Abstract
Thalidomide maintenance has the potential to modulate residual multiple myeloma (MM) after an initial response. This trial compared the effect of thalidomide maintenance and no maintenance on progression-free survival (PFS) and overall survival (OS) in MM patients. After intensive or nonintensive induction therapy, 820 newly diagnosed MM patients were randomized to open-label thalidomide maintenance until progression, or no maintenance. Interphase FISH (iFISH) analysis was performed at study entry. Median PFS was significantly longer with thalidomide maintenance (log-rank P < .001). Median OS was similar between regimens (log-rank P = .40). Patients with favorable iFISH showed improved PFS (P = .004) and a trend toward a late survival benefit. Patients with adverse iFISH receiving thalidomide showed no significant PFS benefit and worse OS (P = .009). Effective relapse therapy enhanced survival after progression, translating into a significant OS benefit. Meta-analysis of this and other studies show a significant late OS benefit (P < .001, 7-year difference hazard ratio = 12.3; 95% confidence interval, 5.5-19.0). Thalidomide maintenance significantly improves PFS and can be associated with improved OS. iFISH testing is important in assessing the clinical impact of maintenance therapy. Overview analysis demonstrated that thalidomide maintenance was associated with a significant late OS benefit. This trial was registered at www.isrctn.org as #ISRCTN68454111.
Introduction
Multiple myeloma (MM) is incurable and relapse is inevitable because of residual disease.1,2 Thalidomide is active in both newly diagnosed3-7 and relapsed or refractory MM.8,9 Its mode of action includes direct apoptotic, antiangiogenic effects, and modulation of the bone marrow microenvironment.10,11 These activities against the myeloma clone suggest that, after induction therapy, either a consolidation block or maintenance therapy with thalidomide may reduce or suppress residual disease, prolonging the disease-free interval and potentially improving survival.
Results of thalidomide maintenance trials are conflicting. After autologous stem cell transplantation, thalidomide maintenance therapy has been associated with improved progression-free survival (PFS).12-16 An overall survival (OS) benefit has not been demonstrated consistently, although a recent meta-analysis did show a trend toward improved OS.17 The outcomes of thalidomide maintenance in specific subgroups of patients, including those defined by cytogenetic characteristics, are also variable.15,16,18 Furthermore, the use and type of relapse therapies also affect the interpretation of maintenance studies. The Medical Research Council (MRC) IX study is a large, randomized study that compared PFS and OS outcomes with thalidomide maintenance versus no maintenance. It included a full iFISH cytogenetic assessment and collation of treatment data at progression.
Methods
Patients
Patients 18 years of age or older with newly diagnosed MM were eligible for inclusion. Exclusion criteria included pregnancy, acute renal failure, asymptomatic myeloma, solitary bone plasmacytoma, extramedullary plasmacytoma, and previous or concurrent active malignancies. A multicenter research ethics committee and local ethics committees of all participating institutions approved the protocol; all patients gave written informed consent in accordance with the Declaration of Helsinki.
Study design
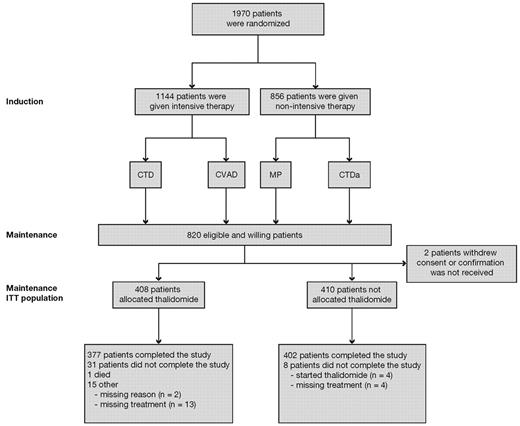
The MRC Myeloma IX study was a multicenter, phase 3, factorial design trial. Patients were assigned to induction treatment via either an intensive or a nonintensive treatment pathway, as determined by performance status, informed discussion, and patient preference (Figure 1). There was no rigid age cut-off.
CONSORT diagram. CTD indicates cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated CTD; CVAD, cyclophosphamide, vincristine, adriamycin, and dexamethasone; ITT, intention-to-treat; and MP, melphalan and prednisolone.
CONSORT diagram. CTD indicates cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated CTD; CVAD, cyclophosphamide, vincristine, adriamycin, and dexamethasone; ITT, intention-to-treat; and MP, melphalan and prednisolone.
In the intensive pathway, patients were randomized to 21-day cycles of CVAD (cyclophosphamide 500 mg/day on days 1, 8, and 15; vincristine 0.4 mg/day on days 1-4; adriamycin 9 mg/m2 per day on days 1-4; and dexamethasone 40 mg/day on days 1-4 and 12-15) or 21-day cycles of CTD (cyclophosphamide 500 mg/day on days 1, 8, and 15; thalidomide 100 mg/day increasing to 200 mg/day if tolerated; and dexamethasone 40 mg/day on days 1-4 and 12-15) both given to maximum response. After 4 to 6 cycles, patients received high-dose melphalan (200 mg/m2) with supporting autologous stem cell transplantation.
Nonintensive pathway patients were randomized to 28-day cycles of MP (melphalan 7 mg/m2 per day and prednisolone 40 mg/day on days 1-4 for 6-9 cycles) or 28-day cycles of an attenuated CTD (CTDa) regimen (cyclophosphamide 500 mg/day on days 1, 8, and 15; thalidomide 50 mg/day increasing to a maximum of 200 mg/day if tolerated; and dexamethasone 20 mg/day on days 1-4 and 15-18). Patients in both pathways were randomized at study entry to either sodium clodronate (C; given continuously at a dose of 1600 mg/day orally) or zoledronic acid (Z; 4 mg as an infusion every 3 or 4 weeks during induction depending on the chemotherapy cycle, and every 4 weeks thereafter). Bisphosphonates were continued at least until progression.
Randomization to maintenance therapy
Patients who completed induction therapy were randomized (1:1) to maintenance therapy with open-label thalidomide (n = 408) or no maintenance (n = 410). Randomization was stratified according to center and previous treatment group from the initial randomization (ie, C + CVAD, C + CTD, Z + CVAD, Z + CTD, C + MP, C + CTDa, Z + MP, or Z + CTDa). Maintenance randomization was not allowed for those showing progressive disease or relapse. However, a few patients in the maintenance arm (n = 9) and no maintenance arm (n = 14) had progressive disease; these patients were randomized in error. The target dose of thalidomide was 50 mg/day increasing to 100 mg/day after 4 weeks, if tolerated, and continuing until progression.
Interphase cytogenetic characterization by FISH
Standard iFISH analysis, performed at study entry, was used for cytogenetic profiling. The number of patients in each subgroup was too small to analyze individually; therefore, patient status was classified as either “favorable” or “adverse.” “Adverse” iFISH was defined as the cytogenetic abnormalities gain(1q), t(4;14), t(14;16), t(14;20), and del(17p)19 ; del(1p32) was an adverse prognostic factor only in younger patients. “Favorable” iFISH was defined by the absence of these cytogenetic abnormalities and predominantly included hyperdiploidy, t(6;14), and t(11;14).
Outcome measures
The primary outcome measures were PFS (the time from maintenance randomization to documented progression or death) and OS (the time from maintenance randomization to death). Progression was defined as relapse from complete response (CR); otherwise, it was progressive disease.20,21
Reporting of the following key adverse events was required for all patients if they occurred during the study period, during disease progression, or until death: thromboembolic events (including deep vein thrombosis, line-related thrombosis, and pulmonary embolism); acute renal failure (unresponsive to up to 72 hours of rehydration, serum creatinine > 500mM, and/or requiring dialysis). Reporting of pregnancies in female patients or a male patient's partner during thalidomide therapy or within 4 weeks after the last thalidomide dose was also required.
Meta-analysis
OS graphs from trials investigating thalidomide maintenance therapy were evaluated to derive individual survival times.12,14,18,22 The papers were scrutinized to ensure that all P values, confidence intervals (CIs), numbers of deaths, and median survival times and durations of patient follow-up matched those reported. In a pooled analysis of all maintenance trials, an OS curve was generated and adjusted for each study or group23 to allow for studies in which more patients were randomized to “no maintenance” than to “maintenance.”
Statistical analysis
The sample size was based on testing the hypothesis that thalidomide maintenance is superior to no maintenance in terms of OS. A total of 762 patients (381 per group) would provide 80% power at a 5% significance level to detect a 10% absolute difference in 5-year survival from start of maintenance treatment (2-tailed test).
The intention-to-treat population (defined as all randomized patients, excluding those who withdrew consent) was used unless otherwise stated. For the primary endpoints of PFS and OS, Cox proportional hazards models were used to compare maintenance treatment groups while adjusting for the minimization factors (stratified by pathway). Patients with missing follow-up data, or those who were not known to have progressed or died at the time of analysis, were censored at the last date they were known to be progression-free or alive, respectively. Cytogenetic analyses were prespecified as exploratory subgroup analyses.
Treatment at progression was not prespecified, but data were collected. A mathematical model, described previously,24 predicted the OS curve resulting from effective salvage therapy at progression. This model was first fitted to the thalidomide maintenance and no maintenance therapy curves for both PFS and survival after progression (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The curves were then combined using numerical integration techniques to produce a single OS curve (supplemental Figure 1C). It was necessary to incorporate the relationship observed between the duration of PFS and subsequent survival after progression, in which patients with a longer PFS had longer survival after progression. As salvage treatment at progression was effective in the no maintenance arm, the model combined the thalidomide maintenance PFS curve with the no maintenance survival-after-progression curve to produce an OS curve (supplemental Figure 1D). This predicts that the effect on OS of adding an effective salvage therapy after PFS benefit was achieved with thalidomide maintenance. It is important to note that, although the mathematical model was designed to enable quantification of resistant disease, in this context it was used purely because it was flexible enough to provide an extremely close fit to both the PFS and survival-after-progression curves.
Statistical analyses were performed with SAS Version 9, or Digital Visual Fortran Version 6.0A software. All hypothesis tests are 2-sided and at the 5% significance level.
Results
Of the 1970 patients recruited from 2003 to 2007, 820 patients underwent maintenance randomization (Figure 1). The majority of exclusions were the result of patient ineligibility (n = 839). In the intensive pathway, 557 patients had been randomized to receive Z and 557 to receive C. In the nonintensive pathway, 430 patients had been randomized to receive Z and 426 to receive C.
Analyses on March 3, 2008, for the independent MRC Leukaemia Data Monitoring and Ethics Committee indicated that thalidomide maintenance had no beneficial effect on OS. Although there were no predetermined limits, the OS data showed a trend against thalidomide, with a P value of the order of 0.1 (hazard ratio [HR] = 0.78, 95% CI, 0.57-1.07, 99.5% CI, 0.50-1.22, P = .11, Cox model). A detailed futility analysis suggested that the chance of thalidomide demonstrating a clinically relevant benefit was negligible. Based on an HR equal to 1.22, which was the upper limit of the 99.5% CI, and a negative exponential survival distribution, no maintenance therapy would be at most 7% worse than thalidomide maintenance therapy at 5 years (P = .005). There was inevitably more toxicity with thalidomide maintenance than with no maintenance treatment. On the basis of these results, together with the observation that there were only approximately 20 patients remaining who would be potentially eligible for randomization, the randomization was closed and treatment with thalidomide stopped. However, the interim analysis raised a few issues to be clarified, and there followed a process of retrospectively collecting data on treatment at progression and of carrying out further scrutiny and analyses (reported in this manuscript). For the present analysis, a cut-off date of October 5, 2009, is used.
Patients
Baseline characteristics were well balanced in the 2 groups; more patients had CR after induction in the thalidomide maintenance group compared with the no maintenance group (38.7% vs 33.9%; Table 1). iFISH results were available for 55% of patients (Table 1). At the time of this analysis, the median follow-up from the start of the study was 46 months and median follow-up from maintenance randomization was 38 months (range, 12-66 months).
Baseline characteristics of the 818 study patients
| Characteristic . | Maintenance (n = 408) . | No maintenance (n = 410) . |
|---|---|---|
| Age, y | ||
| Median | 65 | 64 |
| Range | 34-89 | 31-86 |
| Sex | ||
| Male | 245 (60.0) | 254 (62.0) |
| Female | 163 (40.0) | 156 (38.0) |
| International Staging System at initial randomization | ||
| I | 88 (21.6) | 90 (22.0) |
| II | 155 (38.0) | 143 (34.9) |
| III | 124 (30.4) | 130 (31.7) |
| Missing data | 41 (10.0) | 47 (11.5) |
| Type of myeloma | ||
| IgG | 240 (58.8) | 245 (59.8) |
| IgA | 106 (26.0) | 91 (22.2) |
| IgM | 1 (0.2) | 3 (0.7) |
| No paraprotein | 5 (1.2) | 9 (2.2) |
| IgD | 12 (2.9) | 6 (1.5) |
| Light chain | 42 (10.3) | 51 (12.4) |
| Missing data | 2 (0.5) | 5 (1.2) |
| Induction treatment pathway | ||
| Intensive | 245 (60.0) | 247 (60.2) |
| Nonintensive | 163 (40.0) | 163 (39.8) |
| Time between initial and maintenance randomization, mo | ||
| Median | 8.3 | 8.2 |
| Range | 3.5-21.7 | 3.9-18.6 |
| Randomized induction chemotherapy regimen | ||
| CVAD | 121 (29.7) | 120 (29.3) |
| CTD | 124 (30.4) | 127 (31.0) |
| MP | 79 (19.4) | 82 (20.0) |
| CTDa | 84 (20.6) | 81 (19.8) |
| Response at maintenance randomization* | ||
| Complete response | 158 (38.7) | 139 (33.9) |
| Very good partial response | 65 (15.9) | 79 (19.3) |
| Partial response | 125 (30.6) | 119 (29.0) |
| Minimal response | 17 (4.2) | 23 (5.6) |
| No change | 10 (2.5) | 24 (5.9) |
| Progressive disease | 9 (2.2) | 14 (3.4) |
| Missing data | 24 (5.9) | 12 (2.9) |
| Interphase cytogenetic abnormalities by iFISH, n/N (%) | ||
| Adverse† | 99/225 (44) | 98/227 (43) |
| gain(1q) | 73/191 (38) | 75/199 (38) |
| del(1p32)‡ | 12/117 (10) | 12/111 (11) |
| t(4;14) | 26/221 (12) | 20/225 (9) |
| t(14;16) | 4/219 (2) | 7/225 (3) |
| t(14;20) | 1/217 (0.5) | 3/223 (1) |
| del(17p) | 17/215 (8) | 11/211 (5) |
| Favorable | 126/225 (56) | 129/227 (57) |
| Characteristic . | Maintenance (n = 408) . | No maintenance (n = 410) . |
|---|---|---|
| Age, y | ||
| Median | 65 | 64 |
| Range | 34-89 | 31-86 |
| Sex | ||
| Male | 245 (60.0) | 254 (62.0) |
| Female | 163 (40.0) | 156 (38.0) |
| International Staging System at initial randomization | ||
| I | 88 (21.6) | 90 (22.0) |
| II | 155 (38.0) | 143 (34.9) |
| III | 124 (30.4) | 130 (31.7) |
| Missing data | 41 (10.0) | 47 (11.5) |
| Type of myeloma | ||
| IgG | 240 (58.8) | 245 (59.8) |
| IgA | 106 (26.0) | 91 (22.2) |
| IgM | 1 (0.2) | 3 (0.7) |
| No paraprotein | 5 (1.2) | 9 (2.2) |
| IgD | 12 (2.9) | 6 (1.5) |
| Light chain | 42 (10.3) | 51 (12.4) |
| Missing data | 2 (0.5) | 5 (1.2) |
| Induction treatment pathway | ||
| Intensive | 245 (60.0) | 247 (60.2) |
| Nonintensive | 163 (40.0) | 163 (39.8) |
| Time between initial and maintenance randomization, mo | ||
| Median | 8.3 | 8.2 |
| Range | 3.5-21.7 | 3.9-18.6 |
| Randomized induction chemotherapy regimen | ||
| CVAD | 121 (29.7) | 120 (29.3) |
| CTD | 124 (30.4) | 127 (31.0) |
| MP | 79 (19.4) | 82 (20.0) |
| CTDa | 84 (20.6) | 81 (19.8) |
| Response at maintenance randomization* | ||
| Complete response | 158 (38.7) | 139 (33.9) |
| Very good partial response | 65 (15.9) | 79 (19.3) |
| Partial response | 125 (30.6) | 119 (29.0) |
| Minimal response | 17 (4.2) | 23 (5.6) |
| No change | 10 (2.5) | 24 (5.9) |
| Progressive disease | 9 (2.2) | 14 (3.4) |
| Missing data | 24 (5.9) | 12 (2.9) |
| Interphase cytogenetic abnormalities by iFISH, n/N (%) | ||
| Adverse† | 99/225 (44) | 98/227 (43) |
| gain(1q) | 73/191 (38) | 75/199 (38) |
| del(1p32)‡ | 12/117 (10) | 12/111 (11) |
| t(4;14) | 26/221 (12) | 20/225 (9) |
| t(14;16) | 4/219 (2) | 7/225 (3) |
| t(14;20) | 1/217 (0.5) | 3/223 (1) |
| del(17p) | 17/215 (8) | 11/211 (5) |
| Favorable | 126/225 (56) | 129/227 (57) |
Data are n (%), unless stated otherwise.
CTD indicates cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated CTD; CVAD, cyclophosphamide, vincristine, adriamycin, and dexamethasone; iFISH, interphase fluorescence in situ hybridization; Ig, immunoglobulin; and MP, melphalan and prednisolone.
After induction/high-dose therapy and therefore preceding maintenance randomization.
Multiple abnormalities can be present in the same patient.
Intensive pathway only.
Survival
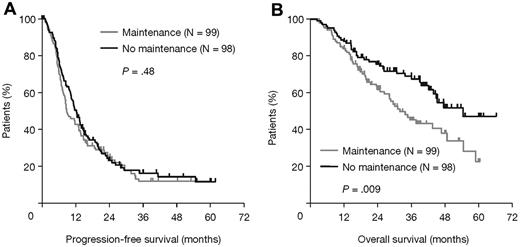
Thalidomide maintenance therapy was associated with a significantly longer PFS than no maintenance (23 vs 15 months; HR = 1.45, 95% CI, 1.22-1.73, log-rank P < .001). There was no significant difference in median OS (HR = 0.91, 95% CI, 0.72-1.17, P = .40; Figure 2).
Survival outcomes in all patients according to thalidomide maintenance therapy. (A) PFS and (B) OS.
Survival outcomes in all patients according to thalidomide maintenance therapy. (A) PFS and (B) OS.
In the intensive pathway, the median PFS was 30 months with thalidomide maintenance therapy and 23 months without maintenance (HR = 1.42, 95% CI, 1.13-1.79, P = .003). The median OS was not reached in either group; however, 3-year survival rates were 75% and 80%, respectively (P = .26). In the nonintensive pathway, PFS was 11 months with maintenance and 9 months without (HR = 1.35, 95% CI, 1.06-1.73, P = .014). The median OS was 38 months with maintenance and 39 months without (HR = 1.00, 95% CI, 0.73-1.38, P = .995).
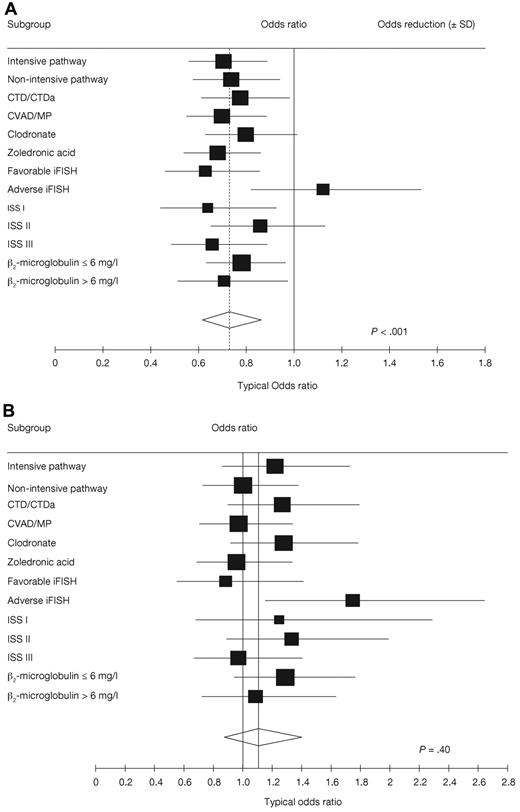
Analyses of the effects of maintenance thalidomide within prognostic and treatment-defined subgroups and pathways showed heterogeneous, probably random, treatment differences in all categories except those defined by iFISH, where there were significant treatment interactions for both PFS (P = .01; Figure 3A) and OS (P = .03; Figure 3B). In patients with favorable iFISH, thalidomide maintenance therapy significantly prolonged median PFS compared with no maintenance (P = .004; Figure 4A), although there was no significant OS benefit (P = .60; Figure 4B). However, there was an apparent late survival benefit that may become significant in subsequent analyses. In patients with adverse iFISH, thalidomide maintenance therapy had no impact on PFS (9 vs 12 months with no maintenance; P = .48; Figure 5A) and was associated with worse OS (P = .009; Figure 5B). This suggests that, in patients with adverse tumor biology, there may be an adverse impact of thalidomide maintenance on OS. In an exploratory analysis of each of the iFISH subgroups making up the “adverse” group, no apparent difference was observed compared with the overall group, possibly because of the low patient numbers.
Forest plots examining the effect of thalidomide maintenance therapy. (A) PFS. (B) OS. ISS indicates International Staging System.
Forest plots examining the effect of thalidomide maintenance therapy. (A) PFS. (B) OS. ISS indicates International Staging System.
Survival outcomes according to thalidomide maintenance therapy in patients with favorable iFISH. (A) PFS and (B) OS.
Survival outcomes according to thalidomide maintenance therapy in patients with favorable iFISH. (A) PFS and (B) OS.
Survival outcomes according to thalidomide maintenance therapy in patients with adverse iFISH. (A) PFS and (B) OS.
Survival outcomes according to thalidomide maintenance therapy in patients with adverse iFISH. (A) PFS and (B) OS.
Consolidation versus maintenance effect
The relationships between response at maintenance randomization and both PFS and OS were evaluated in an attempt to discern whether we are describing a “maintenance” or “consolidation” effect. There was a significant effect on PFS in patients with favorable iFISH who had not achieved a CR with prior induction therapy (P < .001) and an emergent effect on OS (supplemental Figure 2). Although there was no discernable effect of thalidomide maintenance on PFS in patients with favorable iFISH achieving a CR, the numbers were very small (n = 30) and the wide CIs show that the results were not inconsistent with those in patients who had not achieved a CR, making conclusions in this group indeterminate (data not shown). Using electrophoresis and immunofixation as a monitoring technique, there was no difference between the thalidomide maintenance and no maintenance arms in the percentage of patients that upgraded response status over time (P = .19). These analyses incline toward a maintenance effect whereby thalidomide modifies the biology of residual cells in the bone marrow, especially in patients with a greater residual tumor mass, although this issue is still unclear.
Differential effect on PFS of thalidomide at induction and maintenance
To dissect the impact on PFS of thalidomide given at induction from the effect of thalidomide maintenance therapy, we compared the following subgroups of patients: patients who received thalidomide at both induction and maintenance, patients who received thalidomide at induction only, patients who received thalidomide at maintenance only, and patients who did not receive thalidomide at all, also considering treatment pathway. From the time of thalidomide maintenance, the overall results showed that the positive impact of maintenance on PFS is independent of whether thalidomide was also used at induction (regardless of induction, thalidomide maintenance therapy was superior to no maintenance, P = .0011; supplemental Figure 3A). This effect is significant in the intensive induction pathway (P = .03; supplemental Figure 3B). In the nonintensive induction pathway, the situation is less clear, with PFS from maintenance being significantly longer for patients treated with thalidomide at induction or maintenance, and possibly further improved with thalidomide at both these phases, compared with patients who did not receive thalidomide at any treatment phase (P = .0035; supplemental Figure 3C).
Survival after progression
A total of 523 patients experienced disease progression. Of 295 patients with available relapse therapy information, 48% received thalidomide (single agent or in combination), 30% novel agents (bortezomib and lenalidomide), and 27% conventional therapy (supplemental Table 1). The median OS at first progression was significantly shorter in patients randomized to thalidomide maintenance versus no maintenance (P = .005; Figure 6A); this effect was mostly attributed to patients who received thalidomide at disease progression. In these patients, the median OS at first progression was significantly greater in the no maintenance group compared with the thalidomide maintenance group (P = .004; Figure 6B) and most pronounced in patients with adverse iFISH (P = .007; favorable iFISH P = .81). Among patients treated with novel agents at progression, prior thalidomide maintenance therapy had no impact on OS; only patients receiving thalidomide maintenance but not receiving subsequent novel agents experienced a detrimental effect on OS (P < .001; Figure 6C). For the intensive pathway, the median survival after progression was 20 months with thalidomide maintenance therapy compared with 36 months in the no maintenance group (P = .003). For the nonintensive pathway, there was also an effect, although this was not significant (21 vs 26 months with no maintenance; P = .25).
OS at first progression. (A) Impact of maintenance therapy. (B) Type of agents at relapse. (C) Type of therapy at relapse according to prior thalidomide maintenance or no maintenance.
OS at first progression. (A) Impact of maintenance therapy. (B) Type of agents at relapse. (C) Type of therapy at relapse according to prior thalidomide maintenance or no maintenance.
As the OS results were confounded by the treatment choice available at progression, a mathematical model was used to predict the potential impact of effective treatment at progression. The results of this model suggested that a significant OS benefit of 5.5% at 3 years in favor of thalidomide maintenance would have accrued across the trial had all patients received effective therapy at progression (HR = 0.77, 95% CI, 0.60-0.99; supplemental Figure 1).
In an attempt to understand the relatively poor outcome of the 128 patients treated with thalidomide at progression, we generated curves for the impact of thalidomide maintenance in patients with and without thalidomide induction (supplemental Figure 4). Thalidomide induction followed by thalidomide maintenance appears to explain the poor outcome for patients given thalidomide at progression. This finding could be explained by the selection of thalidomide-resistant subclones with the combined use of thalidomide at induction and maintenance.
Safety
The median dosage of thalidomide was approximately 50 mg/day. For patients randomized to thalidomide maintenance therapy who have completed the study to date, the median duration of treatment was 7 months (range, 0-50 months); intensive pathway, 9 months (range, 0-50 months); and nonintensive pathway, 6 months (range, 0-46 months). Among patients randomized to thalidomide maintenance but who discontinued treatment, 52.2% discontinued before progression because of treatment-emergent adverse events, including paresthesia (26.6%), drowsiness (6.8%), constipation (4.1%), eczema/rash (4.1%), hematologic events (1.4%), infection (1.0%), thrombosis (1.0%), and tremor (1.0%). Table 2 summarizes the incidence of adverse events within both pathways of the study.
Adverse events (safety population)
| Event . | Intensive pathway . | Nonintensive pathway . | Overall P† . | ||||
|---|---|---|---|---|---|---|---|
| Maintenance (n = 246)* . | No maintenance (n = 247) . | P . | Maintenance (n = 164)* . | No maintenance (n = 163) . | P . | ||
| Thromboembolic events | 4 (1.6) | 4 (1.6) | 1.0 | 3 (1.8) | 5 (3.1) | .50 | .80 |
| Any serious adverse event‡ | 60 (24.4) | 51 (20.6) | .33 | 56 (34.1) | 33 (20.2) | .0061 | .012 |
| No suspected association with study drugs | 48 (19.5) | 46 (18.6) | .82 | 43 (26.2) | 31 (19.0) | .15 | .26 |
| Any serious adverse reaction§ | 21 (8.5) | 7 (2.8) | .0064 | 16 (9.8) | 4 (2.5) | .0095 | .00014 |
| Hematologic disorders | 0 | 2 (0.8) | .50 | 0 | 0 | NA | .50 |
| Cardiovascular disorders | 2 (0.8) | 1 (0.4) | .62 | 4 (2.4) | 0 | .12 | .12 |
| Fluid and electrolyte disturbance | 0 | 0 | NA | 1 (0.6) | 0 | 1.0 | 1.0 |
| Gastrointestinal disorders | 2 (0.8) | 0 | .25 | 0 | 1 (0.6) | .50 | 1.0 |
| Infection | 6 (2.4) | 0 | .015 | 1 (0.6) | 1 (0.6) | 1.0 | .069 |
| Musculoskeletal, connective tissue, and bone disorders | 1 (0.4) | 3 (1.2) | .62 | 4 (2.4) | 0 | .12 | 1.0 |
| Nervous system disorders | 6 (2.4) | 1 (0.4) | .068 | 3 (1.8) | 2 (1.2) | 1.0 | .14 |
| Renal and urinary disorders | 3 (1.2) | 0 | .12 | 1 (0.6) | 0 | 1.0 | .12 |
| Reproductive system and breast disorders | 1 (0.4) | 0 | .50 | 0 | 0 | NA | 1.0 |
| Skin and subcutaneous disorders | 3 (1.2) | 0 | .12 | 2 (1.1) | 0 | .50 | .062 |
| Event . | Intensive pathway . | Nonintensive pathway . | Overall P† . | ||||
|---|---|---|---|---|---|---|---|
| Maintenance (n = 246)* . | No maintenance (n = 247) . | P . | Maintenance (n = 164)* . | No maintenance (n = 163) . | P . | ||
| Thromboembolic events | 4 (1.6) | 4 (1.6) | 1.0 | 3 (1.8) | 5 (3.1) | .50 | .80 |
| Any serious adverse event‡ | 60 (24.4) | 51 (20.6) | .33 | 56 (34.1) | 33 (20.2) | .0061 | .012 |
| No suspected association with study drugs | 48 (19.5) | 46 (18.6) | .82 | 43 (26.2) | 31 (19.0) | .15 | .26 |
| Any serious adverse reaction§ | 21 (8.5) | 7 (2.8) | .0064 | 16 (9.8) | 4 (2.5) | .0095 | .00014 |
| Hematologic disorders | 0 | 2 (0.8) | .50 | 0 | 0 | NA | .50 |
| Cardiovascular disorders | 2 (0.8) | 1 (0.4) | .62 | 4 (2.4) | 0 | .12 | .12 |
| Fluid and electrolyte disturbance | 0 | 0 | NA | 1 (0.6) | 0 | 1.0 | 1.0 |
| Gastrointestinal disorders | 2 (0.8) | 0 | .25 | 0 | 1 (0.6) | .50 | 1.0 |
| Infection | 6 (2.4) | 0 | .015 | 1 (0.6) | 1 (0.6) | 1.0 | .069 |
| Musculoskeletal, connective tissue, and bone disorders | 1 (0.4) | 3 (1.2) | .62 | 4 (2.4) | 0 | .12 | 1.0 |
| Nervous system disorders | 6 (2.4) | 1 (0.4) | .068 | 3 (1.8) | 2 (1.2) | 1.0 | .14 |
| Renal and urinary disorders | 3 (1.2) | 0 | .12 | 1 (0.6) | 0 | 1.0 | .12 |
| Reproductive system and breast disorders | 1 (0.4) | 0 | .50 | 0 | 0 | NA | 1.0 |
| Skin and subcutaneous disorders | 3 (1.2) | 0 | .12 | 2 (1.1) | 0 | .50 | .062 |
Data are n (%), unless stated otherwise. P values are based on Fisher exact test.
NA indicates not applicable.
Two patients randomized to receive maintenance who were excluded because no consent was received were included in the safety population.
P value for comparison of maintenance (n = 408) versus no maintenance (n = 410).
Irrespective of suspected association with study drugs; patients who had > 1 type of adverse event have been listed against all relevant types of events, but patients who had > 1 occurrence of the same type of event are recorded only once.
Suspected association with study drugs.
Maintenance thalidomide therapy did not result in an increased rate of second primary malignancies, with 12 events observed to date in each arm.
Meta-analysis of thalidomide maintenance trials
The impact of maintenance thalidomide therapy on OS was assessed in the context of other published trials of maintenance thalidomide (supplemental Table 2). There were significant differences between studies (P = .02) explained by the use of thalidomide again at relapse. A meta-analysis, which included the actual survival data from MRC Myeloma IX, demonstrated a significant effect of thalidomide maintenance therapy on OS (P = .047). Furthermore, when including the modeled survival benefit with effective salvage therapy, heterogeneity between studies was removed (P = .24) and a substantial impact of thalidomide maintenance on OS was revealed (odds ratio = 0.75, 95% CI, 0.64-0.87, P < .001; Figure 7A; supplemental Table 2). When analyzed as a composite survival curve,23 a significant late survival benefit was demonstrated (P < .001; Figure 7B).
Meta-analysis of studies including a thalidomide maintenance regimen. (A) Forest plot demonstrates OS with thalidomide maintenance (P < .001).12,14,18,22 In a pooled analysis of these studies, a matching OS curve (B) is generated and adjusted for study/group,23 demonstrating a significant OS advantage for thalidomide maintenance therapy (P < .001).
Meta-analysis of studies including a thalidomide maintenance regimen. (A) Forest plot demonstrates OS with thalidomide maintenance (P < .001).12,14,18,22 In a pooled analysis of these studies, a matching OS curve (B) is generated and adjusted for study/group,23 demonstrating a significant OS advantage for thalidomide maintenance therapy (P < .001).
Discussion
In this study, thalidomide maintenance therapy significantly improved PFS in newly diagnosed MM, and with the use of effective relapse treatment, this benefit can translate into an OS advantage. Furthermore, thalidomide maintenance is associated with a PFS benefit in patients with favorable iFISH, primarily those who had not achieved a CR with prior induction. Interestingly, there was also a trend toward an emergent survival benefit in younger patients with longer median OS. A similar percentage of patients improved their response status over time in both arms. This observation is more consistent with a “maintenance effect” for thalidomide rather than a “consolidation effect” in suboptimal responders, and supports previous investigation,12 but this requires confirmation by flow cytometry.
The use of thalidomide maintenance was associated with PFS benefits and a potential OS benefit in patients with favorable iFISH, but worse OS in patients with adverse iFISH. This important result suggests that thalidomide maintenance therapy may have differential effects dependent on the tumor biology, impairing postrelapse outcome in patients with adverse biology defined by iFISH. This effect was not the result of the relapse therapy, raising the concern that thalidomide maintenance may select clones with more aggressive clinical behavior at relapse in this subgroup. This result contrasts with a similar study where risk status was defined by the use of metaphase cytogenetic analysis by karyotyping patients treated with Total Therapy 2.18 In that study, which used thalidomide throughout, the cases that derived the greatest benefit from thalidomide maintenance were those with adverse metaphase cytogenetics.18,25 It should be noted that iFISH and metaphase cytogenetics capture different cell characteristics that could explain the discrepancy between the 2 studies. These analyses underscore the importance of iFISH-based analysis of patients to define distinct clinical and biologic groups, which aids in the interpretation of the clinical benefits of maintenance therapies.
One of the benefits of a large factorial study, such as this one, is the ability to gain insight into how best to use thalidomide as either induction or maintenance therapy, or combined in both settings. From the time of maintenance, for patients treated via both pathways, the results show that the impact of maintenance therapy on PFS is independent of whether thalidomide was also used at induction. However, looking in more detail at this effect, in the intensive pathway, it is clear that the prior use of either CTD or CVAD induction does not affect PFS from maintenance and that the strongest effect is mediated via maintenance thalidomide. In contrast, in the nonintensive pathway, there are significant differences in PFS dependent on which induction treatment is used. The best PFS is seen for patients treated with thalidomide both at induction and maintenance, with the worst being seen in the group treated with MP and no maintenance. Between these 2 groups are the patients treated with either CTD at induction who received no maintenance and the group treated with MP induction who received thalidomide maintenance; these groups have similar PFS.
In the current study, the initial low availability of novel salvage therapy options may have confounded the OS results. Although survival after progression was worse in patients randomized to thalidomide maintenance therapy, 41% of these patients received thalidomide at first progression. In our modeling analyses, we show that, if patients had been uniformly treated with effective salvage therapy after progression, then a significant survival benefit with thalidomide maintenance would have accrued across the trial as a whole, amounting to 5.5% at 3 years (supplemental Figure 1).
The group with the greatest resistance to thalidomide at relapse was composed of patients treated with thalidomide at both induction and maintenance, and is consistent with the studies of Barlogie et al13 and Lokhorst et al,15 both of which used thalidomide at both induction and maintenance, and showed impairment of survival after progression. This observation also explains the lack of difference in outcome after progression in other maintenance studies that did not use thalidomide as an induction agent.12,14
The duration of thalidomide maintenance was short (median, 7 months), which is well below the median PFS for the study; 52.2% of patients discontinued because of treatment-emergent adverse events. In particular, even at a dose of 50 mg daily, peripheral neuropathy significantly adversely affected patients' ability to remain on an ongoing thalidomide maintenance regimen. Overall, we anticipate that, if patients had been able to tolerate thalidomide maintenance and continued therapy for longer, the effects on PFS would have been greater, and we would have seen an effect on OS without modeling analysis. Therefore, the use of agents with better tolerability profiles, such as lenalidomide, may produce better results.
Meta-analysis results, including the modeled survival benefit with effective salvage therapy, show that thalidomide maintenance therapy significantly reduces the risk of death by 25% (P < .001).12,14,18,22 When analyzed as a composite survival curve,23 a significant late survival benefit, consistent with favorable modulation of the residual myeloma clone, was demonstrated (P < .001). These meta-analysis results suggest a consistent benefit of thalidomide maintenance across all of the studies, especially if effective salvage therapy had been uniformly available.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the patients, investigators, and staff at participating centers who made this study possible; Professor David Bowen; the MRC Leukemia Data Monitoring and Ethics Committee; the MRC Leukaemia Trial Steering Committee; the Haematological Oncology Clinical Studies Group; Myeloma United Kingdom; and the National Institute for Health Research, for support through the National Cancer Research Network. The authors received editorial support from Excerpta Medica in the preparation of this manuscript, funded by Celgene. Anna Georgieva, MD, PhD, provided editorial support. The authors were fully responsible for content and editorial decisions for this manuscript.
This work (the MRC Myeloma IX trial) was supported by the MRC as well as Novartis, Schering Health Care Ltd, Chugai, Pharmion, Celgene, and Ortho Biotech (unrestricted educational grants) via trial coordination and the laboratory studies. None of the funding sources were involved in the study design, data collection, analyses, or interpretation.
Authorship
Contribution: G.J.M., J.A.C., and G.H.J. conceived and designed the study; G.J.M., F.E.D., J.A.C., R.G.O., G.C., N.H.R., C.R., H.R., G.H.J., M.T.D., F.M.R., N.N.C., and S.E.B. collected and assembled the data; W.M.G., G.J.M., J.A.C., G.H.J., S.E.B., N.N.C., J.M.B., M.T.D., A.J.S., and R.G.O. analyzed and interpreted the data; G.J.M. prepared the first draft of the manuscript; and all authors contributed to writing of the manuscript and gave final approval of the manuscript.
Conflict-of-interest disclosure: G.J.M. was a consultant for Novartis and Celgene, received honoraria from Novartis, Celgene, and Ortho Biotech, and received other remuneration from Novartis and Celgene. F.E.D. was a consultant for Novartis and Celgene, received honoraria from Novartis, Celgene, and Ortho Biotech, and received other remuneration from Celgene and Ortho Biotech. G.C. was a consultant for Celgene and Ortho Biotech and received honoraria from Celgene and Ortho Biotech. R.G.O. was a consultant for Celgene and Ortho Biotech and received other remuneration from Celgene and Ortho Biotech. G.H.J. received honoraria from Celgene. S.E.B. received remuneration from Celgene and Ortho Biotech. The remaining authors declare no competing financial interests.
A list of all investigators of the National Cancer Research Institute can be found in the supplemental Appendix.
Correspondence: Gareth J. Morgan, Section of Haemato-Oncology, Institute of Cancer Research, Brookes Lawley Building, 15 Cotswold Road, Belmont, Sutton, Surrey SM2 5NG, United Kingdom; e-mail: gareth.morgan@icr.ac.uk.
References
Author notes
G.J.M. and W.M.G. contributed equally to this study and should both be considered first authors.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal