on reisolated CD45.1+ or control CD45.2+ live NK cells. Results are representative of at least 3 (A-D) or 2 (E) independent experiments with at least 3 mice per group.
Tumor-infiltrating NK cells down-regulate activating receptors and show impaired effector functions. (A) Purified CD3−DX5+ or CD3−NK1.1+ NK cells from CD45.1+ congenic donors were transferred into CD45.2+ recipients. All analyses are gated on live singlet lymphocytes. Within the spleen and tumor of tumor-bearing mice (rows 2 and 3), transferred CD45.1+ NK cells down-regulated NK1.1, NKG2D, and DX5 compared with ex vivo expanded CD45.2+ NK cells (top row). (B) Statistical comparison of events in panel A, showing marked loss of activating receptors on intratumoral NK cells compared with cultured and splenic NK cells; ANOVA with the Dunnett multiple comparison test. (C) IFNγ production is diminished within reisolated NK cells. Gating is based on nonstimulated controls (not shown). (D) Statistical comparison of events in panel C, showing loss of cytokine production on intratumoral NK cells compared with cultured or splenic NK cells; ANOVA with Dunnett multiple comparison test. (E) CD45.1+ cells were sorted from spleen or tumor and assessed for cytotoxicity against chromium-labeled A20 cells. Analyses are gated on reisolated CD45.1+ or control CD45.2+ live NK cells. Results are representative of at least 3 (A-D) or 2 (E) independent experiments with at least 3 mice per group.
Finally, we determined the maturation status of the transferred NK cells by analysis of CD27, CD11b, and KLRG1 expression. Murine NK-cell maturation has been previously shown to progress from CD11b−CD27+ to CD11b+CD27+ to CD11b+CD27− with the latter subset thought to be terminally mature and with impaired effector functionality.25 KLRG1, an inhibitory receptor on NK cells and T cells, is up-regulated on homeostatic proliferation as well as during persistent antigen encounter.26,27 Here we have shown that all reisolated NK cells have markedly up-regulated KLRG1 and have lost CD27 expression compared with control NK cells (supplemental Figure 2D), consistent with a mature, proliferated phenotype.
NK-cell dysfunction is induced by proliferation in the presence of tumor
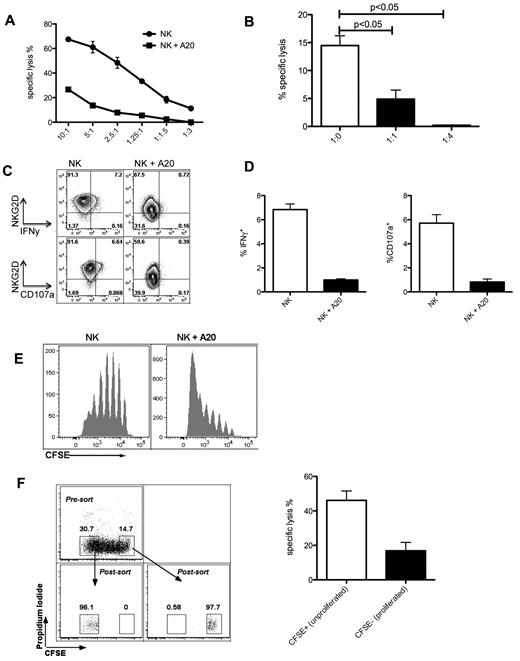
The observed changes in activating receptor expression, cytokine secretion, and cytolytic capacity were present in both tumor and nontumor sites and could result from a global NK-cell defect occurring on homeostatic proliferation after adoptive transfer. Alternatively, NK-cell dysfunction could be induced within the tumor environment followed by recirculation of NK cells to extratumoral sites including the spleen. To discover whether exposure to tumor alone can induce NK-cell dysfunction, we set up an in vitro model of prolonged NK cell–tumor cell contact. Sorted NK cells were cultured with or without irradiated tumor cells for 5 days in the presence of IL-2, then harvested for functional assays. Prolonged culture with tumor led to a loss of cytotoxicity (Figure 5A), and this dysfunction was more marked in the presence of higher numbers of tumor cells (Figure 5B). Cytotoxic function was not regained after a further 5 days in culture without tumor (data not shown). Furthermore, IFNγ production, ability to degranulate and NKG2D expression were lost during prolonged tumor contact (Figure 5C-D). These in vitro observations indicate that tumor exposure alone can induce NK-cell dysfunction. It was previously reported that various tumors can elaborate soluble NKG2D ligands. To discover whether NK-cell dysfunction required contact with target we incubated NK cells directly with irradiated tumor cells or separated by a transwell. After 5 days, cytotoxicity was lost in cells incubated in contact with tumor but preserved in NK cells separated from the tumor (supplemental Figure 3A), arguing against the role of soluble NKG2D ligands in mediating dysfunction in this model. Furthermore, given the observed down-regulation of NKG2D (an important receptor for recognition of several tumors including A20), we sought to investigate whether signaling through this receptor was required to induce NK-cell dysfunction. Baseline cytotoxicity by NKG2D−/− NK cells was lower than that by WT NK cells as expected, but on prolonged tumor exposure both WT and NKG2D−/− lost cytotoxic function, suggesting that signaling through NKG2D was not required for the induction of NK-cell dysfunction (supplemental Figure 3B). By labeling NK cells with CFSE we were able to correlate proliferation with functional phenotype. NK-cell proliferation was increased in the presence of tumor (Figure 5E), with proliferated NK cells exhibiting down-regulation of NKG2D, loss of IFNγ production (supplemental Figure 3C), and loss of cytotoxicity (Figure 5F). These in vitro observations complemented our in vivo findings by highlighting the ability of prolonged tumor exposure to induce NK-cell proliferation, which in turn correlates with loss of function.
NK-cell dysfunction is induced by proliferation in the presence of tumor. (A) Killing of chromium-labeled A20 cells by allogeneic C57BL/6 sorted NK that had been cultured at a 1:1 ratio with or without irradiated A20 for 5 days, in presence of IL-2 750 U/mL; effector:target ratios for the chromium assay are indicated on the x-axis. (B) Killing of chromium-labeled A20 cells by C57BL/6 NK cells that had been cultured at the indicated ratios with irradiated A20 tumor cells for 5 days, effector:target ratio for the chromium release assay was 2.5:1; P = .0009, ANOVA with Dunnett multiple comparison test. (C) Exposure to tumor leads to loss of IFNγ production and degranulation. After a 5 day exposure to irradiated A20 cells, C57BL/6 NK cells were stimulated with plate-bound anti-NK1.1 antibody and stained for IFNγ or CD107a. (D) Quantification of the events in panel C; P < .001 for IFNγ production and P < .01 for CD107a degranulation; 2-tailed unpaired Student t test. (E) Tumor coculture leads to increased NK-cell proliferation. (F) Proliferated, CFSE-low NK cells undergo more marked dysfunction than unproliferated cells. C57BL/6 NK cells were labeled with CFSE and cultured with irradiated A20 tumor cells for 4 days, then sorted on the basis of CFSE dilution; sorted cells were then cultured with chromium-labeled A20 cells at a 1:1 ratio for 18 hours; P = .03 (2-tailed unpaired Student t test). Results were done in triplicate and are representative of 3 (A,C,D) or 2 (B,E,F) experiments.
NK-cell dysfunction is induced by proliferation in the presence of tumor. (A) Killing of chromium-labeled A20 cells by allogeneic C57BL/6 sorted NK that had been cultured at a 1:1 ratio with or without irradiated A20 for 5 days, in presence of IL-2 750 U/mL; effector:target ratios for the chromium assay are indicated on the x-axis. (B) Killing of chromium-labeled A20 cells by C57BL/6 NK cells that had been cultured at the indicated ratios with irradiated A20 tumor cells for 5 days, effector:target ratio for the chromium release assay was 2.5:1; P = .0009, ANOVA with Dunnett multiple comparison test. (C) Exposure to tumor leads to loss of IFNγ production and degranulation. After a 5 day exposure to irradiated A20 cells, C57BL/6 NK cells were stimulated with plate-bound anti-NK1.1 antibody and stained for IFNγ or CD107a. (D) Quantification of the events in panel C; P < .001 for IFNγ production and P < .01 for CD107a degranulation; 2-tailed unpaired Student t test. (E) Tumor coculture leads to increased NK-cell proliferation. (F) Proliferated, CFSE-low NK cells undergo more marked dysfunction than unproliferated cells. C57BL/6 NK cells were labeled with CFSE and cultured with irradiated A20 tumor cells for 4 days, then sorted on the basis of CFSE dilution; sorted cells were then cultured with chromium-labeled A20 cells at a 1:1 ratio for 18 hours; P = .03 (2-tailed unpaired Student t test). Results were done in triplicate and are representative of 3 (A,C,D) or 2 (B,E,F) experiments.
NK-cell dysfunction is induced by homeostatic proliferation
Adoptive transfer of mature NK cells into lymphopenic hosts induces homeostatic proliferation (HP).28 We found that early after adoptive transfer, NK cells maintain the capacity to produce IFNγ, but this function is lost within 5 days in both irradiated WT and lymphodepleted Rag2−/−γc−/− hosts independently of the presence of tumor or the extent of proliferation (Figure 6A). This observation highlights the complex behavior of NK cells in an in vivo setting. Early after adoptive transfer there was increased cytotoxic function (Figure 6B), followed by loss of function at later points (Figure 6C). NK cells proliferating in the absence of tumor were more cytotoxic than nonproliferating NK cells, whereas in the presence of tumor the effect was reversed such that proliferating NK cells were no more, and possibly less cytotoxic than nonproliferating cells (Figure 6D). Although the effect of proliferation on cytotoxicity was not statistically significant, this trend was found across several experiments.
NK-cell dysfunction is induced by homeostatic proliferation. (A) IFNγ production in restimulated NK cells reisolated 1 or 5 days after in vivo transfer with or without disseminated tumor; gates were set using nonstimulated controls (not shown). Injection into Rag2−/−γc−/− recipients in addition to irradiated recipients was performed to account for the effects of radiation. (B) Killing of chromium-labeled A20 cells by freshly isolated naive NK cells or C57BL/6 CD45.1+ NK reisolated 18 hours after transfer into irradiated hosts bearing Hoxa9-Meis1 leukemia, and cultured with A20 tumor cells at a 1:1 ratio for 16 hours. Control cells were sorted CD3−NK1.1+ NK cells from naive spleens; P = .0035 (2-tailed unpaired Student t test). (C) Killing of chromium-labeled A20 cells by freshly isolated naive NK cells or C57BL/6 CD45.1+ NK reisolated 17 days after transfer into irradiated hosts bearing Hoxa9-Meis1 leukemia, and cultured with A20 tumor cells indicated ratios for 16 hours. Control cells were sorted CD3−NK1.1+ NK cells from naive spleens; P = .001 for difference between naive and reisolated NK cells at a 6:1 ET ratio. (D) CFSE-labeled C57BL6 NK cells were injected into Rag2−/−γc−/− recipients alone or with 5 × 106 A20 tumor cells and sorted into CFSE high (unproliferated) or CFSE low (proliferated) populations; these cells were then cultured with chromium-labeled A20 cells for 12 hours at an effector:target ratio of 2:1; ANOVA with the Dunnett multiple comparison test. Results are representative of 2 to 3 experiments with 3 to 4 mice pooled per experiment.
NK-cell dysfunction is induced by homeostatic proliferation. (A) IFNγ production in restimulated NK cells reisolated 1 or 5 days after in vivo transfer with or without disseminated tumor; gates were set using nonstimulated controls (not shown). Injection into Rag2−/−γc−/− recipients in addition to irradiated recipients was performed to account for the effects of radiation. (B) Killing of chromium-labeled A20 cells by freshly isolated naive NK cells or C57BL/6 CD45.1+ NK reisolated 18 hours after transfer into irradiated hosts bearing Hoxa9-Meis1 leukemia, and cultured with A20 tumor cells at a 1:1 ratio for 16 hours. Control cells were sorted CD3−NK1.1+ NK cells from naive spleens; P = .0035 (2-tailed unpaired Student t test). (C) Killing of chromium-labeled A20 cells by freshly isolated naive NK cells or C57BL/6 CD45.1+ NK reisolated 17 days after transfer into irradiated hosts bearing Hoxa9-Meis1 leukemia, and cultured with A20 tumor cells indicated ratios for 16 hours. Control cells were sorted CD3−NK1.1+ NK cells from naive spleens; P = .001 for difference between naive and reisolated NK cells at a 6:1 ET ratio. (D) CFSE-labeled C57BL6 NK cells were injected into Rag2−/−γc−/− recipients alone or with 5 × 106 A20 tumor cells and sorted into CFSE high (unproliferated) or CFSE low (proliferated) populations; these cells were then cultured with chromium-labeled A20 cells for 12 hours at an effector:target ratio of 2:1; ANOVA with the Dunnett multiple comparison test. Results are representative of 2 to 3 experiments with 3 to 4 mice pooled per experiment.
Thus, loss of IFNγ production appears to occur early after adoptive transfer without relation to proliferation or tumor burden, whereas loss of cytotoxicity progresses with proliferation and exposure to tumor.
Transcription factors Eomesodermin and T-bet are down-regulated on exposure to tumor and on proliferation
Eomesodermin (Eomes) and T-bet are Tbox transcription factors with important roles in the development and acquisition of effector functions by NK cells,29-31 suggesting that expression of these transcription factors may correlate with functional changes in mature NK cells. High level expression of both Eomes and T-bet is detectable on naive CD3−NK1.1+ splenic NK cells and increases further on stimulation with IL-2, correlating with IFNγ production in stimulated cells (supplemental Figure 4A). On adoptive transfer of CFSE-labeled NK cells, Eomes and T-bet expression falls over time and is lowest within the most highly proliferated NK cells (Figure 7A). The reduction in transcription factor expression correlated with loss of IFNγ production (Figure 7B) and occurs in adoptively transferred NK cells regardless of the presence of tumor. These results demonstrate the loss of the canonical transcription factors Eomes and T-bet over time with adoptive transfer, correlating with loss of effector functions in line with our other results. Finally, forced expression of Eomes, but not T-bet, in adoptively transferred NK cells led to a reduction in tumor burden and enhanced survival compared with mice treated with control NK cells (Figure 7C-D).
Transcription factors Eomesodermin and T-bet are down-regulated on exposure to tumor and on proliferation. (A) Levels of Eomesodermin and T-bet fall with cell proliferation. Gates were set using isotype controls as shown in the top row for each analysis. Naive NK cells were analyzed fresh and hence were not CFSE-labeled. NK cells reisolated at D+ 15 after adoptive transfer into nontumor-bearing hosts were isolated using the expression of congenic markers, as shown in the left panel. (B) Eomes expression and IFNγ production decrease over time. NK cells were reisolated 1 or 10 days after transfer without or with 1 × 105Hoxa9-Meis1 leukemia cells. Gates are set using isotype controls, as shown in the top row. Naive spleens are depicted for comparison in the left column. (C) Sorted C57BL/6 NK cells were transduced with Eomes, T-bet, or control vector and injected with A20 cells at an E/T ratio of 5:1 into irradiated Balb/c recipients. Overexpression of Eomes, and not T-bet, leads to a significantly prolonged survival compared with control treated NK cells. (D) Tumor burden is reduced in mice receiving Eomes-transduced NK cells. Sorted C57BL/6 NK cells were transduced with Eomes, T-bet, or control vector and injected with luc+ A20 cells at a 5:1 ratio into irradiated Balb/c recipients. BLI on D+8 after injection shows a significant reduction in tumor burden among mice receiving Eomes-transduced NK cells compared with no NK cells. T-bet or control vector treated NK cells did not show a significant reduction in tumor burden. Results are representative of 3 (A) or 2 (B-D) individual experiments, or are a composite of 3 independent experiments with a total of 13 to 14 mice per group (C).
Transcription factors Eomesodermin and T-bet are down-regulated on exposure to tumor and on proliferation. (A) Levels of Eomesodermin and T-bet fall with cell proliferation. Gates were set using isotype controls as shown in the top row for each analysis. Naive NK cells were analyzed fresh and hence were not CFSE-labeled. NK cells reisolated at D+ 15 after adoptive transfer into nontumor-bearing hosts were isolated using the expression of congenic markers, as shown in the left panel. (B) Eomes expression and IFNγ production decrease over time. NK cells were reisolated 1 or 10 days after transfer without or with 1 × 105Hoxa9-Meis1 leukemia cells. Gates are set using isotype controls, as shown in the top row. Naive spleens are depicted for comparison in the left column. (C) Sorted C57BL/6 NK cells were transduced with Eomes, T-bet, or control vector and injected with A20 cells at an E/T ratio of 5:1 into irradiated Balb/c recipients. Overexpression of Eomes, and not T-bet, leads to a significantly prolonged survival compared with control treated NK cells. (D) Tumor burden is reduced in mice receiving Eomes-transduced NK cells. Sorted C57BL/6 NK cells were transduced with Eomes, T-bet, or control vector and injected with luc+ A20 cells at a 5:1 ratio into irradiated Balb/c recipients. BLI on D+8 after injection shows a significant reduction in tumor burden among mice receiving Eomes-transduced NK cells compared with no NK cells. T-bet or control vector treated NK cells did not show a significant reduction in tumor burden. Results are representative of 3 (A) or 2 (B-D) individual experiments, or are a composite of 3 independent experiments with a total of 13 to 14 mice per group (C).
Discussion
NK cells have been proposed to be an attractive cell population for adoptive cancer immunotherapy because of “natural cytotoxicity” that allows prompt non-MHC restricted cytokine production and lysis of target cells without prior stimulation or antigen presentation. In this study, we demonstrated that despite enhancement of function immediately after transfer effector functions are rapidly lost, leading to a critical impairment of the antitumor efficacy of this cell population. This phenomenon is accompanied by down-regulation of activating receptor expression, consistent with previous data on endogenous NK cells in patients with AML and breast cancer,32,33 and is dependent on proliferation and loss of Eomes expression. We have termed this phenomenon “NK-cell exhaustion” by analogy with similar findings in CD8+ T cell biology.17,34 Our findings add to a growing body of knowledge on the parallels between NK cells and CD8+ T cells35 and show for the first time that mature NK cells from healthy donors become rapidly exhausted on adoptive transfer, whether tumor is present or not.
To investigate the disparity between the potent in vitro antitumor effect of NK cells and their limited role in the control of tumor after adoptive transfer, we used several NK-sensitive tumor cell lines, as well as primary mouse AML. Adoptive NK immunotherapy failed to control tumor growth or enhance survival of tumor-bearing mice, despite the use of up to 1000-fold higher numbers of NK cells compared with tumor cells. Several other variables failed to enhance NK-cell in vivo function including the injection of both fresh and ex vivo IL-2 stimulated NK cells, low or high-dose IL-2, syngeneic or allogeneic NK cells, simultaneous or sequential injection of tumor cells and NK cells, intratumoral injection of NK cells and partial depletion of Tregs. We acknowledge that these findings are at some variance with previously published results.6-9 However we attempted to model our experiments on clinically relevant settings including the infusion of no more than clinically achievable NK-cell numbers (2 × 107/kg in clinical reports,36 5 × 107/kg in our experiments) with the administration of NK cells occurring at a site and time distant to tumor implantation.
The intravenous transfer of luciferase-expressing NK cells at a site distant from the tumor allowed us to investigate homing characteristics. We built on previous work showing the normal trafficking behavior of NK cells after adoptive transfer into lymphodepleted hosts18 by visualizing the rapid accumulation of NK cells within the tumor site, with signal increasing over time. These experiments demonstrated that the failure of NK cells to reject tumor in vivo is not because of the lack of migration to the relevant site. We did not investigate the chemokine interactions required to mediate NK homing to tumor in this study, but previous reports demonstrated the importance of CXCR3 and CX3CR1, among other receptors.37,38
Within the tumor site, NK cells were found to down-regulate expression of the activating receptors NKG2D, NK1.1, NKp46, and the integrin CD49b (DX5). These results are in agreement with published data showing that sustained expression of NKG2D ligands leads to down-regulation of NKG2D along with impairment of cytotoxicity,39 and that chronic engagement of NK receptors by their cognate ligands impairs the function of those receptors and downstream pathways.40 Similarly, NK cells in patients with AML show loss of expression of activating receptors during active disease, with reexpression after successful disease eradication.32 Similar findings have been observed in tumor-infiltrating NK cells in patients with breast cancer, where progressive disease correlates with more pronounced dysfunction.33 The latter observations would seem to suggest that adoptive transfer of mature NK cells bearing the full complement of activating receptors and proven antitumor functionality could circumvent endogenous tumor-associated NK-cell dysfunction. However, our findings clearly show that these NK cells too are highly susceptible to induced dysfunction, or exhaustion, that is characterized by loss of activating receptors, IFNγ production, and cytotoxicity.
There is an additional implication of down-regulation of NK1.1, NKp46 and CD49b. As these molecules are used to define NK cells, their down-regulation makes antibody-mediated cytofluorimetric recognition of NK cells problematic, and may potentially skew the results of experiments where only the residual cells still bearing NK1.1 or NKp46 are recognized. In syngeneic settings, only an experimental design with adoptive transfer of NK cells from congenically disparate donors would allow detection of this phenomenon.
Furthermore, we found that dysfunction is time-dependent such that early after adoptive transfer, NK-cell function is enhanced compared with that of baseline naive splenic-derived NK cells (possibly reflecting stimulation from the inflammatory postirradiation milieu), followed by subsequent decline. The early enhancement of function is consistent with published observations showing that NK cells are still functional early after transfer for protection from murine cytomegalovirus (CMV).41 However, the later decline in function contrasts with findings from the same paper, whereby NK cells were shown to retain the ability to proliferate and produce IFNγ in response to CMV infection 2 months after adoptive transfer into lymphodeplete animals. This variance may reflect the longer period of follow-up permitted by a nonmalignant model whereby NK cells may “recover” in an otherwise healthy animal before CMV infection, the different stimuli for NK-cell activation provided by recognition of CMV through the Ly49H receptor, or the fact that cytokine production was assessed in the traditionally defined Ly49H+ NK cells which according to our data may represent a residual population of NK cells not undergoing receptor down-regulation rather than reflecting the total pool of transferred NK cells.
After adoptive transfer, a rapid loss of the transcription factors Eomes and T-bet was associated with the observed NK-cell dysfunction. Loss of T-bet expression was shown to occur in CD8+ T-cell exhaustion during chronic viral infection,17 and although a reciprocal expression pattern is thought to exist between T-bet and Eomes in T cells,42,43 a recent publication revealed that high level expression of microRNA-29 can repress expression of both these transcription factors in T cells simultaneously.44 This situation is reminiscent of recent observations in Treg biology, whereby under certain inflammatory conditions FOXP3 expression is lost and Treg cells acquire a pathogenic phenotype.45 We did not demonstrate increased expression of PD-1, as has been shown for exhausted CD8+ T cells (data not shown).17 NK cells transduced to express Eomes, but not T-bet, provided enhanced tumor control, suggesting that Eomes has a central role in NK-cell exhaustion.
These results may explain why the transfer of up to 1 × 108/kg autologous ex vivo expanded NK cells is unable to mediate meaningful antitumor activity.1 Our findings have clear implications for the field of NK immunotherapy, with the most important next step being to validate these results in NK cells reisolated from patients in ongoing clinical trials of NK immunotherapy. Future work should focus on rational combinations of adoptive NK-cell therapy with cytokines, antibodies, or drugs capable of modulating the proliferation-induced exhaustion phenotype.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
S.G. was supported by a New Investigator Scholarship from the Hematology Society of Australia and New Zealand/Genzyme Corporation and by National Institutes of Health (NIH) training grant T32 HL07952-09.
This study was supported by NIH grant R01 CA125276 to R.S.N.
National Institutes of Health
Authorship
Contribution: S.G. designed research, performed experiments, analysed data and wrote the paper; A.E.V., A.D.S., J.B., A.T.S., H.E.K., K.T., and M.F. performed experiments; K.D.G. provided vital new reagents; D.S.R. designed research and provided vital new reagents; and R.S.N. designed research, analysed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Center for Clinical Sciences Research, 269 W Campus Dr, Rm 2205, Stanford University, Stanford, CA 94305; e-mail: negrs@stanford.edu.