Abstract
There are no plasma biomarkers specific for GVHD of the gastrointestinal (GI) tract, the GVHD target organ most associated with nonrelapse mortality (NRM) following hematopoietic cell transplantation (HCT). Using an unbiased, large-scale, quantitative proteomic discovery approach to identify candidate biomarkers that were increased in plasma from HCT patients with GI GVHD, 74 proteins were increased at least 2-fold; 5 were of GI origin. We validated the lead candidate, REG3α, by ELISA in samples from 1014 HCT patients from 3 transplantation centers. Plasma REG3α concentrations were 3-fold higher in patients at GI GVHD onset than in all other patients and correlated most closely with lower GI GVHD. REG3α concentrations at GVHD onset predicted response to therapy at 4 weeks, 1-year NRM, and 1-year survival (P ≤ .001). In a multivariate analysis, advanced clinical stage, severe histologic damage, and high REG3α concentrations at GVHD diagnosis independently predicted 1-year NRM, which progressively increased with higher numbers of onset risk factors present: 25% for patients with 0 risk factors to 86% with 3 risk factors present (P < .001). REG3α is a plasma biomarker of GI GVHD that can be combined with clinical stage and histologic grade to improve risk stratification of patients.
Introduction
Acute GVHD, a leading cause of nonrelapse mortality (NRM) after allogeneic hematopoietic cell transplantation (HCT), is measured by dysfunction in 3 organ systems: the skin, liver, and gastrointestinal (GI) tract.1-4 Acute GVHD of the GI tract affects up to 60% of patients receiving allogeneic HCT,5,6 causing nausea, vomiting, anorexia, secretory diarrhea, and, in more severe cases, abdominal pain and/or hemorrhage.7 Acute GVHD typically occurs between 2 and 8 weeks after transplantation, but may occur later,4 and is often clinically indistinguishable from other causes of GI dysfunction such as conditioning regimen toxicity, infection, or medication. Endoscopic biopsy is often used to confirm the diagnosis,1,8 but histologic severity on biopsy has not consistently correlated with clinical outcome.3,8-10 Clinical stage II or greater (> 1 L of diarrhea/d) is associated with reduced survival,5,6 but daily stool volume can vary considerably. Lower GI GVHD responds poorly to treatment compared with other target organs,6 and treatment with high-dose systemic steroid therapy carries significant risks, especially infectious complications in profoundly immunosuppressed patients.11,12 A noninvasive, reliable blood biomarker specific for GVHD of the GI tract would thus significantly aid in the management of patients with this disorder.
Methods
Proteomic analysis
Methods for sample preparation, protein fractionation, mass spectrometry (MS) analysis, protein identification, and quantitative analysis of protein concentrations during the intact protein analysis system (IPAS) have been previously reported.15-17
Patients and samples
Heparinized blood samples were collected weekly for 4 weeks after allogeneic HCT, then monthly for 2 months, and also at the time of key clinical events, including the development of symptoms consistent with GVHD (eg, the onset of diarrhea). Plasma samples were collected prospectively under protocols approved by the University of Michigan Institutional Review Board and stored at the University of Michigan. GVHD assessments, sample processing, and storage were performed as previously described.7,17 In Regensburg, Germany, and Kyushu, Japan, serum samples were collected weekly and at the onset of GVHD symptoms, prepared, frozen, and stored per institutional guidelines. Samples were shipped and received frozen on dry ice and no sample was thawed more than twice before analysis. REG3α concentrations were stable in samples frozen for at least 5 years. REG3α concentrations of 12 paired healthy donors plasma and serum were similar (mean ± SEM: 20 ± 3 vs 24 ± 3 ng/mL, respectively).
All patients received pharmacologic GVHD prophylaxis with at least 2 agents, including a calcineurin inhibitor. No donor grafts were depleted of T cells. All patients with available samples were analyzed, including patients who developed other complications of HCT, such as sinusoidal obstruction syndrome (SOS), idiopathic pneumonia syndrome (IPS), and sepsis/bacteremia. Patients were excluded from analysis only if a plasma sample at the time of GVHD onset was not available, or if methylprednisolone > 1 mg/kg (or equivalent) had been administered for > 48 hours at the time of sample acquisition. One sample was analyzed per patient; patients who developed GVHD had samples selected at the time of initial GVHD diagnosis.
The discovery set consisted of plasma samples from 10 HCT patients at the onset of biopsy-proven GI GVHD (clinical stage 1-3) and 10 HCT patients who never developed GVHD and who were matched for key transplantation characteristics (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Patient samples in the discovery set were not included in the validation set.
The University of Michigan validation set consisted of 4 groups: patients with newly diagnosed GVHD involving the GI tract (with or without other organ involvement; GI GVHD); patients at similar time points who never developed GVHD symptoms (no GVHD); patients with GI distress that was inconsistent with GVHD either by clinical or histologic criteria (non-GVHD enteritis); and patients who presented with isolated skin GVHD (skin GVHD). Patient numbers and characteristics are shown in Table 1. Enteritis was determined to be inconsistent with GVHD on clinical grounds by documentation of infected stool and by resolution of symptoms without steroid treatment. The etiologies of non-GVHD enteritis are listed in Table 2.
Patient characteristics of the University of Michigan validation set
| Total, N = 871 . | GI GVHD*†, N = 167 . | No GVHD, N = 362 . | Non-GVHD enteritis‡, N = 52 . | Skin GVHD, N = 290 . | P . |
|---|---|---|---|---|---|
| Median age, y (range) | 50 (0-67) | 46 (0-68) | 48 (3-66) | 49 (0-70) | .003 |
| Disease, % | .002 | ||||
| Malignant | 99 (n = 165) | 92 (n = 334) | 96 (n = 50) | 97 (n = 282) | |
| Other | 1 (n = 2) | 8 (n = 28) | 4 (n = 2) | 3 (n = 8) | |
| Disease status at transplantation, %§ | .63 | ||||
| Other/low/intermediate risk | 64 (n = 105) | 69 (n = 232) | 68 (n = 34) | 68 (n = 192) | |
| High risk | 36 (n = 60) | 31 (n = 102) | 32 (n = 16) | 32 (n = 90) | |
| Donor type, % | < .001 | ||||
| Related donor | 45 (n = 75) | 64 (n = 233) | 54 (n = 28) | 40 (n = 115) | |
| Unrelated donor | 55 (n = 92) | 36 (n = 129) | 46 (n = 24) | 60 (n = 175) | |
| Donor match, % | < .001 | ||||
| Matched donor | 70 (n = 117) | 90 (n = 325) | 92 (n = 48) | 73 (n = 212) | |
| Mismatched donor | 30 (n = 50) | 10 (n = 37) | 8 (n = 4) | 27 (n = 78) | |
| Conditioning regimen intensity, % | .06 | ||||
| High intensity | 57 (n = 95) | 67 (n = 243) | 63 (n = 33) | 57 (n = 165) | |
| Moderate intensity | 43 (n = 72) | 33 (n = 119) | 37 (n = 19) | 43 (n = 125) | |
| Grade of GVHD at onset, % | |||||
| 0 | 0 (n = 0) | 100 (n = 362) | 100 (n = 52) | 0 (n = 0) | |
| I | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 69 (n = 201) | |
| Skin stage 1 | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 41 (n = 118) | |
| Skin stage 2 | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 29 (n = 83) | |
| II | 57 (n = 96) | 0 (n = 0) | 0 (n = 0) | 30 (n = 88) | |
| Isolated skin stage 3 | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 30 (n = 88) | |
| Isolated upper GI stage 1† | 17 (n = 29) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| Lower GI stage 1† | 40 (n = 67) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| III-IV | 43 (n = 71) | 0 (n = 0) | 0 (n = 0) | 1 (n = 1) | |
| Isolated skin stage 4 | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 1 (n = 1) | |
| GI stage 2† | 13 (n = 22) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| GI stage 3† | 16 (n = 27) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| GI stage 4† | 13 (n = 22) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| Median d after HCT (range) | 33 (11-216) | 31 (7-185) | 24 (7-93) | 28 (5-175) | < .001 |
| Total, N = 871 . | GI GVHD*†, N = 167 . | No GVHD, N = 362 . | Non-GVHD enteritis‡, N = 52 . | Skin GVHD, N = 290 . | P . |
|---|---|---|---|---|---|
| Median age, y (range) | 50 (0-67) | 46 (0-68) | 48 (3-66) | 49 (0-70) | .003 |
| Disease, % | .002 | ||||
| Malignant | 99 (n = 165) | 92 (n = 334) | 96 (n = 50) | 97 (n = 282) | |
| Other | 1 (n = 2) | 8 (n = 28) | 4 (n = 2) | 3 (n = 8) | |
| Disease status at transplantation, %§ | .63 | ||||
| Other/low/intermediate risk | 64 (n = 105) | 69 (n = 232) | 68 (n = 34) | 68 (n = 192) | |
| High risk | 36 (n = 60) | 31 (n = 102) | 32 (n = 16) | 32 (n = 90) | |
| Donor type, % | < .001 | ||||
| Related donor | 45 (n = 75) | 64 (n = 233) | 54 (n = 28) | 40 (n = 115) | |
| Unrelated donor | 55 (n = 92) | 36 (n = 129) | 46 (n = 24) | 60 (n = 175) | |
| Donor match, % | < .001 | ||||
| Matched donor | 70 (n = 117) | 90 (n = 325) | 92 (n = 48) | 73 (n = 212) | |
| Mismatched donor | 30 (n = 50) | 10 (n = 37) | 8 (n = 4) | 27 (n = 78) | |
| Conditioning regimen intensity, % | .06 | ||||
| High intensity | 57 (n = 95) | 67 (n = 243) | 63 (n = 33) | 57 (n = 165) | |
| Moderate intensity | 43 (n = 72) | 33 (n = 119) | 37 (n = 19) | 43 (n = 125) | |
| Grade of GVHD at onset, % | |||||
| 0 | 0 (n = 0) | 100 (n = 362) | 100 (n = 52) | 0 (n = 0) | |
| I | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 69 (n = 201) | |
| Skin stage 1 | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 41 (n = 118) | |
| Skin stage 2 | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 29 (n = 83) | |
| II | 57 (n = 96) | 0 (n = 0) | 0 (n = 0) | 30 (n = 88) | |
| Isolated skin stage 3 | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 30 (n = 88) | |
| Isolated upper GI stage 1† | 17 (n = 29) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| Lower GI stage 1† | 40 (n = 67) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| III-IV | 43 (n = 71) | 0 (n = 0) | 0 (n = 0) | 1 (n = 1) | |
| Isolated skin stage 4 | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | 1 (n = 1) | |
| GI stage 2† | 13 (n = 22) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| GI stage 3† | 16 (n = 27) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| GI stage 4† | 13 (n = 22) | 0 (n = 0) | 0 (n = 0) | 0 (n = 0) | |
| Median d after HCT (range) | 33 (11-216) | 31 (7-185) | 24 (7-93) | 28 (5-175) | < .001 |
GI indicates gastrointestinal; HCT, hematopoietic cell transplantation; and CIBMTR, Center for International Blood and Marrow Transplant Research.
Including 29 patients with isolated upper GI GVHD and 138 with lower ± upper GI GVHD.
With or without other GVHD target organ involvement.
Including 13 patients with isolated upper GI non-GVHD enteritis and 39 patients with lower ± upper GI non-GVHD enteritis.
High risk of disease status at HCT is according to CIBMTR guidelines.
Causes of non-GVHD enteritis in the University of Michigan validation set
| Causes of non-GVHD enteritis . | % (n) . |
|---|---|
| Non-GVHD lower GI enteritis ± upper GI symptoms: N = 39 | |
| Clostridium difficile infection | 54 (21) |
| Diarrhea with negative biopsy | 15 (6) |
| Nausea/vomiting and diarrhea with negative biopsies | 28 (11) |
| Ulcerative esophagitis and diarrhea (negative biopsies) | 3 (1) |
| Non-GVHD upper GI enteritis without diarrhea (all biopsy negative): N = 13 | |
| Nausea/vomiting | 54 (7) |
| Anorexia | 15 (2) |
| Chemical gastropathy | 23 (3) |
| Helicobacter pylori gastritis | 8 (1) |
| Causes of non-GVHD enteritis . | % (n) . |
|---|---|
| Non-GVHD lower GI enteritis ± upper GI symptoms: N = 39 | |
| Clostridium difficile infection | 54 (21) |
| Diarrhea with negative biopsy | 15 (6) |
| Nausea/vomiting and diarrhea with negative biopsies | 28 (11) |
| Ulcerative esophagitis and diarrhea (negative biopsies) | 3 (1) |
| Non-GVHD upper GI enteritis without diarrhea (all biopsy negative): N = 13 | |
| Nausea/vomiting | 54 (7) |
| Anorexia | 15 (2) |
| Chemical gastropathy | 23 (3) |
| Helicobacter pylori gastritis | 8 (1) |
GI indicates gastrointestinal.
Patients from the Regensburg/Kyushu validation set were divided into the same 4 groups; patient characteristics are detailed in supplemental Table 2, with causes of non-GVHD enteritis listed in supplemental Table 3.
Histopathology
GI biopsies were obtained and prepared per institutional guidelines. GVHD was histologically confirmed by duodenal/colonic biopsy in 183 of 197 GI GVHD patients and by skin biopsy in an additional 5 patients with both rash and GI symptoms.9 Skin GVHD was confirmed by biopsy in 272 of 341 patients with rashes and by biopsy of another target organ later affected by GVHD in an additional 8 patients. One hundred sixty-two of 197 patients with GI GVHD had diarrhea. One hundred forty of those 162 patients had biopsies (duodenal = 87, colonic = 53) available for formal grading as described by Lerner.18 If both duodenal and colonic biopsies were available, colonic biopsies were graded only if duodenal biopsies were negative. We did not impute values for unavailable biopsies.
ELISAs
REG3α ELISA kits were purchased from MBL International (Ab-Match Assembly Human PAP1 kit and Ab-Match Universal kit), and measurements were performed according to the manufacturer's protocol. Samples (diluted 1:10) and standards were run in duplicate, absorbance was measured with a SpectraMax M2 (Molecular Devices), and results were calculated with SoftMax Pro Version 5.4 (Molecular Devices). Elafin, IL2Rα, HGF, TNFR1, and IL-8 ELISAs were performed in duplicate as previously reported.17,19 Measurements of samples from 66 patients (6.5% of the total population) were repeated in a second ELISA at random intervals and were comparable; correlation coefficient r = 0.82, P < .0001. Details of the assay parameters are provided in supplemental Table 4.
Statistical analysis
The statistical methods used for the IPAS are as previously described.15-17 REG3α and albumin concentrations from individual samples in the discovery and validation sets were compared using 2-sample t tests applied to log-transformed concentrations. Differences in characteristics between patient groups were assessed with a Kruskal-Wallis test for continuous values and χ2 tests of association for categorical values. Receiver operating characteristic (ROC) area under the curves (AUC) were estimated nonparametrically. NRM and relapse mortality were modeled with cumulative incidence regression methods as described by Fine and Gray.20 One-year overall survival (OS) was modeled with Cox regression methods and probability of response was modeled with logistic regression.
Results
Discovery study
We used a proteomics approach to identify candidate biomarkers in a discovery set of pooled plasma samples taken at similar times after HCT from 10 patients with biopsy-proven GI GVHD and 10 patients without GVHD as previously described (supplemental Table 1).15-17 We identified and quantified 562 proteins of which 74 were increased at least 2-fold in patients with GVHD (supplemental Table 5). Five proteins (carboxypeptidase N catalytic chain precursor, pancreatic secretory trypsin inhibitor precursor, palladin, lithostathine 1-α precursor, and regenerating islet-derived 3-alpha) were preferentially expressed in the GI tract based on the relevant literature21-25 and the Human Protein Atlas (http://www.proteinatlas.org/). Commercially available Abs suitable for quantification of plasma concentrations by ELISA were available for only 1 of these 5 proteins, regenerating islet-derived 3-α (REG3α; supplemental Table 5). The MS characteristics of the identified REG3α peptides are shown in supplemental Figure 1 and supplemental Table 6. The plasma concentrations of REG3α in the individual plasma samples in the discovery set were 4 times higher in the patients with GI GVHD than in asymptomatic controls (supplemental Figure 2, P = .01).
Validation study
We next evaluated REG3α plasma concentration as a biomarker of GI GVHD in samples from a validation set of 871 allogeneic HCT recipients from the University of Michigan (Table 1). Older transplant recipients, an underlying diagnosis of malignant disease, graft sources from unrelated and HLA-mismatched donors were overrepresented in the groups with GVHD. The median day of sample acquisition for patients with non-GVHD enteritis was closer to the day of transplantation than for all other groups.
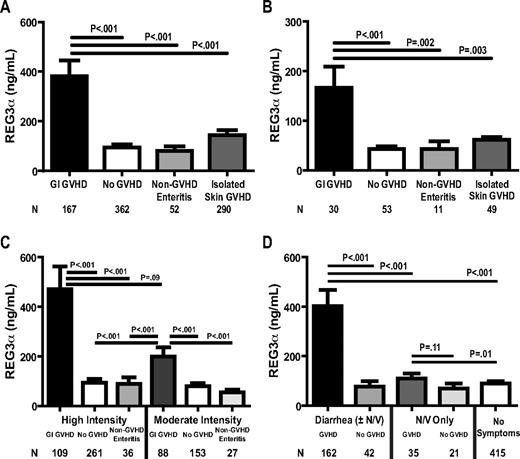
Plasma REG3α concentrations were 3 times higher in patients at the onset of GI GVHD than in all other patients, including those with non-GVHD enteritis (Figure 1A). There was no specific cause of non-GVHD diarrhea associated with higher REG3α concentrations. Serum REG3α concentrations were also higher in GI GVHD in an independent validation set of 143 HCT patients from Regensburg, Germany, and Kyushu, Japan, although the absolute values were lower (Figure 1B). This difference may be because of a center effect that depends on several factors, including variations in transplantation conditioning regimens and supportive care; patients receiving high-intensity conditioning regimens had REG3α concentrations that were twice as high as those receiving moderate intensity conditioning, but this difference did not reach statistical significance (Figure 1C). In addition, all patients in Regensburg and Kyushu received oral antibiotics as GVHD prophylaxis, whereas Michigan patients did not and thus increased GI flora might account for greater REG3α secretion.26 Neither total body irradiation (TBI)–based conditioning nor GVHD prophylaxis regimen significantly impacted REG3α concentrations (data not shown).
REG3α concentrations in plasma samples from HCT patients of 2 independent validation sets. (A) University of Michigan patients (n = 871). (B) Regensburg, Germany, and Kyushu, Japan (n = 143). (C) Plasma REG3α concentrations in patients classified by GI symptoms and histologic diagnosis and categorized by conditioning regimen intensity. High-intensity regimens included: cyclophosphamide ± cytarabine, thiotepa, fludarabine and/or TBI; cyclophosphamide/VP-16/BCNU; busulfan + cytarabine, clofarabine, melphalan, cyclophosphamide/anasacrin, or cytarabine/cyclophosphamide; BCNU/VP-16/cytarabine/melphalan; TBI ± VP-16; melphalan. Moderate-intensity regimens included: fludarabine + busulfan or treosulfan ± TBI, melphalan, zevalin, or anasacrin/cytarabine; fludarabine ± TBI, melphalan, or cyclophosphamide; fludarabine/BCNU/melphalan; TBI. (D) Patients classified by symptoms and etiology (n = 675).
REG3α concentrations in plasma samples from HCT patients of 2 independent validation sets. (A) University of Michigan patients (n = 871). (B) Regensburg, Germany, and Kyushu, Japan (n = 143). (C) Plasma REG3α concentrations in patients classified by GI symptoms and histologic diagnosis and categorized by conditioning regimen intensity. High-intensity regimens included: cyclophosphamide ± cytarabine, thiotepa, fludarabine and/or TBI; cyclophosphamide/VP-16/BCNU; busulfan + cytarabine, clofarabine, melphalan, cyclophosphamide/anasacrin, or cytarabine/cyclophosphamide; BCNU/VP-16/cytarabine/melphalan; TBI ± VP-16; melphalan. Moderate-intensity regimens included: fludarabine + busulfan or treosulfan ± TBI, melphalan, zevalin, or anasacrin/cytarabine; fludarabine ± TBI, melphalan, or cyclophosphamide; fludarabine/BCNU/melphalan; TBI. (D) Patients classified by symptoms and etiology (n = 675).
We next analyzed REG3α concentrations according to diagnosis and type of GI symptom. In patients with diarrhea caused by GVHD, REG3α concentrations at the onset of GVHD were 5 times higher than in patients with diarrhea from other causes (Figure 1D). In patients without diarrhea, REG3α concentrations were 25% higher when attributable to GVHD compared with other causes, a difference that was not statistically significant.
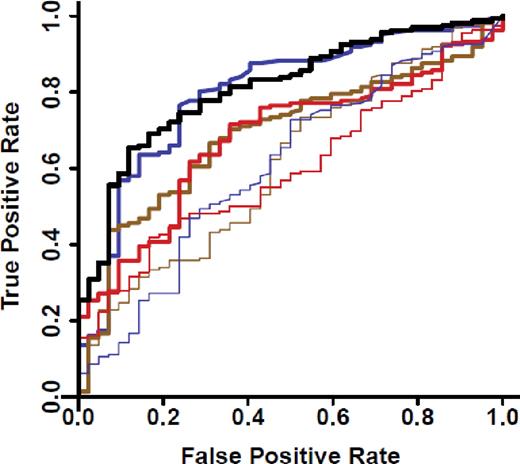
We measured concentrations of 4 previously reported diagnostic markers of systemic acute GVHD (IL2Rα, TNFR1, IL-8, and HGF),19 and of elafin, a biomarker for GVHD of the skin,17 in all patients with diarrhea (Figure 1C, N = 204). ROC curves for these biomarkers distinguished GVHD from non-GVHD with an AUC of 0.80 for REG3α alone and an AUC of 0.81 for a composite panel of all 6 biomarkers (Figure 2). In this analysis, 52% of patients with lower GI GVHD also had skin involvement at onset, and thus the AUC for elafin, which is specific for GVHD of the skin,17 was greater than expected (supplemental Table 7). ROC curves of REG3α concentrations in patients with diarrhea had similar AUCs in both validation sets (supplemental Figure 3). REG3α was therefore the best single diagnostic biomarker at the onset of symptoms of lower GI GVHD, and additional biomarkers provided no further increased sensitivity or specificity. Using REG3α at the median concentration provided a positive predictive value (PPV) of 95% and a negative predictive value (NPV) of 32% for GVHD as the etiology of diarrhea. Additional predictive values at other REG3α concentrations are provided in supplemental Table 8.
ROC curves for patients with post-HCT diarrhea. ROC curves comparing REG3α concentrations for patients with diarrhea caused by GVHD (n = 162) and not caused by GVHD (N = 42). REGα alone (thick blue): AUC = 0.80; IL2Rα (thick brown): AUC = 0.69; Elafin (thick red): AUC = 0.68; IL-8 (thin blue): AUC = 0.61; HGF (thin brown): AUC = 0.61; TNFR1 (thin red): AUC = 0.60; composite of all 6 biomarkers (solid black): AUC = 0.81.
ROC curves for patients with post-HCT diarrhea. ROC curves comparing REG3α concentrations for patients with diarrhea caused by GVHD (n = 162) and not caused by GVHD (N = 42). REGα alone (thick blue): AUC = 0.80; IL2Rα (thick brown): AUC = 0.69; Elafin (thick red): AUC = 0.68; IL-8 (thin blue): AUC = 0.61; HGF (thin brown): AUC = 0.61; TNFR1 (thin red): AUC = 0.60; composite of all 6 biomarkers (solid black): AUC = 0.81.
When we categorized patients by the volume of diarrhea, REG3α concentrations at the onset of symptoms continued to distinguish between GVHD and non-GVHD etiologies (Figure 3A, P < .001) but did not correlate with the clinical stage of GVHD. Twenty-three of 26 patients with clinical stage IV GI GVHD at onset received full-intensity conditioning, and these patients showed a trend toward higher REG3α concentrations than those with stage 1–3 GI GVHD (P = .07; data not shown). Comparing patients who had < 1 L of stool per day because of GVHD versus other causes, the AUC for REG3α was 0.81 (supplemental Figure 4). Plasma REG3α concentrations at the onset of GVHD were significantly higher in patients whose GI biopsies showed evidence of severe GVHD with mucosal denudation (histologic grade 4) compared with less severe GVHD (Figure 3B; P = .03). Hypoalbuminemia is associated with the protein-losing enteropathy in GI GVHD,27 and we analyzed the serum albumin level as a potential marker for loss of intravascular proteins into the intestinal lumen. Albumin levels at the onset of GI GVHD also correlated with both the clinical GI GVHD severity (supplemental Figure 5A) and histopathologic severity (supplemental Figure 5B).
REG3α expression according to severity of GVHD at diagnosis. Patients were classified by volume of diarrhea (A) and histologic grade (B).
REG3α expression according to severity of GVHD at diagnosis. Patients were classified by volume of diarrhea (A) and histologic grade (B).
Prognostic value of REG3α concentrations in patients with lower GI GVHD
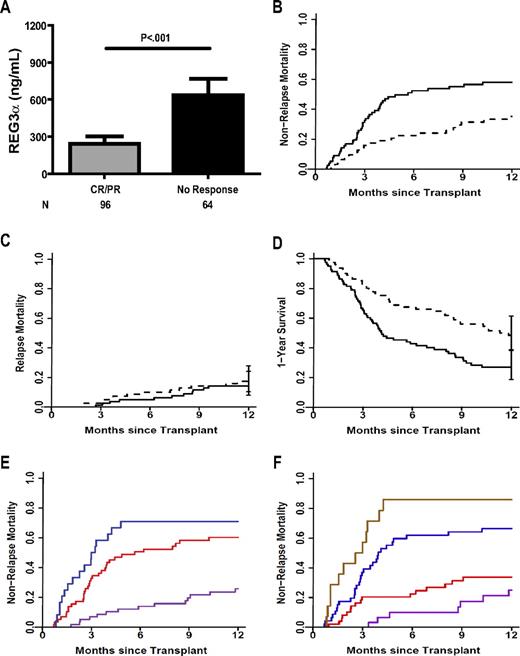
The clinical use of any biomarker is greatly enhanced when it provides prognostic information regarding the future status of a disease and/or patient, for example, the likelihood of response to treatment. We therefore evaluated the prognostic significance of REG3α plasma levels in 162 patients taken at the time of diagnosis of lower GI GVHD. REG3α concentrations were 3-fold higher at the time of GVHD diagnosis in patients who had no response to therapy at 4 weeks28,29 than in patients who experienced a complete or partial response (Figure 4A; P < .001)28,29 ; patients responding to therapy still exhibited REG3α concentrations more than twice that of non-GVHD controls. REG3α concentrations at diagnosis also correlated with eventual maximal clinical stage of GI GVHD (supplemental Figure 6); patients presenting with isolated skin GVHD who later developed GI GVHD had concentrations comparable with those with skin GVHD who never developed GI GVHD (P = .2; data not shown). Because maximal GVHD grade correlates with NRM,11 we hypothesized that the REG3α concentration at GVHD diagnosis would also correlate with NRM. We therefore divided the 162 patients into 2 equal groups based on the median REG3α concentration: high (> 151ng/mL, n = 81) and low (≤ 151 ng/mL, N = 81). NRM was twice as high in patients with high REG3α concentrations, and this difference remained significant after adjusting for known risk factors of donor type, degree of HLA match, conditioning intensity, age, and baseline disease severity (59% [95% confidence interval [CI], 48%-69%] vs 34%[95% CI, 24%-46%], P < .001, Figure 4B). The incidence of relapse mortality was comparable for both groups (14% [95% CI, 8-24] vs 17% [95% CI, 8-24], P = .5; Figure 4C), and thus patients with high REG3α concentrations at the time of GVHD diagnosis experienced significantly inferior 1-year OS (27% [95% CI, 19%-39%] vs 48% [95% CI, 38%-61%], P = .001; Figure 4D). Causes of 1-year mortality for these patients are listed in supplemental Table 9.
Prognostic value of REG3α concentrations at onset of GVHD. (A) Patients were classified by response to GVHD therapy after 4 weeks (N = 160). (B-D) Patients were classified by REG3α concentration: low (≤ 151 ng/mL, n = 81; thin line) and high (> 151 ng/mL, n = 81; thick line). (B) NRM (34% vs 59%, P < .001) (C) Relapse mortality (17% vs 14%, P = .59). (D) One-year survival (48% vs 27%, P = .001). All P values are adjusted for donor source, HLA match, conditioning intensity, recipient age, and baseline disease severity according to the Center for International Blood and Marrow Transplant Research (CIBMTR) guidelines. (E) One-year NRM for patients classified by number of risk factors at GVHD onset, using clinical stage (high risk = stage 2-4) and histologic grade (high risk = grade 4); 0 (purple, NRM = 26%); 1 (red, NRM = 60%); 2 (blue, NRM = 71%); 0 vs 1, P < .001; 1 vs 2, P = .006. (F) One-year NRM for patients classified by number of risk factors at the time of GVHD diagnosis as in panel E and including REG3α concentration (high risk > 151 ng/mL); 0 (purple, NRM = 25%); 1 (red, NRM = 34%); 2 (purple, NRM = 66%); 3 (brown, NRM = 86%); 0 vs 1, P = .2; 1 vs 2, P < .001; 2 vs 3, P < .001.
Prognostic value of REG3α concentrations at onset of GVHD. (A) Patients were classified by response to GVHD therapy after 4 weeks (N = 160). (B-D) Patients were classified by REG3α concentration: low (≤ 151 ng/mL, n = 81; thin line) and high (> 151 ng/mL, n = 81; thick line). (B) NRM (34% vs 59%, P < .001) (C) Relapse mortality (17% vs 14%, P = .59). (D) One-year survival (48% vs 27%, P = .001). All P values are adjusted for donor source, HLA match, conditioning intensity, recipient age, and baseline disease severity according to the Center for International Blood and Marrow Transplant Research (CIBMTR) guidelines. (E) One-year NRM for patients classified by number of risk factors at GVHD onset, using clinical stage (high risk = stage 2-4) and histologic grade (high risk = grade 4); 0 (purple, NRM = 26%); 1 (red, NRM = 60%); 2 (blue, NRM = 71%); 0 vs 1, P < .001; 1 vs 2, P = .006. (F) One-year NRM for patients classified by number of risk factors at the time of GVHD diagnosis as in panel E and including REG3α concentration (high risk > 151 ng/mL); 0 (purple, NRM = 25%); 1 (red, NRM = 34%); 2 (purple, NRM = 66%); 3 (brown, NRM = 86%); 0 vs 1, P = .2; 1 vs 2, P < .001; 2 vs 3, P < .001.
Of the 162 patients with diarrhea at the onset of GVHD, we possessed all 4 data points of clinical stage, histologic grade, REG3α concentration, and serum albumin level in 140 patients. As shown in Table 3, the plasma concentration of REG3α, the clinical severity of GVHD, the histologic severity, and serum albumin level at GVHD diagnosis independently predicted lack of response to GVHD therapy 4 weeks following treatment after adjustment for the aforementioned risk factors (odds ratios: 4.8, 3.9, 18.9, and 2.5, respectively). When lack of response to therapy and NRM were modeled simultaneously on all 4 parameters, all but albumin remained statistically significant. When only advanced clinical stage and severe histologic grade were considered, NRM was 71% (Figure 4E). The inclusion of high REG3α concentration further risk-stratified patients who had either advanced clinical stage or histologic severity (Figure 4F; 34% vs 66% for 1 or 2 risk factors, respectively, P < .001), and patients who had all 3 risk factors experienced significantly greater NRM than those with any 2 of the risk factors (86% vs 66%, P < .001). Details of patient risk factors are listed in supplemental Table 10; NRM by all other risk factor combinations are shown in supplemental Figure 7.
REG3α concentrations and characteristics at onset of GVHD diarrhea predict 4-week response to GVHD therapy and 1-year NRM
| . | Independent . | Simultaneous . | ||
|---|---|---|---|---|
| Ratio . | P* . | Ratio . | P* . | |
| No response to treatment (at 4 wk) | Odds | Odds | ||
| REG3α (high vs low) | 4.8 | < .001 | 5.7 | .001 |
| GVHD GI onset stage (2-4 vs 1) | 3.9 | .001 | 3.0 | .027 |
| Histologic grade (4 vs 1-3) | 18.9 | < .001 | 16.7 | < .001 |
| Albumin (low vs high) | 2.5 | .02 | 1.4 | .5 |
| 1-y NRM | Hazard | Hazard | ||
| REG3α (high vs low) | 2.2 | .003 | 2.4 | .002 |
| GVHD GI onset stage (2-4 vs 1) | 3.0 | < .001 | 3.1 | < .001 |
| Histologic grade (4 vs 1-3) | 3.6 | < .001 | 2.9 | < .001 |
| Albumin (low vs high) | 2.3 | .004 | 1.6 | .2 |
| . | Independent . | Simultaneous . | ||
|---|---|---|---|---|
| Ratio . | P* . | Ratio . | P* . | |
| No response to treatment (at 4 wk) | Odds | Odds | ||
| REG3α (high vs low) | 4.8 | < .001 | 5.7 | .001 |
| GVHD GI onset stage (2-4 vs 1) | 3.9 | .001 | 3.0 | .027 |
| Histologic grade (4 vs 1-3) | 18.9 | < .001 | 16.7 | < .001 |
| Albumin (low vs high) | 2.5 | .02 | 1.4 | .5 |
| 1-y NRM | Hazard | Hazard | ||
| REG3α (high vs low) | 2.2 | .003 | 2.4 | .002 |
| GVHD GI onset stage (2-4 vs 1) | 3.0 | < .001 | 3.1 | < .001 |
| Histologic grade (4 vs 1-3) | 3.6 | < .001 | 2.9 | < .001 |
| Albumin (low vs high) | 2.3 | .004 | 1.6 | .2 |
NRM indicates nonrelapse mortality; and GI, gastrointestinal.
Adjusted for age, donor type, HLA match, conditioning intensity, and disease status at transplantation.
Discussion
The etiology of diarrhea following HCT presents a common diagnostic dilemma.30,31 We identified REG3α as a candidate biomarker specific for lower GI GVHD through an unbiased, in-depth tandem MS-based discovery approach that can quantify proteins at low concentrations and that we previously used successfully to identify elafin as a plasma biomarker specific for GVHD of the skin.17 Our discovery approach identified 74 proteins that were increased at least 2-fold in the plasma from patients with GI GVHD. Of note, the list did not include cytokeratin-18 (KRT18), which has been reported to be specific for both liver and intestinal GVHD.32 This discrepancy may be explained by limitations in proteomics technology and the significantly later acquisition times of samples in the earlier report.
REG proteins act downstream of IL-22 to protect the epithelial barrier function of the intestinal mucosa33,34 through the binding of bacterial peptidoglycans.13 Intestinal stem cells (ISCs) are principal cellular targets of GVHD in the GI tract,3,35 where intestinal flora are critical for amplification of GVHD damage.36,37 A leading hypothesis is that ISCs are protected by antibacterial proteins such as REG3α secreted by neighboring Paneth cells into the crypt microenvironment.38 If death of an ISC eventually manifests itself as denudation of the mucosa, the patchy nature of GVHD histologic damage may be explained as the lack of mucosal regeneration following the dropout of individual ISCs.3,35 REG3α reduces the inflammation of human intestinal crypts in vitro,14,39 and its administration protects ISCs and prevents GI epithelial damage in vivo,34 raising interesting therapeutic possibilities for this molecule.
REG3α plasma concentrations correlate with disease activity in inflammatory bowel disease, and can distinguish infectious and autoimmune causes of diarrhea.14 The correlation of mucosal denudation (histologic grade 4) with high REG3α concentrations suggests that microscopic breaches in the mucosal epithelial barrier caused by severe GVHD permit REG3α to traverse into the systemic circulation. The tight proximity of Paneth cells with ISCs concentrates their secretory contents in that vicinity, so that mucosal barrier disruption caused by stem cell dropout may preferentially allow Paneth cell secretions, including REG3α, to traverse into the bloodstream. We hypothesize that plasma levels of REG3α may therefore serve as a surrogate marker for the cumulative area of these breaches to GI mucosal barrier integrity, a parameter impossible to measure by individual tissue biopsies. Such an estimate of total damage to the mucosal barrier may also help explain the prognostic value of REG3α with respect to therapy responsiveness and NRM.
In this study, 3 high-risk parameters each independently correlated with lack of response to treatment and to higher NRM: elevated plasma REG3α concentration, higher clinical stage of GVHD at diagnosis and grade 4 histologic severity. All 3 of these values thus provided important prognostic information before the initiation of therapy rather than at the time of maximum grade of GVHD, which by definition includes responsiveness to therapy.5,6,11 This study confirms earlier reports where higher clinical stage of GI GVHD5,6 and more severe histology correlated with worse survival.10 In our study the 1-year NRM was 33% (22 of 67 patients) in patients with clinical stage I lower GI GVHD when considering clinical severity alone. Seven of 8 patients (88%) who had the 2 other high risk factors present experienced 1-year NRM while 25% (15 of 59) of patients with 1 or no risk factors experienced 1-year NRM. In this regard it should be noted that REG3α levels did not obviate the need for biopsy. If the prognostic value of REG3α is confirmed in additional patients, we believe the integration of clinical stage, histologic grade and REG3α plasma concentrations into a single grading system will permit better risk stratification and rapid identification of those patients with severe GI damage in whom standard treatment is likely to be insufficient.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr David Beer for critical reading of the manuscript, the clinicians of the University of Michigan Blood and Marrow Transplantation division: Daniel Couriel, Greg Yanik, Carrie Kitko, James Connelly, Craig Byersdorfer, Shin Mineishi, John Magenau, Ed Peres, and Attaphol Pawarode; David Hanauer for assistance with data abstraction using the EMERSE tool; the University of Michigan clinical research support staff: Joel Whitfield, Dawn Jones, and Connie Varner; the University of Michigan BMT division data managers: Pamela Jones, Rachel Young, Charlotte Zinkus, Katherine Archangeli, Tamara Cummings, Sean Kelley, and Jennifer Lay-Luskin; the members of the Paczesny laboratory: Aurelie Gomez, Megan Conlon, and Jeffrey Crawford; the members of the Hanash laboratory: Hong Wang, Vitor Faca, and Sharon Pitteri; and the Seattle Proteomics Core, particularly Phillip Gafken and Jason Hogan.
This work was supported by National Institutes of Health (NIH) grants RC1-HL-101102, P01-CA039542, T32-HL007622, the Hartwell Foundation, and the Doris Duke Charitable Foundation. Informatics assistance with data abstraction and the EMERSE tool was provided by the University of Michigan Cancer Center's (UMCC) Biomedical Informatics Core with partial support from the NIH through the UMCC Support grant (CA46592). Partial funding for the LTQ-FT mass spectrometer used in this work was generously provided by the M. J. Murdock Charitable Trust.
J.L.M.F. is a clinical research professor of the American Cancer Society and a visiting fellow of the Oxford All Souls College. S.P. is an investigator of the Eric Hartwell fund and the Amy Strelzer Manasevit Research Program.
National Institutes of Health
Authorship
Contribution: J.L.M.F. planned the study, interpreted the data, and wrote the manuscript; A.C.H. designed and planned the experiments, performed research, performed data collection and quality assurance, analyzed data, and wrote the manuscript; J.K.G. and E. Huber performed pathology evaluations and wrote the manuscript; T.M.B. was the study statistician and wrote the manuscript; E. Holler, T.T., J.E.L., S.W.J.C., K. L., K.A., and P.R. contributed to patient accrual, clinical data collection and quality assurance, research discussion, and wrote the manuscript; M.V.L. performed experiments and wrote the manuscript; A.C., Q.Z., and S.H. performed the proteomics experiments, interpreted data, and wrote the manuscript; and S.P. conceived and planned the study design, performed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Sophie Paczesny, Blood and Marrow Transplant Program, University of Michigan Comprehensive Cancer Center, Rm 6410, 1500 E Medical Center Dr, Ann Arbor, MI, 48109-5942; e-mail: sophiep@med.umich.edu.
References
Author notes
J.L.M.F. and A.C.H. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal