Abstract
Acute and chronic graft-versus-host disease (GVHD) are potentially lethal complications after stem cell transplantation (SCT). Steroids are the appropriate first-line treatment for both. However, if patients do not adequately benefit from steroid therapy, mortality is high and standardized treatment algorithms are lacking. This is mainly because of limited data from prospective, randomized clinical trials. In addition, most of the available treatment options only induce clinical benefits in a limited proportion of patients. Thus, there is an urgent clinical need to develop more potent immunosuppressive treatment strategies for patients suffering from acute or chronic steroid-refractory GVHD while maintaining the graft versus tumor effect to avoid a potential rise in relapse-related mortality. The increasing knowledge about host- as well as donor-derived variables favoring GVHD development and the increasing armamentarium of immune-modulatory agents entering preclinical and clinical research will probably allow more effective treatment of GVHD in the future. This review describes novel developments in the treatment of steroid-refractory GVHD, with a special focus on the rationale behind promising pharmacologic compounds or up-coming cellular therapies.
Introduction
In 1957, E. D. Thomas and colleagues first described the infusion of bone marrow cells into patients after prior radio- or chemotherapy in their seminal New England Journal of Medicine paper.1 This work initiated 5 decades of basic and clinical research in the field of stem cell transplantation (SCT) and nowadays SCT is the treatment of choice for many malignant and benign hematopoietic diseases. It was known before 1957 from preclinical animals studies that tranplantation of splenocytes from noncongenic donor strains, while facilitating hematopoietic recovery, induced a severe illness, characterized by progressive weight loss, hunched posture, and diarrhea.2 In the following years it has become clear that this was not primarily because of the conditioning therapy, but that it was an immune-mediated syndrome, which is now referred to as graft versus host disease (GVHD). GVHD nowadays remains not only a major cause of non-relapse mortality, but also induces substantial morbidity, which can severely affect quality of life. GVHD is a very complex immunologic disorder characterized by a plethora of clinical presentations.3 Importantly, the predominant use of peripheral blood stem cells (PBSC) rather than bone marrow (BM) and the increasing proportion of reduced intensity conditioning (RIC) regimens has made multifacetted chronic GVHD (cGVHD) more and more clinically relevant.4,5 The incidence of GVHD thus depends on several variables, including the donor type (related versus unrelated, matched versus mismatched or haploidentical), the type of conditioning (total boby irradiation [TBI] versus nonTBI), the donor's sex (female or male, versus all other sex combinations) and the stem cell source (PBSC versus BM).6 Despite the increasing importance of cGVHD in clinical practice, preclinical animal cGVHD models are scarce. This is in contrast to acute GVHD (aGVHD), which can be adaquately described by several animal models7 enabling researchers to study its immune pathogenesis and test novel treatment approaches.
Steroids are still the first-line GVHD treatment8 and patients not responding to steroid therapy are at high risk of dying from GVHD or its related complications.9 Currently, there is no common standard treatment strategy for steroid-refractory GVHD patients. This review will focus on novel developments in the prevention of GVHD development and the treatment of steroid-refractory GVHD, with a special focus on promising innovative compounds as well as on up-coming cellular therapies.
Pharmacologic approaches
Modification of conditioning-induced tissue damage and danger signals
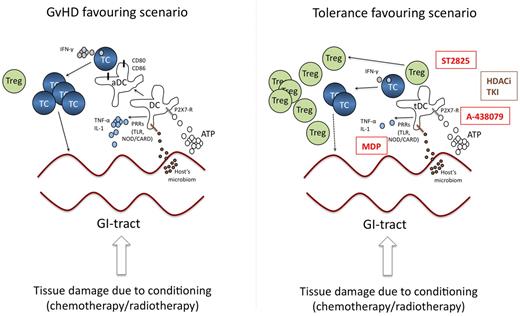
Conditioning of the recipient before the donor's stem cells are transferred is usually done by chemo- or radio-chemotherapy, both of which induce significant tissue damage. This results in the accumulation of “danger signals,” which are signals released from injured, stressed, or dying cells and which in turn activate antigen-presenting cells.10 The gastrointestinal (GI) tract is a predisposed organ for the development of aGVHD, because tissue damage induces partial disruption of the barrier function between the sterile submucosal area and the colonized intraluminal space allowing the transit of bacteria. In addition, local release of danger signals from activated T cells11 may activate innate and adaptive immune cells. Both, bacterial components and extracellular danger signals transmit their signals via toll-like receptors (TLRs) or via NOD-like receptors expressed on innate immune cells.12 Zeiser and colleagues elegantly demonstrated in a murine aGVHD model that high levels of adenosine triphosphate (ATP), an important extracellular danger signal released by dying cells, can be detected in the GI tract of animals receiving TBI and in the peritoneal fluid of patients receiving TBI before SCT.13 ATP induces higher expression of the costimulatory molecules CD80 and CD86 on antigen-presenting cells (APC) which leads to a more efficient priming of allo-specific T cells. The receptor for ATP on recipient's APC is P2X7R. This receptor has been described to activate the inflammasome, thereby also allowing more potent allo-stimulatory T-cell priming. Accordingly, pharmacologic blockade of P2X7R or reconstitution of animals with P2X7R-deficient APC before SCT substantially reduced the incidence of GVHD, increased the number of regulatory T cells (Treg) and decreased IFN-γ producing effector T cells.13 These observations underscore the importance of ATP-mediated danger signaling in tipping the balance towards a more allo-reactive and pro-inflammatory milieu. SNP data from 152 patients who underwent matched sibling allogeneic SCT support the clinical relevance of these findings. Here, overall survival was significantly shorter in recipients carrying the CC genotype in the P2X7R gene and in patients receiving stem cells from donors carrying the CC genotype. The CC genotype was also identified as an independent prognostic factor for overall survival.14 Thus, inhibition of P2X7R signaling by targeted drugs (such as A-438079) might represent a promising strategy for preventing aGVHD caused by tissue damage during conditioning (Figure 1).
Targeting danger signaling and APCs. The left panel highlights the central role of danger signals and APC activation during GVHD. Tissue damage induced by the conditioning regimen induces the release of ATP and transit of bacteria/bacterial components, which in turn induces activation of DC (aDC) leading to priming of allo-reactive T cells. The right panel illustrates potential strategies interfering with these processes of APC activation to inhibit or prevent GVHD development. Promising strategies include TLR-inhibition by MyD88-inhibitors (ST825), APC modification by HDACi or TKI or blockade of ATP-signaling by P2X7-inhibitors (A-438079). Moreover, stimulation of APC by MDP might induce a tolerogenic APC-phenotype. Compounds in clinical testing are shown in brown, promising strategies to be tested in the future in humans are given in red. TC indicates T-cell; DC, dendritic cell; aDC, activated DC; Treg, regulatory T-cell; PRR, pattern recognition receptor; TLR, toll-like receptor; HDACi, histone deacetylase inhibitor; TKI, tyrosine kinase inhibitor; NOD/CARD, nucleotidebinding oligomerization domain containing/caspase recruitment domain; tDC, tolerogenic DC; and MDP, muramyl-dipeptide.
Targeting danger signaling and APCs. The left panel highlights the central role of danger signals and APC activation during GVHD. Tissue damage induced by the conditioning regimen induces the release of ATP and transit of bacteria/bacterial components, which in turn induces activation of DC (aDC) leading to priming of allo-reactive T cells. The right panel illustrates potential strategies interfering with these processes of APC activation to inhibit or prevent GVHD development. Promising strategies include TLR-inhibition by MyD88-inhibitors (ST825), APC modification by HDACi or TKI or blockade of ATP-signaling by P2X7-inhibitors (A-438079). Moreover, stimulation of APC by MDP might induce a tolerogenic APC-phenotype. Compounds in clinical testing are shown in brown, promising strategies to be tested in the future in humans are given in red. TC indicates T-cell; DC, dendritic cell; aDC, activated DC; Treg, regulatory T-cell; PRR, pattern recognition receptor; TLR, toll-like receptor; HDACi, histone deacetylase inhibitor; TKI, tyrosine kinase inhibitor; NOD/CARD, nucleotidebinding oligomerization domain containing/caspase recruitment domain; tDC, tolerogenic DC; and MDP, muramyl-dipeptide.
During this early process of danger signaling other nonpurinergic signaling cascades might be as important as free ATP. Among these, pattern recognition receptors (PRRs) sensing microbial components such as lipopolysaccharide (LPS) or flagellin15 and extracellular matrix components16 are important as well. Genetic association studies linking TLR4 polymorphism (Thr399Ile) in patients and donors with an increased the risk for severe aGVHD17 suggest an important role for TLRs in allo-SCT. Moreover, cotreatment of animals with TLR9 agonistic CPG oligonucleotides aggravated GVHD lethality because it increased host-derived IFN-γ production.18 Functional data using mice deficient in the TLR signaling components MyD88 and TRIF or in TLR9 support the role of TLRs in GVHD development.19 Deficiency in TLR9 may even led to increased survival.19 Thus, preclinical testing of novel MyD88-inhibitors such as ST282520 might be a rational approach to modify microorganism-mediated TLR activation during GVHD (Figure 1).
In addition to TLRs, NOD/CARD15 also regulates GVHD pathogenesis by sensing bacterial products. Polymorphisms of NOD/CARD15 are linked to GVHD pathogenesis.21 Single nucleotide polymorphisms (SNPs) resulting in reduced function of NOD/CARD15 induce a hyperactive phenotype of APC,22 which might contribute to hyperactivation of allo-reactive T cells during GVHD. In line with this idea, NOD2-deficiency in mice induces an increased allo-priming of T cells.23 Thus, inter-individual differences in the sensitivity toward pathogen-associated microbial patterns (PAMPs) as a consequence of SNPs probably play a role in the deregulation of the immune system during allo-reaction/GVHD. As a consequence, actual concepts transferred from models for inflammatory bowel disease24 are testing the value of continuous NOD2 stimulation via its cognate ligand muramyl-dipeptide to prevent GVHD (Figure 1).
Targeting recipient and donor type APC
The previous topic of danger signal-mediated regulation of inflammatory responses already highlighted the important role of APC as central regulators of allogeneic T-cell priming. Host APC survive for a considerable time within the recipient and are very potent allogeneic T-cell activators because they express minor histocompatibility antigens (miHA) that induce allo-miHA-specific T-cell activation. Even after sublethal irradiation, 30% of host dendritic cells (DC) are still present in the spleen 24 hours after challenge,25 allowing rapid activation of donor T cells. Because of their high degree of radioresistance, tissue-specific DCs including Langerhans cells (LCs) in the skin undergo a markedly less dynamic turnover and can still be found months after SCT.26 LCs are predominatly replaced in case of an ongoing skin GVHD.27 After replacement of the DC compartment by donor-derived DCs, CD8+ T cells are primarily activated via crosspresentation of miHA by CD103+ or CD8+ DCs. There are various approaches to target DCs or DC function to modify the course of GVHD. Notably, currently used immunosuppressive agents including cyclosporin, mycophenolate mofetil and steroids also modify DC function.28-30 However, more innovative strategies have been proposed in more recent reports. An interesting and clinically feasible strategy is the in vivo tailoring of DCs into a tolerogenic phenotype. Targeting the process of histone acteylation for example appears to be a very interesting approach not only for direct elimination of malignant T cells, but also for immune-modulation. We could recently demonstrate that histone deacetylase inhibitors (HDACi) skew DC differentiation by preventing the acquisition of the DC hallmark CD1a as well as by affecting the expression of costimulatory and adhesion molecules.31 The observed defects in DC function on exposure to HDACi obviously reflect impaired signaling through nuclear factor-kappa B, IRF-3 and IRF-8.31 In addition, HDACi exert substantial effects on the APC-induced cytokine storm during GVHD (that is, reduced TNF-α, IFN-γ, IL-1β, and IL-12 levels) and they impair TLR-induced expression of CD40 and CD80 as well as the APC's allo-stimulatory capacity.32 In line with these in vitro effects, injection of in vitro HDACi-treated DCs reduced experimental GVHD in murine SCT models, mainly because of induction of IDO, a well known suppressor of DC and T-cell function.32
Moreover, HDACi promote Treg expansion, as FoxP3 interacts with HDAC 7 and 9, which maintain the Treg phenotype, and with the histone acetyltransferase TIP60.33 Accordingly, mice exposed to the HDACi trichostatin A have increased numbers and functionally improved Treg, increasing donor-specific allograft tolerance.34 Thus, the impact of HDACi on DCs as well as on the Treg compartment might at least in part explain the promising effects of suberoylanilide hydroxamic acid and ITF 2357 in murine GVHD models.32,33 Together, the available preclinical data suggest that HDACi might have a huge potential for prevention and treatment of GVHD and currently various clinical phase 1/2 trials testing the efficacy of single agent panobinostat or its combination with steroids in the treatment or prevention of aGvHD are ongoing (Figure 1).
Finally, tyrosine kinase inhibitors (TKI) also exert beneficial effects in the treatment of GVHD. The most frequently used TKI is imatinib, which primarily induces responses in sclerodermiformal skin and pulmonary cGVHD.35 The obvious rational for TKIs in cGVHD is based on their inhition of platelet-derived growth factor-receptor (PDGF-R) signaling, which is assumed to regulate tissue scarring triggered by chronic inflammation,36 because patients with cGVHD have been described to exhibit elevated levels of agonistic antibodies binding to the PDGF-R.37 As a result of this finding, imatinib, an inhibitor of PDGF intracellular signaling, has been studied in various patient populations with fibrotic cGVHD. One hundred mg/d imatinib induced a response rate of 79% in a prospective study including 19 patients with refractory cGVHD involving the skin, lung and bowel. Seven of 19 patients achieved a complete remission (CR).38 Toxicity included fluid retention and myelosuppression and only 3 patients discontinued because of toxicity. Another group found a somewhat lower response rate (50%) in a retrospective study including 14 patients with extensive cGVHD of the skin and joints. Here, the median dose was 400 mg/d leading to a higher rate of toxicity and subsequent treatment discontinuation.39
In addition to its well known effects on PDGF-R signaling, we and others have demonstrated that imatinib inhibits DCs and monocyte/macrophage function,40,41 and potentially affects T cells.42 In more detail, imatinib reduces human monocyte-derived DCs differentiation by inhibiting the phosphatidylinositol 3-kinase/Akt and the NF kappa B pathway, which is paralleled by a reduced capacity of TKI-exposed DCs to activate T cells.40 We further extended these finding to monocytes and macrophages, which are similarly characterized by a markedly impaired cytokine response to TLR-agonists in the presence of imatinib.41 Others have provided evidence that imatinib and the second generation TKI nilotinib also inhibit T-cell responses.43 So far, no correlative immunologic data from GVHD patients receiving imatinib are available. However, the immunomodulatory function of TKI might at least in part explain their beneficial effects seen in cGVHD and a more systematic testing of these compounds either as monotherapy or in combination with other treatment modalities (ie, rituximab) in cGVHD is currently ongoing (Figure 1).
Targeting the T-cell compartment
Various CD4+ T-cell subpopulations are involved in the pathogenesis of GVHD. Each individual GVHD manifestation appears to have a distinct T-cell milieu, which is determined not only by the target structures but also by the respective inflammatory environment. The understanding of the role of T-cell subsets in humans is derived from cytokine quantifications in peripheral blood, lesional tissue biopsies or from SNPs in cytokine genes and their association with GVHD.44-46 Murine models allow a more concise description of the functional involvement of CD4+ T-cell supopulations in aGVHD. Donor TH1 cells predominantly mediate GI and hepatic aGVHD.47 When IFN-γ deficient CD4+ T cells are transferred, T cells differentiate into TH2 and TH17 cells which aggravate pulmonary and skin aGVHD. In addition, absence of IL-4 and IFN-γ augments TH17 differentiation inducing skin aGVHD.47 Finally, if cells deficient in IFN-γ and IL-17 are transferred, augmented TH2 differentiation induced idiopathic pneumonia.47 This data supports the model that tissue-specific migration patterns of TH1, TH2, and TH17 cells occur, which mediate organ-specific GVHD. In humans, the picture might be more complex: the TH17/Treg ratio detected in lesional biopsies from the GI tract and skin is positively correlated with severity of aGVHD.48 In addition, sole quantification of TH17 did not show a difference between affected and healthy skin. In contrast, significantly more IFN-γ producing T cells were detected in biopsies from affected areas.48 This data suggest that detection of individual T-cell subpopulations might not be sufficient to reflect the complex immunologic processes in GVHD.
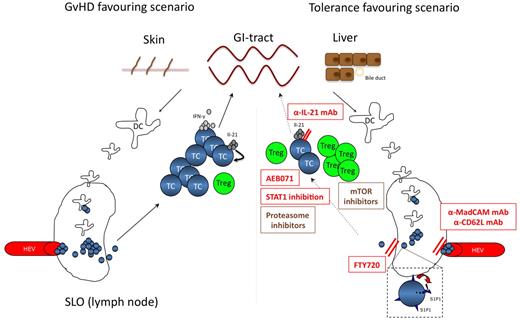
However, it is tempting to speculate that fine-tuning of CD4+ T-cell diversification might influence the course of GVHD. Recent reports emphasized the importance of IL-21 derived from donor T cells for GVHD development.49,50 Disruption of the IL-21 pathway in donor CD4+ T cells markedly impairs development of GI aGVHD while preserving the graft versus tumor effect.50 Surprisingly, disruption of IL-21 signaling had no impact on IL-17 production, impaired IFN-γ secretion in colon-infiltrating T cells and increased TH2 and induced Treg (iTreg).49 Recent reports also emphasized signaling molecules as interesting targets for fine-tuning of T-cell differentiation within the recipient. STAT1 for example interferes with TH1 differentiation, as reflected by an impaired in vivo activation of the cells with subsequent reduction of IFN-γ production.51 Moreover, STAT1 is a natural inhibitor of FoxP3 and its inhibition boosts in vivo iTreg conversion and naturally occurring Treg (nTreg) expansion. Future development of more specific STAT1 inhibitors will allow pre-clinical testing of this concept in murine GVHD models (Figure 2).
Targeting the T-cell compartment. The left panel highlights the central role of T-cell activation within SLO for GVHD initiation. APC activate and expand allo-reactive T cells, which then migrate to target organs. Secretion of pro-inflammatory cytokines, such as IFN-γ and IL-21 are hallmarks of a sustained alloreactive T-cell response. In this GVHD-favouring scenario, the balance within the T-cell compartment is on the effector T-cell side. The right panel depicts potential strategies inhibiting T-cell activation or SLO occupancy to inhibit or prevent GVHD development. Antibodies inhibiting SLO entry might help to prevent T-cell priming, whereas blockade of T-cell exit by FTY720 inhibits the migration of GVHD-mediating T cells to GVHD target organs. Other drugs help to tip the balance toward the Treg compartment, such as mTOR inhibitors, STAT1 inhibitors or antibodies blocking IL-21. The activation process of T cells can also be directly inhibited by PKC-inhibitors (eg AEB071). Compounds in clinical testing are shown in brown, promising T-cell targeting strategies to be tested in future clinical trials are given in red. TC indicates T-cell; Treg, regulatory T-cell; HEV, high endothelial venules; mTOR, mammalian target of rapamycin; mAb, monoclonal antibody; MadCAM, mucosal addressin cell adhesion molecule; and SLO, secondary lymphoid organ.
Targeting the T-cell compartment. The left panel highlights the central role of T-cell activation within SLO for GVHD initiation. APC activate and expand allo-reactive T cells, which then migrate to target organs. Secretion of pro-inflammatory cytokines, such as IFN-γ and IL-21 are hallmarks of a sustained alloreactive T-cell response. In this GVHD-favouring scenario, the balance within the T-cell compartment is on the effector T-cell side. The right panel depicts potential strategies inhibiting T-cell activation or SLO occupancy to inhibit or prevent GVHD development. Antibodies inhibiting SLO entry might help to prevent T-cell priming, whereas blockade of T-cell exit by FTY720 inhibits the migration of GVHD-mediating T cells to GVHD target organs. Other drugs help to tip the balance toward the Treg compartment, such as mTOR inhibitors, STAT1 inhibitors or antibodies blocking IL-21. The activation process of T cells can also be directly inhibited by PKC-inhibitors (eg AEB071). Compounds in clinical testing are shown in brown, promising T-cell targeting strategies to be tested in future clinical trials are given in red. TC indicates T-cell; Treg, regulatory T-cell; HEV, high endothelial venules; mTOR, mammalian target of rapamycin; mAb, monoclonal antibody; MadCAM, mucosal addressin cell adhesion molecule; and SLO, secondary lymphoid organ.
A more useful, already druggable target is protein kinase C (PKC)θ. PKCθ is critical for T-cell survival and regulates the T-cell activation threshold.52 Genetic mouse models enabled validation of PKCθ as promising therapeutic target for GVHD prevention.53 When focusing on clinical translation of these findings, AEB071 represents a potent and selective inhibitor of novel and classic PKCs, decreasing IL-2 and IFN-γ production in T cells.54 In preclinical monkey studies, oral AEB071 mono- or combination-therapy prolongs survival of rat heart and kidney allografts.55 Most importantly, AEB071 blocks T-cell activation through mechanisms distinct from calcineurin inhibitors (CNI), the current standard therapy in GVHD prophylaxis and may therefore lack toxicities associated with CNIs. Consequently, AEB071 is currently tested in combination with tacrolimus versus mycophenolate mofetil plus tacrolimus in solid organ transplantion. AEB071 might also be a promising drug for GVHD treatment or prevention after allogeneic SCT in humans (Figure 2).
Another interesting group of novel agents potentially limiting alloreactivity of T cells are compunds interfering with T-cell localization, as T cells enter secondary lymphoid organs (SLO) for T-cell priming and subsequently GVHD target organs to induce tissue damage.56 As an example, FTY720, a sphingosine analog has been shown to prevent GVHD57 by blocking sphingosine 1-phosphate receptor-mediated T-cell egress from SLO normally induced by a sphingosine 1-phosphate gradient from blood and lymph fluid to tissue.58 As an alternative approach, Beilhack and coworkers extensively tested strategies of blocking T-cell access to SLO.59 It is generally assumed that primed T cells are instructed to migrate to the respective target organs.60 In GVHD, lack of specific priming sites did not affect disease severity in the respective target organ and a single priming site is sufficient for aGVHD induction.59 Intriguingly, blockade of SLO access by application of blocking mAbs to MAdCAM-1 and CD62L to splenectomized mice almost completely prevented aGVHD lethality, supporting the importance of SLO occupancy by donor-derived T cells for their priming.59 This data shows that blockade of SLO access might be a rational approach to prevent the development of aGVHD (Figure 2).
Finally, proteasome inhibitors are also a novel group of agents potentially reversing alloreactivity of T cells toward tolerance. Bortezomib, the first in kind proteasome inhibitor widely applied for treatment of multiple myeloma, has been shown in vitro to selectively induce apoptosis in activated alloreactive T cells via caspase activation and cleavage of the antiapoptotic bcl-2 protein.61 Notably, there was no inhibition of the anti-tumor effect in the mouse model,62 although a slight concern regarding possible aggravation of GI symptoms was raised.63 Thus, soon clinical case reports on myeloma patients with progressive disease after allo-SCT followed: Mateos-Mazon et al reported on 5 patients with relapsed myeloma and GVHD treated with bortezomib.64 Four of these patients showed an improvement of their GVHD. Based on these promising results, first clinical studies are being performed including bortezomib as part of the GVHD prophylaxis regimen demonstrating its feasibility and minimal toxicity.65 Randomized trials have yet to be published.
Targeting GVHD-associated neovascularization
Infused allogeneic T cells primarily interact with circulating cells within the blood stream and with endothelial cells lining the inner face of the vessel. The endothelial cell (EC) compartment can be damaged by conditioning regiments, especially by TBI. Because of endothelial damage, host ECs are at least in part replaced by donor-derived endothelium by vasculogenesis.66 Interestingly, some types of ECs (that is, sinusoidal endothelial cells) have been demonstrated to excert immuno-modulatory effects.67 Besides, the vessel endothelium can directly activate alloreactive CD8+ T cells in vitro and in vivo in the absence of classic APC.68 It is conceivable that this first step of allo-activation within the host is an important event for priming and expansion of allo-reactive T cells, which subsequently invade the interstitial space and target epithelial structures. Thus, conditioning-mediated endothelial damage and replacement of EC because of recruitment of endothelial progenitor cells represents an interesting therapeutic target in GVHD. Of note, in cGVHD of the skin the vascularization process is rather insufficient, causing rarification of vessels within the involved areas.69 In aGVHD models, treatment of recipient animals with the anti-vascular endothelial-cadherin antibody E4G10 inhibited neovascularization by donor-derived cells and inhibited GVHD and tumor growth with a subsequent increase of survival rates.70 This study provides a strong rationale for further testing of anti-angiogenic strategies in GVHD.
In addition, vascular endothelial growth factor (VEGF) expression in GVHD is negatively associated with GVHD severity and non-relapse mortality.71 Recent data further demonstrated that angiopoetin-2 and thrombomodulin are increased in steroid-refractory GVHD.72 The correlative data of a negative impact of increased VEGF levels for GVHD are somehow contradicted by pre-clinical results showing that VEGF-R1 or R2 blockade not only aggravated GVHD severity, but also reduces engraftment and increased mortality.70 Thus, blocking the VEGF/VEGF-R appears not to be the appropriate approach for aGVHD treatment, but obviously more pre-clinical and translational clinical data are needed to explore the potential of angiogenesis/vasculogenesis targeting in the management of GVHD, especially because recent data show that various anti-angiogenic compounds targeting the VEGF or PDGF pathways seem to be capable of modulating immune responses.73
Similarly, the anti-angiogenic agent thalidomide has yielded discrepant results: while early studies in cGVHD have been very promising,74 later publications only confirmed an overall response rate around 40% in cGVHD,75 no efficacy in acute GVHD76 and a possibly detrimental effect in GVHD prophylaxis.77 In this context it might not be surprising, that the thalidomide-derivative lenalidomide given within 6 months after allo-SCT, rather than showing protective properties, led to an almost 50% discontinuation rate because of GVHD and was therefore not considered feasible by the authors.78
Targeting or using cellular compartments
Donor Treg infusion (DTI)
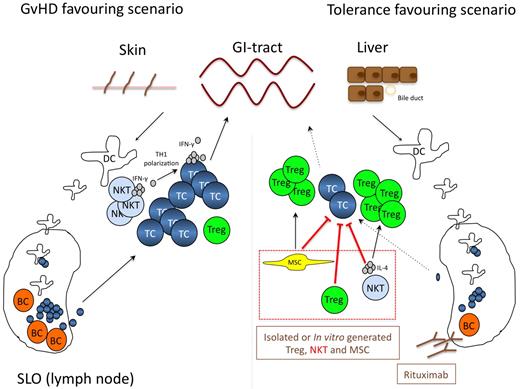
Natural occurring CD4+CD25+ Treg tip the balance between auto- and tumor-immunity. Treg also regulate allo-reactivity in vitro and in vivo.79 In murine models adoptively transferred Treg prevent the development of aGVHD without affecting the graft versus tumor response.80 Accordingly, Treg content in the graft or the donor's blood correlates with aGVHD incidence after HLA-identical sibling SCT.81,82 Of note, relapse-related mortality or infections were not increased in patients receiving grafts with high Treg numbers. As a consequence, recently the first clinical trial of adoptive Treg transfer for prevention of aGVHD was reported.83 Twenty-eight patients undergoing haploidentical stem cell transplantation received freshly isolated donor Treg on day −4, which was then followed by transfer of highly purified CD34+ stem cells together with conventional T cells. Patients did not receive any prophylactic immunosuppression. For safety reasons of this pilot trial, patients received only 25% conventional T cells compared with Treg. None of the first 4 patients developed aGVHD, and therefore, conventional T cells were escalated to 50% of the Treg cell dose. Rapid and stable engraftment was seen and only 2 patients developed grade II aGVHD. So far, none of the patients has developed cGVHD. When compared with a dataset of 152 patients receiving haploidentical SCT without Treg transfer, the approach rather promoted lymphoid reconstitution and improved immunity to opportunistic pathogens. Fourteen patients died during the study and only 1 patient in this high risk patient population died from relapse. The study is small, the median follow-up is short and thus the data too premature to draw definite conclusions. However, DTI appears to be feasible and safe in terms of GVHD prevention. Randomized studies are now needed to test the preventive as well as the therapeutic potential of DTI and to evaluate optimized application schedules (Figure 3).
Cellular therapies including MSC, Treg, NKT-, and B cell targeting. The left panel highlights the central role of various cell types including B and NKT cells for effector T-cell activation during GVHD. In this GVHD favoring scenario NKT-cell–derived IFN-γ and B cells function as T-cell activators, while Treg expansion is suppressed. The right panel depicts potential strategies how this processes can be manipulated to favor a more tolerogenic environment. Adoptive transfer of in vitro generated or donor-derived Treg, MSC or NKT cells either directly inhibits effector T-cell activation and expansion, or indirectly tips the balance toward a tolerogenic milieu by inducing Treg expansion. Moreover, cellular compartments known to be involved in the activation of alloreactive T cells, such as B cells can be depleted by monoclonal antibodies directly targeting B cells (ie rituximab). Therapeutic strategies currently in clincial testing are shown in brown, preclinical concepts in red. MSC indicates mesenchymal stem cell; Treg, regulatory T-cell; NKT, natural killer T-cell; BC, B cell; DC, dendritic cell; TC, T-cell; and SLO, secondary lymphoid organ.
Cellular therapies including MSC, Treg, NKT-, and B cell targeting. The left panel highlights the central role of various cell types including B and NKT cells for effector T-cell activation during GVHD. In this GVHD favoring scenario NKT-cell–derived IFN-γ and B cells function as T-cell activators, while Treg expansion is suppressed. The right panel depicts potential strategies how this processes can be manipulated to favor a more tolerogenic environment. Adoptive transfer of in vitro generated or donor-derived Treg, MSC or NKT cells either directly inhibits effector T-cell activation and expansion, or indirectly tips the balance toward a tolerogenic milieu by inducing Treg expansion. Moreover, cellular compartments known to be involved in the activation of alloreactive T cells, such as B cells can be depleted by monoclonal antibodies directly targeting B cells (ie rituximab). Therapeutic strategies currently in clincial testing are shown in brown, preclinical concepts in red. MSC indicates mesenchymal stem cell; Treg, regulatory T-cell; NKT, natural killer T-cell; BC, B cell; DC, dendritic cell; TC, T-cell; and SLO, secondary lymphoid organ.
In vivo Treg expansion—GVHD treatment and prevention by mTOR inhibition
In addition to adoptive Treg therapies, the Treg compartment can also be modified by pharmacologic intervention. We recently demonstrated that expansion of adoptively transferred Treg in vivo is critical for their GVHD suppressive activity.84 The mammalian target of rapamycin (mTOR) functions as rheostat regulating this process. mTOR regulation is a dynamic and oscillatory process characterized by an early down-regulation of the leptin-mTOR pathway followed by a rapid increase, which is a prerequisite for Treg expandability.85 Rapamycin induces in vitro expansion of nTreg,86 which has also recently been used to establish GMP-conform expansion protocols for human CD4+CD25+ T cells.87 Data from murine in vivo SCT models support the observation of Treg-supportive effects of rapamycin88 including increased generation of thymic Treg89 and infiltration into GVHD target organs.90 Of note, Treg expansion by rapamycin preserves the graft-versus- leukemia effect. Very recent data support the importance of iTreg in addition to nTreg to allow complete coverage of autoreactive T-cell clones, thus enabling rescue of foxp3-deficient animals from auto-immunity.91 Interestingly, rapamycin together with IL-2 enables expansion of donor-derived nTreg and conversion of CD25− T cells to iTreg by IL-2, which potently inhibits development of GVHD in vivo.92 Thus, combination of adoptive Treg transfers with in vivo application of rapamycin, or the in vivo application of rapamycin in combination with IL-2 to get higher yields of nTreg and iTreg represents a promising strategy of pharmacologic Treg modification to treat or prevent GVHD (Figure 3).
The clinical potential of mTOR inhibition is emphasized by various data from small clinical trials. As an example, when combined with mycophenolate mofetil and anti-thymocyte globulin (ATG), CNIs can be replaced by sirolimus within the FLAMSA-RIC protocol.93 Using this approach, 21% of patients developed grade II-IV aGVHD, 30% cGVHD and nonrelapse mortality rate was 14%. Of note, in this setting sirolimus did not induce transplant-associated thrombotic microangiopathy (TA-TMA). Seventy-five percent of the patients were alive after a median follow-up of 10 months.93 In contrast, when combined with CNIs, various groups recently reported that sirolimus-based GVHD prophylaxis in patients receiving myeloablative or RIC-SCT induces severe toxicities, such as transplantation associated microangiopathy or sinusoidal obstructive syndrome.94,95
When used for treatment but not prophylaxis of GVHD, sirolimus appears to be a promising agent even in steroid-refractory aGVHD.96 Overall response rate of a recently reported study including 34 patients was 76% with 42% CR in steroid-refractory and 67% of steroid-intolerant patients leading to a 1-year survival of 44%. Sirolimus might even be used as front-line therapy of aGVHD instead of steroids, inducing CR in up to 50% of patients.97 With respect to treatment of cGVHD, a retrospective analysis including 34 patients with severe sclerodermatous cGVHD treated with sirolimus or everolimus (including 12 receiving only mTOR inhibitors) also suggest substantial clinical efficacy in cGVHD.98 The overall response rate was again 76%, including CR and PR in 6 and 20 patients, respectively. Steroids could be tapered and stopped in a significant number of patients. Relevant side effects included hyperlipidemia, impaired wound healing and TA-TMA in 2 patients. After a median follow-up of 723 days, 26 of the 34 patients were still alive. Overall survival at 3 years since initiation of mTOR-inhibitor therapy is 72%. Unfortunately, data on immunologic surrogate providing biomarkers for clinical responses to mTOR inhibition are not available so far. Future studies will have to clarify the role of sirolimus in GVHD treatment and prophylaxis, but combination with CNI should be used with caution, because of increased toxicity and because CNIs antagonize the effects of mTOR inhibitors on the generation of Tregs by inhibiting IL-2 production.
NKT cells
Natural Killer T (NKT) cells express an invariant Vα24 TCR-chain (Vα14 in mice) and secrete large amounts of IL-4 and/or IFN-γ after activation via the T-cell receptor, which recognizes glycolipids presented by the nonpolymorphic CD1 days antigen-presenting molecule.99-101 Various reports provide evidence that both, host- and donor-derived NKT cells protect from GVHD.102-104 This effect primarily depends on NKT-cell secreted IL-4, which is known to drive TH2-polarization of conventional donor-derived T cells,105,106 attenuating their capacity to mediate GVHD.105,107,108 Thus, NKT cells expressing IL-4 can be referred to as another regulatory lymphocyte population. Activation of host NKT cells appears to be crucial for GVHD prevention, as transplantation of CD1 days- and Jα-18–deficient host mice with wild-type transplants allowed graft versus leukemia reaction but failed to prevent GVHD.103 In a murine SCT setting modeling human aGVHD-protective RIC protocols using total lymphoid irradiation and anti-thymocyte globulin (ATG) as conditioning regimen, host NKT cells cooperated with donor-Treg to induce IL-4–dependent in vivo Treg expansion and prevented lethal GVHD.109 Moreover, in mice highly purified NKT-cell infusion protected from GVHD development by limiting T cell–mediated secretion of pro-inflammatory cytokines, such as IFN-γ and TNF-α, while the graft- versus-leukemia effect was preserved.102 This observation underscores the therapeutic potential of donor NKT cell for immune-modulation within the recipient and their potential as cellular immuno-suppressant, possibly in combination with DTI. However, data from transplant models showing reduced GVHD severity in recipients of NKT-deficient grafts,110 demonstrate that donor NKT cells might under certain conditions also contribute to the pathogenesis of GVHD. Thus, further research is needed to determine how NKT cells are optimally modulated to skew the T-cell response toward TH2 cells, as they might also boost GVHD.
Mesenchymal stem cells
The bone marrow contains an adherent bone marrow–derived fibroblast-like cell, which as a consequence of its multipotency (ie, differentiation into adipocytes, osteoblasts and chondrocytes), has been referred to as mesenchymal stem/stromal cells (MSC).111 MSC are potent immune-regulators. This information set the stage for their clinical testing as cellular immunosuppressants in GVHD.112 MSC primarily affect T-cell biology; however, the exact mechanisms of immunregulation are not well defined. MSC-mediated inhibition of T-cell proliferation is assumed to be mediated by several mechanisms including secretion of TGF-ß, HGF, PGE2, galectin, and shaping of DC toward a more tolerogenic phenotype.113 Moreover, MSC express IDO, which in turn depletes the essential amino acid tryptophan leading to an accumulation of the T-cell toxic metabolite kynurenin.114 Recent data demonstrated that MSC also induce Treg.115 Interestingly, it is the possible to transplant third-party MSC without any need for HLA-identity, making this treatment quite convenient. The reason for this phenomenon might be the lack of MHC class I and II and low costimulatory molecule expression.116 Importantly, not all reports confirmed the effects of MSC in treatment of refractory aGVHD,117 but the largest phase 2 trial available so far demonstrated high efficacy of this approach. This trial included 30 of 55 patients achieving complete resolution of steroid-refractory aGVHD.118 Of note, the median dose in this trial was 50% higher than in the negative trial. None of the patients experienced relevant side-effects, especially no increase in infection or relapse rates. This is in line with previous observations that MSC predominantly inhibit allogeneic but not anti-viral T-cell responses in vivo.119 Moreover, complete response was associated with increased overall survival. The currently ongoing phase 3 trial is not yet fully published but will help to define the value of MSC in the treatment of steroid-refractory aGVHD more accurately (Figure 3). Finally, early clinical data also suggest a clincial potential of MSC in the treatment of cGVHD.120
B-cell targeting
B cells have mostly been appreciated for their ability to produce highly pathogen-specific antibodies. However, in recent years physiologic antibody-independent immune functions such as antigen presentation to T cells, immune regulation and cytokine secretion have been investigated.121 Correspondingly, in several autoimmune diseases antibody-independent B-cell functions have been described to play an important role.122 In patients with autoimmune diseases antigen presentation of auto-reactive B to T cells was found.123 Similarly, a dysfunctional B-cell compartment has been detected in cGVHD: Patients with cGvHD have a high number of activated memory (CD27+) B-cells, higher levels of B cell–activating factor of the tumor necrosis family and donor-derived allo-antibodies. Besides, they show a faster B-cell reconstitution.124,125
Although the involvement of B cells in the pathogenesis of aGVHD is less well studied, it is correlated with the number of B cells in the transplant and depletion of B cells resulted in a reduction of aGVHD.126-128 Still, as GVHD is predominantly T cell–mediated, it remains unclear whether occurrence of allo-antibodies represents a bystander effect or is a relevant cofactor.
In the light of the sometimes rapid response of GVHD to B-cell depletion and the serum half-life of immunoglobulins a predominantly antibody-mediated mechanism of GVHD seems unlikely, rendering a direct effect on B cells possible. This is supported by the fact, that other treatment modalities like mycophenolate mofetil, steroids, extracorporal photophoresis and ATG also have direct effects on B cells. ATG, one of the oldest immunosuppressive agents, not only targets T cells but also an array of epitopes expressed by B cells including CD19, CD20 and many others.129 Interestingly, HMG-CoA-Reductase inhibitors have also been demonstrated to inhibit aGVHD130 potentially by targeting B cells among other cellular compartments.131 Rituximab as a B cell–specific agent has shown considerable clinical benefit in patients with auto-immune disease, probably by preventing B cell–mediated T-cell activation in addition to B-cell depletion.132 Correspondingly, several small trials showed clinical effectiveness of rituximab in cGVHD. GVHD of the skin responded better than cGVHD of the visceral organs,133 but successful treatment of cGVHD of inner organs has been reported anecdotally.134 As CD20 is a promising target not only in malignant disease several alternative, humanized or human antibodies such as ocrelizumab, GA101 and ofatumumab are currently being tested and should also be investigated in GVHD. Thus, B cells represent exciting targets for the prevention and treatment of GVHD. The development of B-cell–specific agents in other fields will rapidly offer an arsenal of therapeutics that need to be investigated in the context of GVHD.
Summary and outlook
Acute and chronic GVHD are a potentially lethal complications and still limit the success of SCT in a considerable proportion of patients. Steroids are the current treatment standard for GVHD. However, if it does not resolve and the patient is steroid-refractory, the mortality rate is high. We here reviewed pre-clinical and early clinical approaches representing potential future strategies for treating GVHD. This includes novel pharmacologic agents (eg, HDAC inhibitors, proteasome inhibitors, antibodies targeting IL-21 or adhesion molecules) or up-coming cellular therapeutics such as Treg, NKT, MSC, or B-cell targeting. The recent presentation of promising first clinical data from Treg or MSC therapy support the feasibility of this concept. It remains critical that well designed clinical trials will be performed to define the value of the respective novel treatment strategy in steroid-refractory GVHD.
Acknowledgments
D.W. is supported by the Austrian Science Fund FWF, the OEGHO, and the TILAK. P.B. is supported by DFG and Deutsche Krebshilfe.
Authorship
Contribution: D.W., M.v.L.T., and P.B. wrote the first draft of the paper; A.M.W. wrote the Treg part; M.S. the mTOR section, M.v.B.B. the B-cell part; and S.A.E.H. the APC section; and all authors wrote the final version of the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Brossart, MD, or Dominik Wolf, MD, Department of Hematology and Oncology, University Hospital Bonn, Wilhelmstraße 35, 53111 Bonn, Germany; e-mail: peter.brossart@ukb.uni-bonn.de or dominik.wolf@i-med.ac.at.