Abstract
Integrins are integral membrane proteins that mediate cell-matrix and cell-cell adhesion. They are important for vascular development and hematopoiesis, immune and inflammatory responses, and hemostasis. Integrins are also signaling receptors that can transmit information bidirectionally across plasma membranes. Research in the past 2 decades has made progress in unraveling the mechanisms of integrin signaling and brings the field to the moment of attempting synthetic reconstruction of the signaling pathways in vitro. Reconstruction of biologic processes provides stringent tests of our understanding of the process, as evidenced by studies of other biologic machines, such as ATP synthase, lactose permease, and G-protein–coupled receptors. Here, we review recent progress in reconstructing integrin signaling and the insights that we have gained through these experiments.
Introduction
What I cannot create, I do not understand. Richard Feynman
Regulation of cell adhesion through cell-matrix or cell-cell interaction is a critical step in various physiologic processes, such as cell migration and anchoring during development of the blood-forming organs, recruitment of cells into sites of inflammation, and aggregation and adhesion of platelets. Integrins are cell surface receptors composed of type I heterodimeric transmembrane proteins, formed by a combination of 18 α-subunits and 8 β-subunits. Twenty-four integrins have been identified so far.1 In blood cells, integrins are usually in a resting (inactive) state with low affinity for their ligands; they can quickly switch to an activated, high-affinity state in response to agonists, such as proteases or adenine nucleotides, a process often referred to as inside-out activation or inside-out signaling.1-5 Disruption of integrin function causes several hematologic diseases. For example, loss of integrin-αIIbβ3 (GPIIb-IIIa), the most abundant platelet integrin, causes Glanzmann thrombasthenia, a hereditary hemorrhagic disorder.6-9 In the early 1990s, investigators also identified Glanzmann thrombasthenia patients whose platelet αIIbβ3, although expressed in normal amount, cannot be activated by agonists because of mutations in the β3 cytoplasmic domain.10,11 These mutations, although not fully explained until the recent understanding of talin and kindlin function in integrin regulation, provided important early insight indicating that integrins were regulated from inside-out (reviewed in the last section).
Similarly, a subset of integrins, such as β2-integrins and α4-integrins, are expressed in leukocytes and mediate their adhesion to endothelium during various stages of extravasation during inflammatory responses. Loss of integrin-β2 (CD18) expression causes leukocyte adhesion deficiency I (LAD I), a disease characterized by recurrent infections.12 Certain patients with LAD symptoms have normal levels of β2-integrins combined with a bleeding diathesis. These patients' leukocytes are defective in β2- and β1-integrin activation, whereas their platelets also exhibit defects in activation of αIIbβ3; this variant is termed LAD III, or Lad1v.13-16 The defective integrin activation in these LAD patients is caused by kindlin-3 mutations.13-15,17 Some groups have also suggested the name of integrin activation deficiency disease for these conditions.14 Furthermore, Kindler syndrome, a skin blistering disease, is the result of defective β1-integrin activation caused by kindlin-1 mutations.18,19
As proteins involved in multiple biologic processes and located at the cell surface, integrins are also readily accessible therapeutic targets. For example, inhibitors of integrin-αIIbβ3 are currently used in the prevention and treatment of arterial thrombosis in the acute settings of percutaneous coronary intervention.20 Other integrin-blocking agents against α4 are currently used for multiple sclerosis and Crohn disease, and those against αvβ3 and α5β1 are being tested for cancer and osteoporosis.21 Thus, understanding the mechanism of integrin activation can help identify new therapeutic targets. Platelet integrin-αIIbβ3 and leukocyte β2 integrins are the prototypes for studying integrin activation because of the dramatic changes in their affinity for ligands after inside-out signaling.
Reconstruction seeks to synthetically re-create a biologic process with the required components. Reconstruction has played an important role in our understanding of other membrane proteins, such as the ATP synthase,22 G-protein coupled receptors,23 lactose permease,24 and ion channels.25 For example, reconstructed ATPase on nickel-coated glass chips provided crucial microscopic evidence for the rotary mechanism of ATP synthase,26-28 whereas reconstituted lactose permease liposomes made it possible to measure the kinetics of H+ and lactose transport.24 Similarly, reconstitution of integrin inside-out signaling in cell-based systems was useful in mapping inside-out signaling pathways.29-31 Studies on integrin activation re-created by various other means provided important insights into the mechanism of integrin activation at atomic,32-38 molecular,39-42 and macromolecular levels.43 In this review, we describe the experiments that reconstructed integrin inside-out signaling pathways and the insights we have gained from these studies.
Cell-based reconstitution of inside-out integrin activation
Although agonist-induced integrin activation results in dramatically increased integrin affinities in many cell types, primary leukocytes and platelets are less amenable to genetic manipulations. Thus, genetic analysis of molecular mechanisms of inside-out signaling was facilitated by development of a tractable cell-based system. Chinese hamster ovary (CHO) cells stably expressing wild-type or mutant αIIbβ3 integrins44 have become a basic building block for such work. The function of various cytoplasmic factors in integrin activation can be studied by measuring integrin activation after overexpression or knockdown of various proteins in these engineered CHO cells.
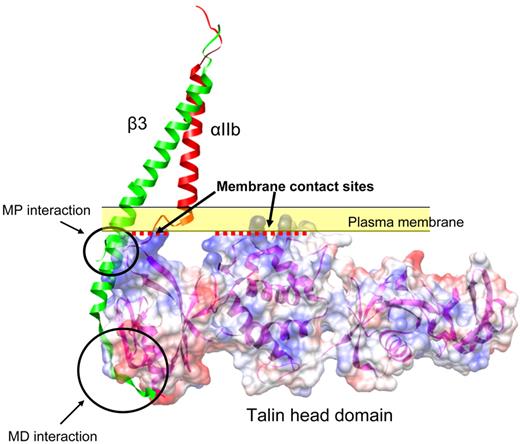
Calderwood et al found that overexpressed talin head domain (THD) could strongly activate αIIbβ3 in CHO cells.45 This was followed by a series of studies leading to the conclusion that talin binding to the integrin-β tail is a final common step in inside-out integrin activation.46 Drawing on insight from integrin-talin complex structures, a large number of mutational studies were also completed in this cell-based system that proved the importance of talin-integrin interaction in integrin inside-out signaling.36,38,47,48 Talin interacts with 2 sites in integrin-β tail and makes contact with the plasma membrane (Figure 1). These interactions position talin in such a way that talin Lys324 competes for binding to β3 Asp723, breaking the electrostatic interaction between αIIb Arg995 with β3 Asp723 that stabilizes the resting state. Thus, disrupting this Arg995-Asp723 interaction contributes to integrin activation.36 Recently, kindlins have been identified as important modulators of integrin inside-out signaling through genetic studies.13-15,17,49-52 This CHO system can be used to investigate the mechanisms of kindlin function.53-57 Although overexpression of kindlins in CHO cells does not activate integrins by itself, kindlin-1 and kindlin-2 can synergize with THD in activating αIIbβ3. Furthermore, kindlin-integrin-β tail interactions are important for kindlin function, as mutations that disrupt kindlin-integrin binding blocked the effects of kindlins.56-58
Structural model of talin-integrin interaction at the plasma membrane. Talin binds to 2 sites in the integrin-β3 tail: the membrane distal (MD) interaction site centered on NPxY747 motif and a membrane proximal (MP) site. Talin also makes contact with the plasma membrane at its interface with the cytosol (yellow band) through the positively charged residues on its surface (represented as blue; red dotted lines represent the membrane contact interfaces). Red and green represent the integrin-αIIb and β3 TM domains from the recent NMR structure (PDB entry 2k9j). Talin head domain (PDB entry 3IVF) is shown in surface representation. The ribbon representation of β3 was extended to the cytoplasmic domain by aligning the β3 TM domain with the β1D cytoplasmic domain structure from a β1D-Talin F2F3 (PDB entry 3G9W).
Structural model of talin-integrin interaction at the plasma membrane. Talin binds to 2 sites in the integrin-β3 tail: the membrane distal (MD) interaction site centered on NPxY747 motif and a membrane proximal (MP) site. Talin also makes contact with the plasma membrane at its interface with the cytosol (yellow band) through the positively charged residues on its surface (represented as blue; red dotted lines represent the membrane contact interfaces). Red and green represent the integrin-αIIb and β3 TM domains from the recent NMR structure (PDB entry 2k9j). Talin head domain (PDB entry 3IVF) is shown in surface representation. The ribbon representation of β3 was extended to the cytoplasmic domain by aligning the β3 TM domain with the β1D cytoplasmic domain structure from a β1D-Talin F2F3 (PDB entry 3G9W).
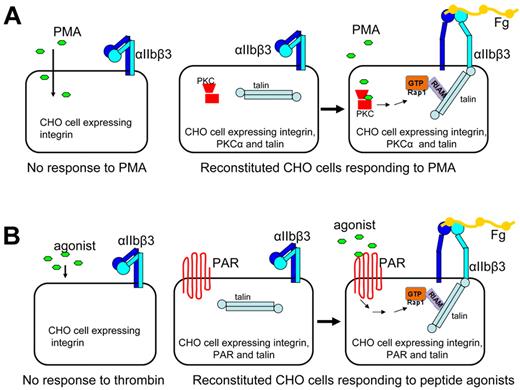
Despite the utility of CHO cells expressing αIIbβ3 in dissecting the final steps of integrin activation, they are limited in providing insights about the signaling cascades that regulate the process, as CHO cells do not respond to agonist stimulation.44 Two approaches were taken to re-create agonist-induced physiologic inside-out integrin activation. In the first approach, protein kinase C and talin were transiently overexpressed in αIIbβ3-expressing CHO cells to the levels approximating those in platelets. These cells then produced a robust response to phorbol myristate acetate (PMA)29 (Figure 2A). In this engineered CHO cell system, one can now pinpoint the role and position of a particular cytoplasmic factor in the signaling cascade using knockdown strategies or other specific deactivating reagents. Indeed, inhibition of Rap1 GTPase, a protein proven important in platelet integrin inside-out activation,59 blocked PMA-induced αIIbβ3 activation in this system, whereas activated Rap1A(G12V) bypassed the requirement for PKC, establishing that Rap1 is downstream of PKC. Furthermore, siRNA-mediated knockdown of RIAM, a protein that mediates effect of Rap1 on integrins,60 blocked integrin activation even in the presence of activated Rap1A(G12V), confirming that RIAM is downstream of Rap1a.29 Thus, the signaling pathway from agonist (PMA) to αIIbβ3 through PKC, Rap1a, RIAM, and talin was reconstructed in CHO cells.
Agonist-induced inside-out integrin signaling system reconstituted in CHO cells. (A) CHO cells normally do not respond to PMA. CHO cells overexpressing integrin-αIIbβ3, PKC-α, and talin responded to PMA, resulting in αIIbβ3 activation. (B) CHO cells do not respond to thrombin or related peptide agonists, CHO cells overexpressing αIIbβ3, PAR, and talin responded to thrombin receptor peptide agonist, leading to activation of Rap1, formation of a Rap1-RIAM-talin ternary complex, and αIIbβ3 activation.
Agonist-induced inside-out integrin signaling system reconstituted in CHO cells. (A) CHO cells normally do not respond to PMA. CHO cells overexpressing integrin-αIIbβ3, PKC-α, and talin responded to PMA, resulting in αIIbβ3 activation. (B) CHO cells do not respond to thrombin or related peptide agonists, CHO cells overexpressing αIIbβ3, PAR, and talin responded to thrombin receptor peptide agonist, leading to activation of Rap1, formation of a Rap1-RIAM-talin ternary complex, and αIIbβ3 activation.
Platelets and megakaryocytes express protease-activated receptors (PARs), a family of G protein-coupled receptors activated by thrombin and thus can respond to agonists, such as thrombin or other proteases. To extend the reconstruction of intracellular signaling, CHO cells expressing αIIbβ3 were engineered to express talin and PAR1.31 Stimulation of these cells with PAR agonist peptide resulted in increased αIIbβ3 activation (Figure 2B). By monitoring the response to agonist stimulation when certain signaling components are depleted by siRNA, this reconstituted system can assess the required components in the agonist-induced signaling pathway. Indeed, knockdown of either Rap1a or RIAM inhibited αIIbβ3 activation induced by PAR agonist,31 confirming the importance of Rap1a and RIAM in inside-out integrin signaling29,59,60
These 2 CHO cell-based systems recapitulate agonist-induced integrin activation; however, there remain technical limitations. For example, because talin is overexpressed, basal integrin activation is elevated in these cells. Thus, the integrin activation response to agonist stimulation is not as robust as that observed in the platelets. Further engineering will be required to provide precise control of expression of each component (the integrin, talin, the agonist receptor) and of other components in the pathway for this powerful technology to realize its full value and to come into more widespread use.
Reconstruction of inside-out integrin activation in a purified system
Although reconstituted cellular systems have been valuable tools to understand integrin inside-out signaling, they have their limitations. First, a limited number of exogenous components can be introduced into the cells. As more cDNAs and siRNAs are introduced to study the complex signaling network, cotransfections become inefficient. Second, as more exogenous components were inserted into the genome to construct stable cell lines, the risk of disrupting function of endogenous genes increases. Third, these studies are subject to contributions of unknown factors in the cellular milieu. Clearly, a reconstituted in vitro inside-out integrin activation system using purified proteins will be more tractable, can avoid these problems of cell-based systems, and will also allow more quantitative analysis of signaling pathways.
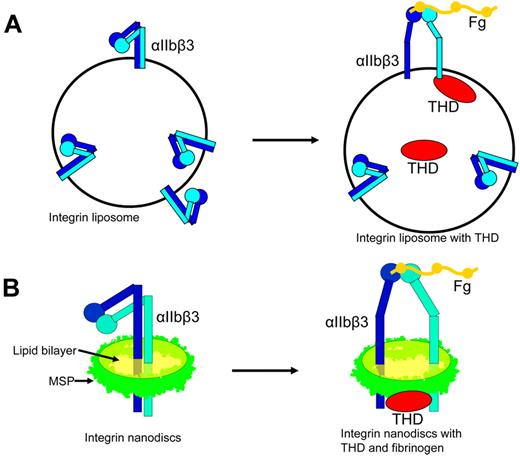
Two such in vitro reconstructions of inside-out integrin activation have recently been developed. The first such system was developed with reconstituted integrin liposomes. Integrin liposomes are normally reconstituted by mixing detergent-solubilized integrin and lipids and then removing the detergent with either biobeads or dialysis.61-64 Because the integrins are inserted into liposomes with random orientation, approximately half of the integrins face outside and are accessible to ligands for affinity measurements. Integrin regulators, such as talin, can be incorporated inside the liposome when added to the lipid protein mixture before removing the detergent. The talin inside the liposome can bind to the cytoplasmic tail of the outside-facing integrins, and the resulting change in integrin affinity can be measured by ligand binding using flow cytometry43 (Figure 3A). In this purified system, THD activated integrin-αIIbβ3, establishing that talin alone is sufficient to activate integrins and that the integrin must be inserted into a lipid bilayer for activation to occur (Figure 3A). Mutations that disrupt either talin-integrin tail interaction or talin lipid contacts blocked the ability of talin to activate integrins, confirming the requirement for binding of talin to integrin tails and lipids in inside-out activation.43 Some semiquantitative data are obtainable in this system. For example, Coomassie-stained SDS-PAGE gel revealed that the talin to integrin ratio is about 2:1, approximating that in platelets.
Complete reconstruction of integrin inside-out activation with liposomes and nanodiscs. (A) Incorporation of THD into integrin liposomes resulted in activation of the externally oriented αIIbβ3. (B) Integrin-αIIbβ3 in nanodiscs is in an inactive bent conformation. Addition of THD resulted in the activation of αIIbβ3 leading to increased binding affinity for its ligand and a shift toward the extended conformation.
Complete reconstruction of integrin inside-out activation with liposomes and nanodiscs. (A) Incorporation of THD into integrin liposomes resulted in activation of the externally oriented αIIbβ3. (B) Integrin-αIIbβ3 in nanodiscs is in an inactive bent conformation. Addition of THD resulted in the activation of αIIbβ3 leading to increased binding affinity for its ligand and a shift toward the extended conformation.
In the second strategy, integrins were inserted into the phospholipid nanodiscs. Nanodiscs are discoid lipid bilayers of 10 to 13 nm in diameter encircled by engineered apolipoprotein A1 (termed membrane scaffold protein [MSP]).65 The integrin nanodiscs have both the cytoplasmic tail and extracellular domains accessible. Thus, one can add integrin regulators and assess the changes in integrin affinity in simple in vitro assays, such as ELISA43 (Figure 3B). The presence of talin head increased the activation as measured with the activation specific antibody PAC1, confirming that THD is sufficient to activate αIIbβ3 in a purified system (Figure 3B). The activating effects of THD on integrin nanodiscs also required the binding of talin to the 2 sites on integrin-β tail and lipid bilayer, as mutations disrupting any of these interactions inhibited the capacity of THD to activate the integrin nanodiscs.43 The concentration of THD added to the system is known, and half-maximal αIIbβ3 activation was observed at a THD concentration of 700nM, a value in remarkable agreement with the 400nM Kd of talin for integrin-αIIbβ3 measured by dynamic light scattering.43 Thus, the final step of physiologic integrin inside-out signaling has been reconstructed. Studies with integrin nanodiscs of different size formed with various lengths of MSPs (10 nm with MSP1 and 13 nm with MSP1E3)65 showed similar results, although 13-nm integrin nanodiscs were more difficult to separate from empty nanodiscs because of smaller differences in their Stokes radii. To study more complex inside-out signaling complexes, the system can be expanded to include multiple components, potentially allowing in vitro reconstruction of the complex signaling pathways by controlling the composition, ratio, and timing of addition of the regulators. Furthermore, the integrin nanodiscs can be used to study the effects of lipid composition on integrin activation. The lipid composition used in the initial study was determined by a few factors: (1) saturated lipids were used to avoid potential oxidation during a lengthy procedure; (2) lipids were chosen to have a fatty acid chain length as close to natural lipids as possible and to retain a phase transition temperature below room temperature; and (3) the lipids were chosen for high miscibility. However, further studies on the effects of lipid composition may be complicated by limited lipid miscibility66,67 (also see the Avanti Polar Lipids Web site: http://avantilipids.com/index.php?option = com_content&view = article&id = 1701&Itemid = 420), solubility of lipids in detergent, and the varying rate of integrin incorporation to different lipids.
Reconstruction of integrin inside-out activation with nanodiscs also facilitates high-resolution electron microscopy (EM) studies thanks to a specimen thickness at < 100 nm. The EM studies presented a unique opportunity to correlate previous structures of integrin ectodomain, transmembrane domain (reviewed in the next section), and talin-integrin tail complexes with different integrin activation stages during inside-out signaling. The vast majority of the integrins in lipid nanodiscs are in a bent conformation. Addition of THD resulted in a modest increase in the extended conformation to approximately 25% of integrins The rest of the integrins in nanodiscs, although still in the bent conformation, are discernibly different from the bent conformation in the absence of THD. Whether this different form of bent conformation represents a truly different structural state or is a result of different specimen orientation on the EM grid remains to be investigated. On binding to fibrin, the percentage in the extended conformation in the THD-activated integrin nanodiscs further increased.43 These structures can be correlated with bent and extended conformations observed with integrin ectodomains reviewed in the next section. These studies clearly demonstrate the potential of reconstituted purified systems to track integrin structural changes during inside-out signaling.
Exogenous integrin activation reconstructed with activating antibodies, metal ions, and reducing reagents
Exogenous integrin-activating reagents act on the integrin extracellular domains to induce integrin activation regardless of integrin transmembrane and cytoplasmic interactions. They enable (1) structural studies of integrin activation by simply comparing integrin structure in the presence and absence of an activating reagent; and (2) functional studies of activated integrins with purified integrins or integrin ectodomains. The combination of these 2 factors has proven extremely useful in studying the structural mechanisms of integrin activation.
Mn2+ re-created integrin activation
Binding of integrins to their ligands requires the presence of divalent cations,68 and different cations can strikingly alter integrin affinities to fibronectin.62,69 In early studies, Mn2+ produced the most striking increase in α5β1-integrin affinity for fibronectin compared with other divalent cations (Mg2+ or Ca2+). This result has been confirmed with a wide variety of integrins, including α3β1,70 αVβ3,71 αLβ2,72 αIIbβ3,73 α6β1,74 α1β1 and α2β1,75 α4β1,76 and α4β7.77 Subsequently, Mn2+ has been widely used as a positive control for integrin activation.
Mn2+-recreated integrin activation was thought to mimic physiologic integrin activation because both activate integrins in the absence of a bound ligand and induce similar epitope exposure.78 Takagi et al42 first reported that Mn2+ induced a global conformational change of an engineered αVβ3 ectodomain from a bent conformation to an extended conformation. This was supported by both Stokes radius measurements and negatively stained EM images.42 However, a crystal structure of the αVβ3 extracellular domain in the presence of Mn2+ and small peptide ligand revealed a bent conformation similar to the inactive αVβ3 ectodomain structure, with Mn2+ occupying the metal ion-dependent adhesion site, adjacent metal ion-dependent adhesion site, and ligand-associated metal-binding site.33,34 A subsequent EM study using a complex between the αVβ3 ectodomain and its ligand (fibronectin domain 7-10) in the presence of Mn2+ confirmed that the ectodomain can have a bent conformation with physiologic ligand in the absence of crystal contact.39 A later study investigated the global conformational changes of liposome-reconstituted integrin induced by Mn2+ but found that these integrins remain the same height, suggesting that Mn2+ does not induce global structural rearrangement.61 Kim et al studied the effect of Mn2+ on integrins in live cells by measuring the fluorescence resonance energy transfer (FRET) between α- and β-subunits fused at their C terminus to fluorescent proteins.79 Mn2+ alone did not result in altered FRET between the 2 integrin subunits, whereas physiologic activation, such as PMA or overexpression of THD, did.79 In another FRET study with α4β1, Mn2+ reduced the FRET efficiency between fluorescent dyes bound to integrin ectodomain and to the lipid bilayer, indicating a possible large-scale conformational change. However, the changes induced by Mn2+ are different from that induced by chemokine.80
Although Mn2+ can increase the ligand binding affinity of purified αIIbβ3, the activation is not maximal81 and Mn2+ reduced the initial association rates of ligand with integrins αIIbβ3.82 Surprisingly, Mn2+ was unable to support agonist induced platelet aggregation. At 0.5mM Mn2+ concentration, ADP-induced platelet aggregation was almost completely absent.82 In contrast, Mn2+ induced both high level of ligand association and increased initial velocity rates for ligand association with integrin-αvβ3.82 Kamata et al found that swap of αv calf-2 domain to αIIbβ3, a domain with no known cation binding site, can result in a high level of αIIbβ3 activation in response to Mn2+ stimulation.81 A model that Mn2+ disrupts the membrane proximal stalk interface was proposed.81
Thus, it appears that Mn2+–re-created integrin activation has multiple forms depending on the integrin isoforms and the context. It is probable that Mn2+-induced integrin activation is somewhat different from the physiologic inside-out integrin signaling process. The debates about the nature of Mn2+-induced integrin activation and its relevance to physiologic inside-out integrin signaling process will probably continue.
Re-creation of integrin activation with antibodies
It was discovered early on that antibodies generated against integrins can recognize specific epitopes exposed during different integrin activation states. Studies using those antibodies suggested that integrins have at least 3 conformational states: inactive (resting state), activated, and activated and ligand occupied.83,84 It was later found that some of the activation specific antibodies can also drive integrins toward an activated form when used at high concentrations, presumably by binding to integrins and locking them in an activated form. Conversely, some antibodies inhibit integrin activation. A large inventory of such antibodies have been developed for β1, β2, and β3 integrins.85
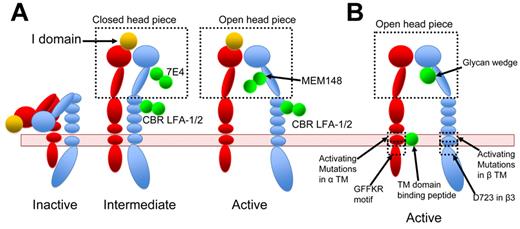
Activating antibodies are convenient tools to exogenously re-create integrin activation. Kim et al reported that an activating antibody against β2, CBR LFA1/2, as well as PMA stimulation, alter FRET between the αL and β2 cytoplasmic tail, whereas Mn2+ alone did not, indicating that antibody–re-created activation might better mimic physiologic inside-out signaling.79 Because antibodies are large molecules and can greatly facilitate molecular imaging, they also presented opportunities to correlate structure and function in integrin activation. Nishida et al obtained negative stained EM images of αXβ2 ectodomain in complex with CBR LFA1/2.41 These EM images, with both integrin density and antibody density clearly visible, established that αXβ2 is in an extended conformation when bound by the activating antibody.41 A subsequent study from the same group further correlated the affinity states of αXβ2 enforced by multiple antibodies and the conformations of αXβ2 integrin determined in EM images. Different combinations of antibodies allowed the authors to re-create multiple distinctive conformations and functional states: bent low affinity state, intermediate affinity state of extended αXβ2 with either open or closed head piece, and high affinity state of extended αXβ2 with open head piece40 (Figure 4A). The advantages of integrin activation recreated through antibodies rather than through Mn2+ were demonstrated in the study, as combination of various stimulatory and inhibitory antibodies created a spectrum of integrin activation states.
Integrin activation by various means. Red represents α-subunit; and blue, β-subunit. Activating agents are shown in green. (A) αLβ2-Integrin activation recreated by antibodies. CBR LFA-1/2 locks β2-integrin in extended conformation. MEM148 stabilizes the head piece in an open active conformation, whereas 7E4 locks it in a closed inactive conformation. Using a different combination of antibodies, Chen et al stabilized integrins in inactive, intermediate, or active state.40 For a complete description of antibodies, the reader is referred to Byron et al.85 (B) Integrin activation induced by synthetic peptides that bind to integrin TMD, by mutations, or by a glycan wedge.
Integrin activation by various means. Red represents α-subunit; and blue, β-subunit. Activating agents are shown in green. (A) αLβ2-Integrin activation recreated by antibodies. CBR LFA-1/2 locks β2-integrin in extended conformation. MEM148 stabilizes the head piece in an open active conformation, whereas 7E4 locks it in a closed inactive conformation. Using a different combination of antibodies, Chen et al stabilized integrins in inactive, intermediate, or active state.40 For a complete description of antibodies, the reader is referred to Byron et al.85 (B) Integrin activation induced by synthetic peptides that bind to integrin TMD, by mutations, or by a glycan wedge.
Re-creation of integrin activation with synthetic peptides directed against TMDs
Earlier mutational studies on the effects of G708N substitution, in the midpoint of the β3 transmembrane domain (TMD), suggested that oligomerization of integrin-β3 TMD can shift integrin conformational equilibrium toward the activated form.86 Subsequently, homodimerization of αIIb TM domain was also reported, and addition of αIIb TM domain peptide to platelets induced integrin activation and platelet aggregation.87,88 Later work also raised the possibility that αIIb TMD peptide might activate αIIbβ3 by competing with the αIIb subunit for β3 TMD binding.89 The earlier works established the concept of disrupting α-β interaction with a synthetic peptide. Yin et al further developed this concept by showing that computationally designed αIIb TMD-binding peptides induced platelet aggregation, whereas αv binding peptides activated integrin-αvβ3.90 These peptides were proposed to disrupt integrin-α-β interaction by binding to a site in the αIIb or αV TM helix that physically blocks their interaction with the β TMD (Figure 4B). Thus, these peptides could be a convenient tool to enforce the activated integrin conformation.
Re-creation of integrin activation with RGD peptides or reducing reagents
Integrin activation can also be re-created by reducing reagents, such as dithiothreitol (DTT).91 Compared with Mn2+, DTT is a more potent integrin activator and is normally irreversible.92 This too has become widely used as a positive control for the highest level of integrin activation and as a way of rescuing integrin activation exogenously. DTT induces a ligand-occupied conformation in β1,78 and in α4β1 DTT-induced integrin activation may be independent of and additive to agonist-induced integrin activation.93 The role of endogenous reducing agents, analogous to DTT, in physiologic integrin activation remains a question of current interest.
Integrin activation can also be recreated with small peptide ligands. Preincubating resting αIIbβ3 with an RGD peptide and then removing the peptide ligand through dialysis leave αIIbβ3 in a high-affinity state for fibrinogen.94 Several structural studies have tried to take advantage of this phenomenon to re-create and capture activated integins bound to small peptides or a peptide mimetic ligand.32,34 But small peptide ligands can bind to both activated and resting integrins in some contexts and be activation sensitive in others. For example, integrin binding of polyacrylonitrile beads conjugated with RGD can be activation sensitive depending on the spacer length between RGD and the beads. A Gly-Gly-Gly linker between the beads and RGD peptide resulted in selective binding of high-affinity integrins, whereas a longer linker resulted in nondiscriminative binding.95 Small peptides can induce ligand-induced binding sites of integrins on the cell surface83 and cause molecular extension of engineered αvβ3 ectodomain constructs.42 Thus, RGD peptide binding can be either activation dependent or activation independent, and one needs to independently validate the intended activation state (ie, with an activation specific antibody) when using small peptide ligands to re-create integrin activation.
Integrin activation re-created with integrin mutations
Mutational studies have guided research efforts in integrin inside-out signaling. The idea that integrins are regulated from inside-out was suggested in mutational studies where mutation of the conserved αIIb cytoplasmic tail GFFKR motif strongly activated αIIbβ3,96 α2β1,97 and αLβ298 (Figure 4B). Chimeric integrins, such as αIIbα5β3, where αIIb cytoplasmic tail has been replaced by that of α5, are also constitutively active96,99 in certain cells. Thus, cytoplasmic domains are important to maintain integrins in a resting state.
Mutations have also been identified in integrin-β cytoplasmic tails that can re-create or abolish integrin activation. Although chimeric integrins, such as αIIbα5β3, are constitutively active, their activation depends on the presence of integrin-β tails as these active integrins revert to a resting state with the truncation of β-tails.99 Thus, integrins are regulated by both α- and β-cytoplasmic tails. Integrin-α subunits have an Arg at the end of conserved GFFKR motif (Arg995 in αIIb), which can electrostatically interact with an Asp in the opposing integrin-β subunit (Asp723 in β3) at the membrane interface (Figure 4B). Integrins can be activated by mutating either of the 2 interacting residues, whereas combined charge reversals at both positions preserved the low-affinity activation state,100 a result later confirmed in β2-integrins.98 Thus, the importance of α- and β-cytoplasmic domains and their interactions as an inside-out regulatory mechanism were established by re-creating different integrin activation states with integrin mutations.
Mutational studies in the integrin TMDs added to our understanding of inside-out integrin regulation. Substitution of β3 Gly-708 in the TMD to bulky amino acids, such as Asn86 or Ile,101 activates integrin-αIIbβ3. Gly residues are also found in the GXXXG motif of α-subunits in close apposition to the β-subunit Gly708. Mutating either Gly residue in the GXXXG motif to bulky amino acids also resulted in a constitutively active integrin.101,102 These mutational data suggest that αβ-helical packing centered on the Gly residues is important to keep integrins in a resting state. An integrin-αIIbβ3 TMD structure confirmed this interaction interface in integrin TMD37 and was termed the “outer membrane clasp.” Another packing interface was also found between the 2 Phe residues in the αIIb GFFKR motif and the β3 Trp715 by both mutagenesis and NMR37,100 ; this packing interface in combination with the aforementioned αIIb(R995)-β3(D723) electrostatic interaction contributes to the formation of an “inner membrane clasp.” No structures of the mutation-re-created active integrins are currently available, but many of the mutations disrupt the α- and β-transmembrane and cytoplasmic domain interactions, which are a central mechanism of inside-out activation induced by agonist stimulation and talin binding.79,89
Many mutations in the extracellular domain that can activate integrins have also been reported. Approximately half of the integrins have an α-subunit I domain (or A domain) that contains a ligand-binding site. For integrins without I domain, ligand binding occurs in an I-like domain in the β-subunit and at the interface between α- and β-subunit in the integrin head piece.103 Structural studies revealed 2 conformation for I domain: an open active conformation and a closed inactive conformation.104-106 Corresponding active open and inactive close conformations have also been observed in integrins without an α-subunit I domain.32,107,108 Luo et al introduced a glycosylation site at Asn303 through a mutation of Asn305 to Thr in β3.109 The bulky N-glycan was thought to act as a wedge to stabilize the active open head piece conformation109 (Figure 4B). Indeed, the glycan wedge mutation resulted in constitutive activation of αIIbβ3, αVβ3, and α5β1. Activation mutations can also be found in the I domains. Mutation F302W in αM I domain stabilized the open conformation of I domain and conferred ligand-binding activity to αMβ2,110 although the degree of activation was debated.111 The corresponding mutation in α2 I domain, E318W112 or E318A,97 also increased the activation of α2β1-integrin. Others have successfully used computational methods to design mutations that can stabilize the active open conformation of αM I-domain or αIIbβ3 head piece.111,113 In a different strategy, engineered disulfide bond in I domains through cysteine mutation has been used to lock the αL and αM I domains into closed or open conformers with low or high affinity for ligand.114-117 Disulfide bonds in β3 have also been engineered to lock αIIbβ3 into an inactive or activated state.118 Similarly, a disulfide bond locking αIIb into the bent form reduced αIIbβ3 activation in response to certain activating antibodies that bind to αIIb (antibody PT25-2).119 Thus, integrin activation re-created by extracellular domain mutations have provided insights into the structural mechanism of integrin activation.
Perhaps the most informative mutations are those loss-of-function mutations that abolished inside-out integrin signaling. A naturally occurring Ser752 to Pro mutation11,99 and a truncation mutant at residue 724 in β3 tails10 disrupted integrin inside-out signaling. Later work in reconstructed systems showed that the S752P mutation disrupted kindlin binding,51,57 whereas truncation of β3 tails at 724 eliminated both talin and kindlin binding sites.38
In conclusion, great progress has been made in understanding and reconstructing inside-out integrin activation, but there remain unanswered questions. As work in genetic and cell-based integrin activation studies has shown, inside-out signaling is a complex process involving a number of signaling and adaptor molecules. Reconstruction experiments have indicated that PKC signals via Rap1a, which in turn binds the adaptor protein RIAM and forms a Rap1-RIAM-talin complex targeting talin to plasma membrane.29-31 Talin and probably kindlin are the final players that bind to integrin tail, trigger a conformational change of the integrins, and switch the integrins to a high-affinity state.4,120 Reconstitution of the whole system in vitro using purified components would put our understanding of the pathway to the ultimate test but will require careful engineering and the testing of numerous experimental conditions. So far, we have synthetically reconstructed integrin inside-out signaling with THD, integrin, and ligand (fibrin, PAC1).43 The basic building blocks and protocols have been established to completely reconstruct the agonist-stimulated inside-out signaling complexes in the future. Complete reconstitution would also create opportunities to obtain high-resolution molecular models by subjecting the reconstituted signaling complex to EM imaging. The era of engineering and re-engineering of integrin activation pathways is on us and holds the promise of a much deeper understanding of this process of critical importance in the functions of cells of the blood and vasculature.
Acknowledgments
The authors thank Dr Brian Petrich for reading the manuscript and for his valuable suggestions.
F.Y. was supported by the American Heart Association (postdoctoral fellowship 09POST2180011). Work described from the laboratory of M.H.G. was supported by HL 57900, HL 078784, and AR 27214 from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: F.Y., C.K., and M.H.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark H. Ginsberg, Department of Medicine, University of California–San Diego, 9500 Gilman Dr, La Jolla, CA 92093; e-mail: mhginsberg@ucsd.edu.
References
Author notes
F.Y. and C.K. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal