Abstract
Human mature dendritic cells (DCs) can efficiently stimulate natural killer (NK)–cell responses without being targeted by their cytotoxicity. To understand this important regulatory crosstalk, we characterized the development of the immunologic synapse between mature DCs and resting NK cells. Conjugates between these 2 innate leukocyte populations formed rapidly, persisted for prolonged time periods and matured with DC-derived f-actin polymerization at the synapse. Polarization of IL-12 and IL-12R to the synapse coincided with f-actin polymerization, while other activating and inhibitory molecules were enriched at the interface between DCs and NK cells earlier. Functional assays revealed that inhibition of f-actin polymerization in mature synapses led to an increase of IFN-γ secretion and cytotoxicity by NK cells. This elevated NK-cell reactivity resulted from decreased inhibitory signaling in the absence of MHC class I polarization at the interface, which was observed on inhibition of f-actin polymerization in DCs. Thus, inhibitory signaling is stabilized by f-actin at the synapse between mature DCs and resting NK cells.
Introduction
Dendritic cells (DCs) are key players in the immune system as they bridge innate and adaptive immunity.1 In their immature form, DCs reside as sentinels for pathogens and stress signals in almost all organs of the human body. On interaction with so-called maturation stimuli, DCs migrate at increased frequency to secondary lymphoid organs for the initiation of immune responses.2 DC maturation leads also to the up-regulation of antigen presentation on MHC molecules,3 of chemokine receptors and of costimulatory molecules,4 as well as to the release of cytokines.5,6 In secondary lymphoid tissues, mature DCs are able to alert and activate cells of the adaptive immune system, like T cells, and also of the innate immune system, like natural killer (NK) cells.7-9 Indeed, NK-cell activation by DCs is required for many immune responses.10-15 On interaction with mature DCs and the cytokines that they produce, resting NK cells, preferentially those resident in secondary lymphoid organs, secrete IFN-γ, TNF, and GM-CSF. This effect is mostly mediated via IL-12 and IL-18 production by DCs.7,16-18 Moreover, cytokines secreted by DC-activated NK cells induce further maturation of DCs in secondary lymphoid tissues, prompting them to efficiently stimulate CTL responses. Furthermore, type I IFN of mature DCs up-regulates cytotoxicity of NK cells for powerful anti-tumor19 and anti-viral immune responses.17,20 Finally, the interaction with mature DCs also elicits resting NK-cell priming,10 survival21,22 and proliferation,7 via DC produced IL-15. Thus, mature DCs secrete several cytokines that stimulate distinct NK-cell functions.
Up-regulation of NK-cell cytotoxicity through this interaction could lead to the killing of DCs, thereby compromising the priming of efficient adaptive immune control by these antigen presenting cells. To minimize DC lysis, mature DCs have developed mechanisms that prevent the cytotoxic effect of activated lymphocytes, while immature DCs can be edited by activated NK cells via NKp30 mediated recognition.3 Among these protective mechanisms against cell-mediated cytotoxicity, mature DCs express members of the serpin-family of serin protease-inhibitors that prevent apoptosis induction by granzyme B.23 Moreover, maturation leads to the up-regulation of MHC class I molecules on the surface of DCs. MHC class I molecules interact with inhibitory receptors on the NK-cell surface, controlling the activation of these lymphocytes.3 Thus, a balance between activating and inhibitory signals seems to exist at the immunologic synapse between mature DCs and resting NK cells.21 In this way, resting NK cells can be efficiently activated by DCs and, at the same time, mature DCs are protected from being killed.
To characterize the development of the regulatory synapse between mature DCs and resting NK cells, we describe here the kinetics of distribution of cytoskeletal elements, as well as activating and inhibitory molecules in conjugates of mature DCs with resting NK cells. These studies demonstrate sequential polarization of these molecules to the interface, and a novel role for the filamentous actin (f-actin) cytoskeleton of DCs in stabilizing inhibitory rather than activating signals at the synapse with NK cells.
Methods
Antibodies and labeling reagents
The reagents used are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Human DCs and NK cells
The preparation and purification of monocyte-derived and blood DCs as well as the NK-cell populations from human PBMCs are outlined in the supplemental Methods. The use of blood from healthy volunteers has been approved by the cantonal ethics committee (KEK) for this study.
K562 cells and their HLA class I transfection
The K562 culture conditions and transfectant generation are summarized in supplemental Methods.
Stealth siRNA duplexes and electroporation of DCs
The procedure of siRNA mediated silencing in DCs can be found in supplemental Methods.
Human mature DC/resting NK-cell co-cultures and conjugation assays
Conjugate formation between human DCs and NK cells was performed according to the protocol contained in supplemental Methods.
DC protein extracts and Western blot
Protein extract generation and Western blotting were executed according to the description in supplemental Methods.
Live cell imaging
The live cell imaging protocol used can be obtained from supplemental Methods.
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed as described in supplemental Methods.
Immunofluorescence microscopy and analysis
The immunofluorescence techniques are delineated in supplemental Methods.
Flow cytometry
Cells were acquired on a BD LSR II flow cytometer using FACSDiva Version 6.1.3 software (BD Biosciences) or a FACSCanto II (BD Biosciences) and all flow cytometry analyses were performed with FlowJo Version 9.3.1 software (Tristar).
Statistics
Statistical analyses were performed with the Mann Whitney test, nonparametric, and bi-caudal. P values < .05 were considered significant. Plotted data are displayed as median ± interquartile ranges.
Results
The regulatory synapse between human mature DCs and autologous resting NK cells matures with DC-derived f-actin polymerization
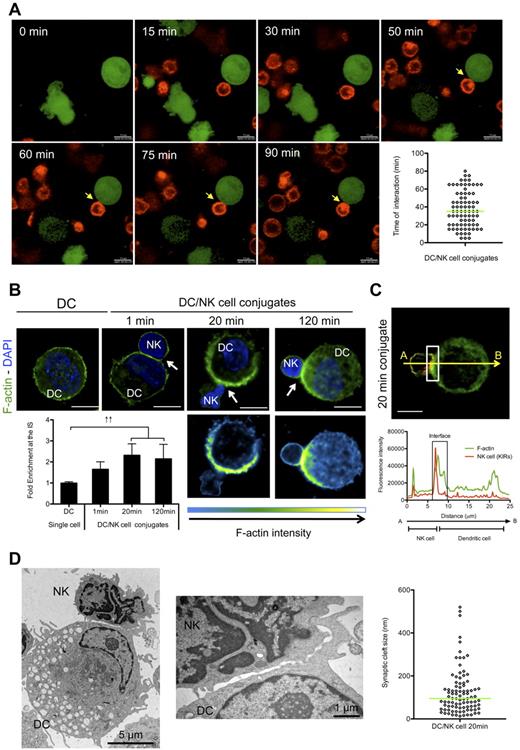
Our previous results showed a rapid formation of conjugates between human DCs and NK cells.21 To analyze the duration of this interaction, we visualized poly(I:C) matured monocyte-derived DCs and autologous resting NK-cell cocultures in extracellular matrix by live cell imaging. We determined that the majority of the conjugates between these 2 leukocyte populations lasted for at least 20 minutes (Figure 1A and supplemental Video 1). Therefore, the contacts between human mature DCs and resting NK cells seem to be long-lived.
The formation of the regulatory synapse between mature DCs and resting NK cells is a fast event and matures with DC-derived f-actin polymerization at the synapse. (A) CSFE-labeled mature DCs (green) were co-cultured with PKH26 labeled NK cells (red) in extracellular matrix. Conjugate formation was visualized by live cell imaging (see supplemental Video 1). Pictures were acquired every 5 minutes for at least 90 minutes. Arrows indicate the immunologic synapse. A representative conjugate is shown. The graph represents the duration of different synapses between DCs and NK cells. (B) Mature DC single cell cultures or mature DC/resting NK-cell cocultures were fixed after 1, 20 minutes or 120 minutes of interaction and f-actin was stained with bodipy conjugated phallacidin (green). DAPI was used to stain nuclear DNA (blue). Arrows indicate the synapse. The graph represents the quantification of f-actin staining intensity at the synapse compared with the staining at the opposite side of the same DC. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (C) Mature DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and stained with bodipy conjugated phallacidin to stain f-actin (green) and anti-KIRs antibodies (red). The fluorescence intensities of KIRs and f-actin stainings were plotted along the indicated trajectory, from A to B. The synapse area in cellular conjugates is indicated by boxes. (D) Mature DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and treated for TEM analysis. Original magnifications are 4200× and 17 500×, respectively from left to right. The graph represents the size of several regions in the synaptic clefts of 5 DC/NK-cell conjugates. Images are representative of 1 experiment for TEM images and at least 3 independent experiments for the other techniques. Original magnifications are 100× for all the light microscopy images. Values on graph bars are medians and error bars represent interquartile ranges from the analysis of at least 100 conjugates from at least 3 independent experiments. ↑↑ indicates fold increase > 2. Scale bars are 10 μm unless specified otherwise.
The formation of the regulatory synapse between mature DCs and resting NK cells is a fast event and matures with DC-derived f-actin polymerization at the synapse. (A) CSFE-labeled mature DCs (green) were co-cultured with PKH26 labeled NK cells (red) in extracellular matrix. Conjugate formation was visualized by live cell imaging (see supplemental Video 1). Pictures were acquired every 5 minutes for at least 90 minutes. Arrows indicate the immunologic synapse. A representative conjugate is shown. The graph represents the duration of different synapses between DCs and NK cells. (B) Mature DC single cell cultures or mature DC/resting NK-cell cocultures were fixed after 1, 20 minutes or 120 minutes of interaction and f-actin was stained with bodipy conjugated phallacidin (green). DAPI was used to stain nuclear DNA (blue). Arrows indicate the synapse. The graph represents the quantification of f-actin staining intensity at the synapse compared with the staining at the opposite side of the same DC. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (C) Mature DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and stained with bodipy conjugated phallacidin to stain f-actin (green) and anti-KIRs antibodies (red). The fluorescence intensities of KIRs and f-actin stainings were plotted along the indicated trajectory, from A to B. The synapse area in cellular conjugates is indicated by boxes. (D) Mature DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and treated for TEM analysis. Original magnifications are 4200× and 17 500×, respectively from left to right. The graph represents the size of several regions in the synaptic clefts of 5 DC/NK-cell conjugates. Images are representative of 1 experiment for TEM images and at least 3 independent experiments for the other techniques. Original magnifications are 100× for all the light microscopy images. Values on graph bars are medians and error bars represent interquartile ranges from the analysis of at least 100 conjugates from at least 3 independent experiments. ↑↑ indicates fold increase > 2. Scale bars are 10 μm unless specified otherwise.
To study the development of these interactions, we investigated the kinetics of cytoskeletal remodeling in DC conjugates with NK cells. F-actin polymerization at the synapse is involved in the generation of tight interactions, and represents a hallmark of cytotoxic NK-cell synapses.24,25 Our previous results showed that there is no significant polymerization of f-actin at the synapse between mature DCs and resting NK cells after 1 to 5 minutes,21 but information on longitudinal f-actin remodeling at this regulatory synapse is still lacking. Therefore, we allowed mature DCs to conjugate with autologous resting NK cells for different time periods (1 to 120 minutes). We confirmed no significant f-actin accumulation at the DC/NK-cell synapse after 1 to 5 minutes of incubation (Figure 1B). However, a progressive enrichment of f-actin at the interface could be observed, reaching the highest values after 15 to 20 minutes of interaction (Figure 1B). A significant clustering of f-actin was still visible after 120 minutes of interaction (Figure 1B). Similar results were obtained using DCs matured with proinflammatory cytokines (R.B.d.S. and C.M., unpublished data, March 2008). As there are 2 major subsets of NK cells in the human peripheral blood (the CD56brightCD16− and the CD56dimCD16+ subsets), we studied the distribution of f-actin in conjugates between mature DCs and NK cells from these 2 populations. Both NK-cell subsets formed synapses with mature DCs. However, CD56brightCD16− NK cells conjugated more readily with mature DCs, than did CD56dimCD16+ NK cells (supplemental Figure 1A).21 Furthermore, our results show that there is an enrichment of f-actin in conjugates of mature DCs with both CD56brightCD16− and CD56dimCD16+ NK cells after 20 minutes of interaction (supplemental Figure 1B).
To clarify which conjugation partner mainly contributed to this f-actin enrichment at the synapse, we costained f-actin and killer immunoglobulin like receptors (KIRs) of NK cells on fixed DC/NK-cell conjugates (Figure 1C). Next, we plotted the fluorescence intensity of KIRs (red) and f-actin (green) staining along trajectories perpendicular to the synapse, as illustrated in Figure 1C. F-actin accumulation was predominantly observed on the KIR-negative side (86.6 ± 2.9% of 53 analyzed conjugates, P < .001; Figure 1C). This finding suggests that DCs stabilize the DC/NK-cell synapse by f-actin polymerization after 20 minutes of interaction. In contrast, when KIRs and f-actin were stained in K562 conjugates with resting NK cells (supplemental Figure 1C-D), we could confirm f-actin polarization from the NK-cell side (97.2% ± 0.3% of the 37 analyzed conjugates, P < .05), as previously described.24,26,27
Membrane intercalation in cell conjugates has been described to happen in cytotoxic NK-cell synapses.28 Indeed, this event could lead to a misevaluation of the cellular contribution for f-actin enrichment at the synapse, considering the resolution of 60 nm in light microscopy used in our study. Therefore, we studied the morphology of DC/NK-cell conjugates by transmission electron microscopy (TEM). The results indicate that the synaptic cleft between DCs and NK cells measured between 13 and 520 nm (median = 94.8; Figure 1D). Interestingly, TEM images from K562 conjugates with NK cells revealed that the synaptic cleft in this cytotoxic synapse was narrower (1 to 96 nm, median = 33.8) than in the regulatory synapses between mature DCs and resting NK cells (supplemental Figure 1E), indicating tighter interactions during cytolysis. However, no significant membrane intercalation at the interface between DCs or K562 cells and NK cells could be observed (Figure 1D and supplemental Figure 1E). These results indicate that the interaction between mature DCs and autologous resting NK cells occurs rapidly and matures with the polymerization of f-actin, primarily from the DC side of the synapse.
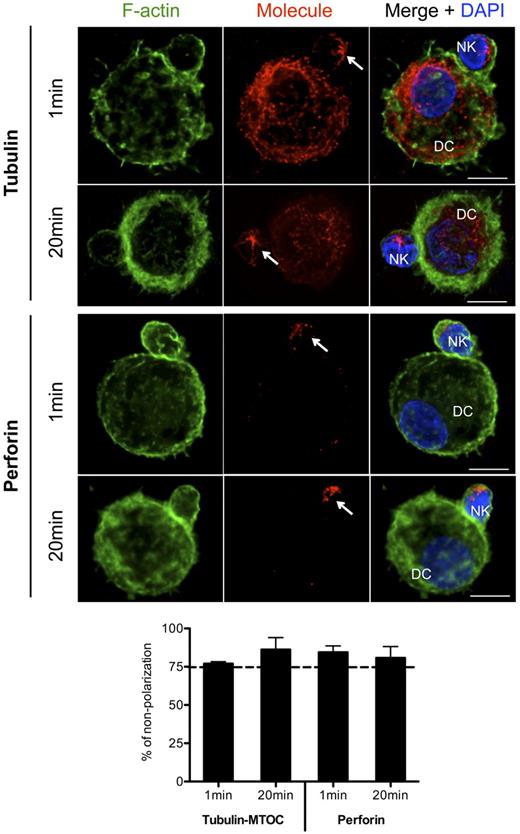
In addition to the f-actin cytoskeleton, we studied the position of the microtubule organizing center (MTOC) and perforin granules of the conjugated NK cells. The movement of the MTOC to the interface of an activated lymphocyte with a target cell is essential for the delivery of perforin-containing cytotoxic granules to the target cell.24,29,30 Our results show that the majority of conjugates between mature DCs and NK cells did not show MTOC and perforin polarization in NK cells to the synapse, even after maturation of the interactions (Figure 2), and no significant differences between conjugates of mature DCs and autologous CD56brightCD16− or CD56dimCD16+ NK cells were observed (supplemental Figure 1B). In contrast, we could see a fast (after only 1 to 5 minutes of interaction) polarization of MTOCs in conjugates between NK cells and MHC class I negative K562 cells (supplemental Figure 2A). The lack of MTOC and perforin polarization further supports our previous findings that the interaction of NK cells with mature DCs is not cytotoxic.3 To investigate the role of MHC class I/KIR inhibitory signaling in the NK-cell cytoskeleton dynamics, we conjugated KIR3DL1+ and KIR3DL1− NK cells with K562 cells stably expressing HLA-Bw4 molecules (HLA-B*3701; supplemental Figure 2B). Conjugates between KIR3DL1+ NK cells and HLA-Bw4 expressing K562 cells presented reduced NK cell–derived f-actin polymerization at the interface of the cells and the minority of these conjugates exhibit MTOC polarization at the synapse (supplemental Figure 2C). In contrast, KIR3DL1− NK cells polymerized their f-actin and MTOCs at the contact side with K562-Bw4 cells, in a similar fashion as did NK cells with MHC class I negative K562 cells (supplemental Figure 2A,C). These studies support a role of MHC class I/KIR inhibitory signaling in the regulation of the NK-cell cytoskeleton dynamics.
MTOC and perforin granules do not polarize to the synapse of autologous mature DCs with resting NK cells. Co-cultures of mature DCs with resting NK cells were fixed after 1 or 20 minutes of interaction. F-actin (green), nuclear DNA (blue) and α-tubulin or perforin (red) were stained. Arrows indicates MTOC (top) and perforin granules (bottom) of NK cells. The number of conjugates with MTOCs or perforin granules distant from the synapse was plotted in the graph as percentages of total number of conjugates analyzed. Original magnifications are 100× for all the microscopy images. A total of at least 200 conjugates from 3 independent experiments were analyzed. Values on graph bars are medians and error bars represent interquartile ranges. *P < .05. Statistics were performed with a null value of 75%, representing random distribution. Scale bars are 10 μm.
MTOC and perforin granules do not polarize to the synapse of autologous mature DCs with resting NK cells. Co-cultures of mature DCs with resting NK cells were fixed after 1 or 20 minutes of interaction. F-actin (green), nuclear DNA (blue) and α-tubulin or perforin (red) were stained. Arrows indicates MTOC (top) and perforin granules (bottom) of NK cells. The number of conjugates with MTOCs or perforin granules distant from the synapse was plotted in the graph as percentages of total number of conjugates analyzed. Original magnifications are 100× for all the microscopy images. A total of at least 200 conjugates from 3 independent experiments were analyzed. Values on graph bars are medians and error bars represent interquartile ranges. *P < .05. Statistics were performed with a null value of 75%, representing random distribution. Scale bars are 10 μm.
The analysis of the cytoskeleton rearrangements in conjugates of mature DCs with resting NK cells revealed a role of DC-derived f-actin in the maturation of the interaction between these 2 cells and corroborated its noncytotoxic character.
The regulatory synapse between mature DCs and resting NK cells matures with sequential polarization of both activating and inhibitory molecules
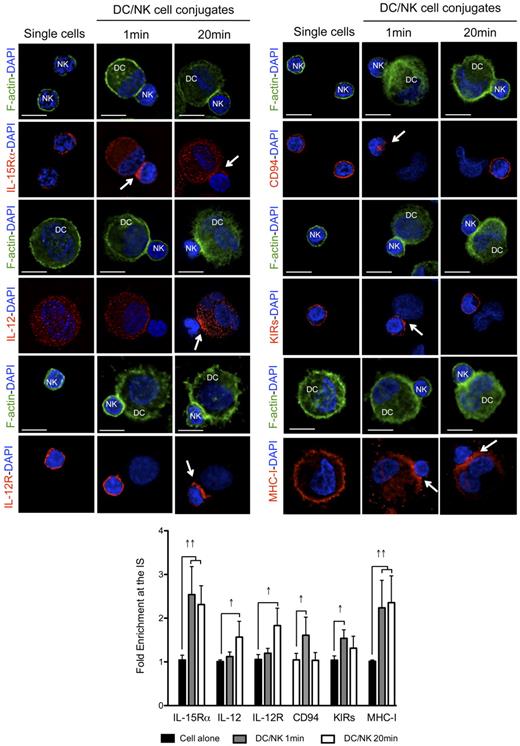
To analyze the kinetics of activating and inhibitory receptor polarization to the synapse between mature DCs and resting NK cells, we allowed the cells to conjugate for different periods of time, which could be distinguished by the f-actin polarization status (no f-actin polymerization at 1 minute and polarization at 20 minutes). We reasoned that the kinetics of polarization for activating and inhibitory molecules at these different time points could indicate the sequential occurrence of distinct signaling events at the synapse. Among the most important activating molecules involved in this crosstalk, we studied the distribution of the α chain of the receptor for IL-15 (involved in NK survival, priming and proliferation),10,13 as well as IL-12 and IL-12R (relevant for NK cell-mediated cytokine production).7,31 Apart from these activating interactions, we studied the distribution of the NK-cell inhibitory receptors KIRs and CD94, binding to MHC class I molecules on target cells.21,32 As previously described, IL-15Rα, CD94, KIRs and MHC class I molecules polarized rapidly to the synapse (Figure 3 one minute columns). The median fold enrichments of the molecules at the synapse, when compared with a similar region on the opposite site of the cell were 2.54, 1.61, 1.54, and 2.24 for IL-15Rα, CD94, KIRs, and MHC class I, respectively. All these molecules were found to remain clustered at the synapse after 10 minutes of interaction (R.B.d.S. and C.M., unpublished data, April 2008). DC-derived IL-12 and IL-12R in NK cells, in contrast, did not polarize significantly after 1 minute of interaction with a fold increase of 1.12 and 1.20, respectively (Figure 3). Next, mature conjugates that presented significant f-actin polymerization at the synapse were analyzed after 20 minutes (Figure 3 twenty minutes columns). In these conjugates, IL-15Rα and MHC class I were still enriched at the contact site between the cells (fold increases were 2.31 and 2.35, respectively), whereas CD94 and KIR distribution patterns were more similar to the ones presented by nonconjugated NK cells, with clustering values with a < 1.5 fold increase in the majority of the conjugates analyzed (Figure 3). These results suggest that these molecules might redistribute after the first fast polarization to the synapse. DC-derived IL-12 and NK-cell expressed IL-12R polarization was well visible after 20 minutes of interaction (fold increase 1.57 and 1.83, respectively). These results were reproduced with proinflammatory cytokine matured DCs (R.B.d.S. and C.M., unpublished data, April 2008). IL-12 polarization, however, was only observed in proinflammatory cytokine matured DCs, because the IL-12 signal was too abundant in the cytoplasm of poly(I:C) matured DCs to detect partial enrichment at the synapse (fold increase 1.10; supplemental Figure 3A). Furthermore, MHC class II molecules were not found clustered at the synapse of mature DCs with resting NK cells at any of the time points analyzed (supplemental Figure 3B), suggesting that the observed molecule enrichments did not result from bulk membrane accumulation at the synapse interface. These results indicate that IL-15Rα as stimulatory, and CD94, KIRs, and MHC class I as inhibitory molecules, polarize early after conjugation to the synapse between mature DCs and resting NK cells, while IL-12 and IL-12R enrichment coincides with later f-actin polymerization at the synapse.
Activating and inhibitory molecules polarize with different kinetics to the interface of conjugates between mature DCs and resting NK cells. Single cell cultures or cocultures of mature DCs with resting NK cells were fixed after 1 or 20 minutes of interaction and f-actin (green), nuclear DNA (blue) and IL-15Rα, IL-12, IL-12R, CD94, KIRs, or MHC class I (red) were stained. Arrows indicate molecule enrichment at the synapse. The graph represents the quantification of molecule staining intensity at the synapse, compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. Images are representative of at least 3 independent experiments. Original magnifications are 100× for all the microscopy images. Values on graph bars represent medians from the analysis of at least 100 conjugates, from at least 3 independent experiments. Error bars indicate interquartile ranges. ↑↑ indicates fold enrichment > 2 for molecules that exist in both DC and NK cell (IL-15Rα and MHC class I); and ↑, fold enrichment > 1.5 for molecules that exist in only 1 cell (IL-12, IL-12R, CD94, and KIRs). Scale bars are 10 μm.
Activating and inhibitory molecules polarize with different kinetics to the interface of conjugates between mature DCs and resting NK cells. Single cell cultures or cocultures of mature DCs with resting NK cells were fixed after 1 or 20 minutes of interaction and f-actin (green), nuclear DNA (blue) and IL-15Rα, IL-12, IL-12R, CD94, KIRs, or MHC class I (red) were stained. Arrows indicate molecule enrichment at the synapse. The graph represents the quantification of molecule staining intensity at the synapse, compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. Images are representative of at least 3 independent experiments. Original magnifications are 100× for all the microscopy images. Values on graph bars represent medians from the analysis of at least 100 conjugates, from at least 3 independent experiments. Error bars indicate interquartile ranges. ↑↑ indicates fold enrichment > 2 for molecules that exist in both DC and NK cell (IL-15Rα and MHC class I); and ↑, fold enrichment > 1.5 for molecules that exist in only 1 cell (IL-12, IL-12R, CD94, and KIRs). Scale bars are 10 μm.
DC-derived f-actin polymerization supports inhibition of NK cell function
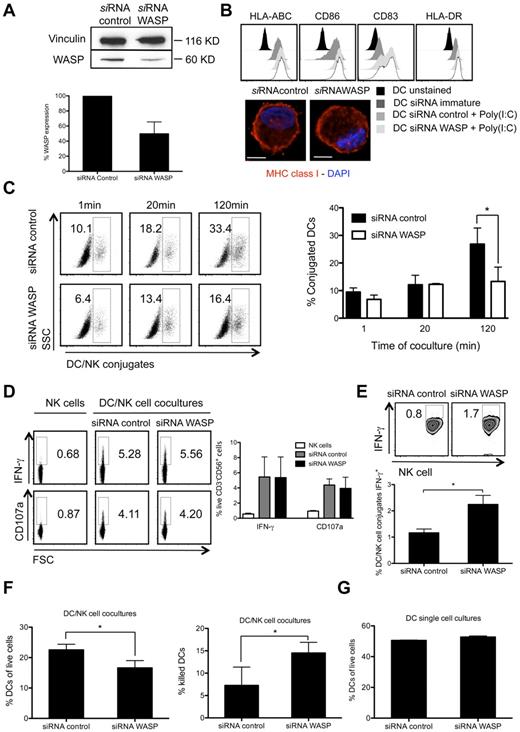
The differences in inhibitory and activating molecule polarization to the synapse suggested that they are differently stabilized by cytoskeletal components. Because we preferentially observed f-actin polymerization by DCs, we aimed to selectively compromise it in these cells. To do so, polymerization of f-actin in DCs was down-regulated by targeting the Wiskott-Aldrich syndrome protein (WASP) by siRNA mediated silencing (ΔWASP DCs) and, as a control, DCs were transfected with control siRNA (control DCs). WASP is a key regulator of f-actin polymerization.33 SiRNA transfection of DCs lead to roughly 50% decrease in WASP protein content, as determined by Western blot quantification (Figure 4A). The electroporation of DCs and silencing of WASP did not alter the phenotypical maturation of DCs after poly(I:C) treatment, including the levels of MHC class I (HLA-ABC), CD86, CD83, and MHC class II (HLA-DR; Figure 4B). Poly(I:C) matured ΔWASP or control DCs were then cocultured with autologous resting NK cells. Notably, mature ΔWASP DCs conjugated less efficiently with NK cells, but this difference became only significant at time points later than 20 minutes and, after 120 minutes of co-culture, a decrease of roughly 50% in the number of conjugated DCs was observed in WASP silencing conditions (Figure 4C). Interestingly, these fewer ΔWASP DC conjugates with NK cells were able to induce as much IFN-γ production and degranulation of total NK cells as obtained with control DCs (Figure 4D). More importantly, the percentage of DC conjugated NK cells with IFN-γ production was higher in WASP silencing conditions (Figure 4E), contrary to unconjugated NK cells (supplemental Figure 4A), suggesting a role of DC-derived f-actin polymerization in the inhibition of NK-cell activation. In addition to elevated cytokine production per conjugate, enhanced NK-cell cytotoxicity against WASP deficient DCs was detected. After 6 hours of coculture, less ΔWASP DCs than control DCs could be recovered from cocultures of mature DCs with resting NK cells (Figure 4F left graph), and ΔWASP DCs were more readily killed by activated NK cells (Figure 4F right graph). Furthermore, WASP down-regulation in DCs did not alter their survival capacity, as after 6 hours of culture without NK cells, no differences were observed in the percentage of live cells between ΔWASP DCs and control DCs conditions (Figure 4G). Therefore, selective inhibition of f-actin polymerization in mature DCs seemed to convert these cells to targets of NK-cell cytotoxicity.
DC-derived f-actin dynamics play a role in the maturation of interactions with resting NK cells and DC survival on NK cell activation. (A) Mature DCs treated with siRNA control or siRNA targeting WASP were lysed and protein extracts were run on SDS-page gels. Vinculin (as a loading control) and WASP were detected by Western blot and protein levels were quantified by ImageJ. (B) Immature or mature control and ΔWASP DCs were stained with fluorochrome conjugated anti–HLA-ABC, -CD86, -CD83 and –HLA-DR antibodies and compared with unstained DCs by flow cytometry. For microscopy images, mature control or ΔWASP DCs were fixed and MHC class I molecules (red) and nuclear DNA (blue) were stained. (C) Mature control DCs, mature ΔWASP DCs and resting NK cells were prestained with vital dyes and cocultured for 1, 20, or 120 minutes. The percentage of NK cell-conjugated DCs was determined by flow cytometry. (D) NK cells were cultured alone or with mature control or ΔWASP DCs for 6 hours, in the presence of BFA. IFN-γ production by NK cells, as well as degranulation (CD107a staining) were assessed by ICS in flow cytometry. NK cells were gated as live, individual, CD3−CD56+ cells. (E) Mature control DCs, mature ΔWASP DCs and resting NK cells were prestained with vital dyes and cocultured for 6 hours, in the presence of BFA, and IFN-γ production by DC-conjugated NK cells was assessed by ICS in flow cytometry, above the high autofluorescence of nonconjugated DCs. (F) Mature control and ΔWASP DCs were cultured with resting NK cells for 6 hours, and the percentage of surviving DCs (% DCs of live cells) was assessed by flow cytometry, using the Fixable Aqua Live/Dead reagent (left graph). Mature control and ΔWASP DCs were prestained with vital dyes and cultured alone or with IL-2 activated NK cells, for 6 hours. The percentage of killed DCs (% killed DCs) was assessed by flow cytometry, using the TO-PRO-3 reagent (right graph). Medians ± interquartile ranges were plotted in the graphs, after subtracting the values of spontaneous lysis of DC single cell cultures. (G) Mature control and ΔWASP DCs were cultured alone for 6 hours, and the percentage of surviving DCs (% DCs of live cells) was assessed by flow cytometry, using the Fixable Aqua Live/Dead reagent. Plots are representative of at least 3 independent experiments. Values on graph bars represent medians from at least 3 independent experiments with duplicates. Error bars indicate interquartile ranges. Original magnifications are 100× for all the microscopy images. *P < .05. P values from Mann-Whitney test, nonparametric and bi-caudal. Scale bars are 10 μm.
DC-derived f-actin dynamics play a role in the maturation of interactions with resting NK cells and DC survival on NK cell activation. (A) Mature DCs treated with siRNA control or siRNA targeting WASP were lysed and protein extracts were run on SDS-page gels. Vinculin (as a loading control) and WASP were detected by Western blot and protein levels were quantified by ImageJ. (B) Immature or mature control and ΔWASP DCs were stained with fluorochrome conjugated anti–HLA-ABC, -CD86, -CD83 and –HLA-DR antibodies and compared with unstained DCs by flow cytometry. For microscopy images, mature control or ΔWASP DCs were fixed and MHC class I molecules (red) and nuclear DNA (blue) were stained. (C) Mature control DCs, mature ΔWASP DCs and resting NK cells were prestained with vital dyes and cocultured for 1, 20, or 120 minutes. The percentage of NK cell-conjugated DCs was determined by flow cytometry. (D) NK cells were cultured alone or with mature control or ΔWASP DCs for 6 hours, in the presence of BFA. IFN-γ production by NK cells, as well as degranulation (CD107a staining) were assessed by ICS in flow cytometry. NK cells were gated as live, individual, CD3−CD56+ cells. (E) Mature control DCs, mature ΔWASP DCs and resting NK cells were prestained with vital dyes and cocultured for 6 hours, in the presence of BFA, and IFN-γ production by DC-conjugated NK cells was assessed by ICS in flow cytometry, above the high autofluorescence of nonconjugated DCs. (F) Mature control and ΔWASP DCs were cultured with resting NK cells for 6 hours, and the percentage of surviving DCs (% DCs of live cells) was assessed by flow cytometry, using the Fixable Aqua Live/Dead reagent (left graph). Mature control and ΔWASP DCs were prestained with vital dyes and cultured alone or with IL-2 activated NK cells, for 6 hours. The percentage of killed DCs (% killed DCs) was assessed by flow cytometry, using the TO-PRO-3 reagent (right graph). Medians ± interquartile ranges were plotted in the graphs, after subtracting the values of spontaneous lysis of DC single cell cultures. (G) Mature control and ΔWASP DCs were cultured alone for 6 hours, and the percentage of surviving DCs (% DCs of live cells) was assessed by flow cytometry, using the Fixable Aqua Live/Dead reagent. Plots are representative of at least 3 independent experiments. Values on graph bars represent medians from at least 3 independent experiments with duplicates. Error bars indicate interquartile ranges. Original magnifications are 100× for all the microscopy images. *P < .05. P values from Mann-Whitney test, nonparametric and bi-caudal. Scale bars are 10 μm.
Inhibition of DC-derived f-actin polymerization compromises MHC class I polarization and results in a cytotoxic synapse with NK cells
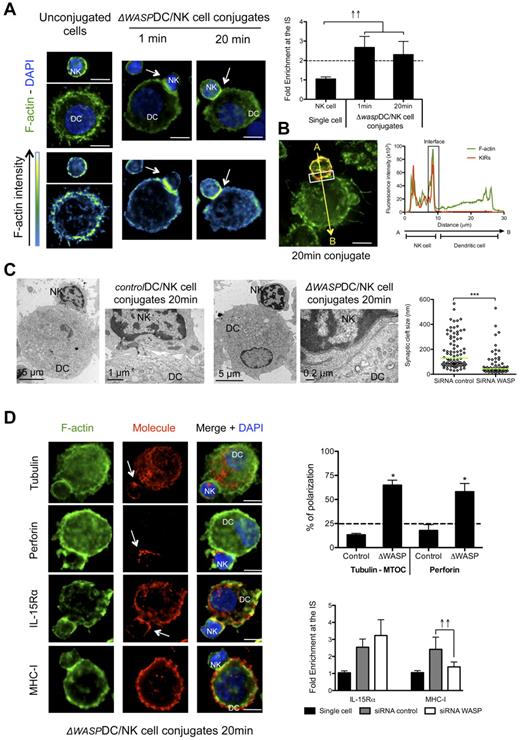
To further test the hypothesis that DC-derived f-actin polymerization was required for inhibitory interactions at the synapse between mature DC and resting NK cells, we studied the distribution of f-actin, perforin granules and MTOCs in the conjugated NK cells. Interestingly, WASP reduction in DCs favored f-actin enrichment on the NK-cell side of the synapse. This f-actin polymerization occurred with accelerated kinetics, right after the formation of the synapse (1 minute) and persisted after 20 minutes of interaction (Figure 5A). Moreover, it overlapped with the staining of KIR molecules in 77.4% ± 1.3% of the 41 analyzed conjugates (P < .05; Figure 5B), indicating a NK cell-derived enrichment of f-actin at the synapse. TEM images from conjugates of mature ΔWASP DCs with resting NK cells revealed narrow synaptic clefts (between 14 and 530 nm, median = 44.1), while the morphology of control conjugates was similar to cocultures without siRNA electroporation (Figure 1D), displaying wider synaptic clefts between 29 and 566 nm (median = 130.7; Figure 5C). These f-actin characteristics and kinetics, as well as the ultrastructure of synapses between mature ΔWASP DCs and resting NK cells resembled the features observed for the cytotoxic synapse of NK cells with MHC class I negative K562 cells (supplemental Figure 1C-E). Consistent with this, NK cell–derived MTOC and perforin granules were found adjacent at the synapse between mature ΔWASP DCs and resting NK cells (Figure 5D). Therefore, DC-derived f-actin polymerization seems to play an important role in the maintenance of inhibitory signals. As the inhibitory signaling mediated by KIR recognition of MHC class I molecules is crucial for the control of NK-cell activation and IFN-γ production, we analyzed maintenance of MHC class I accumulation at the synapse in WASP deficient DCs. We found that mature ΔWASP DCs were unable to maintain polarization of MHC class I molecules at the synapse (Figure 5D). As a second indication that lack of KIR engagement leads to a cytolytic synapse between mature DCs and NK cells, we studied the immunologic synapse between allogeneic mature HLA-Bw4 mismatched DCs and NK cells. The synapse between KIR3DL1 positive NK cells and HLA-Bw4 negative DCs developed with NK cell–derived f-actin polymerization at the synapse between the 2 cells (supplemental Figure 4B). In addition, the majority of the conjugates presented MTOC polarization in NK cells. Therefore, polymerization of DC-derived f-actin and maturation of interactions with resting NK cells seem to be crucial for the stabilization of MHC class I molecules at the synapse, which are important for the inhibition of IFN-γ production and cytotoxicity of NK cells in response to mature DCs.
DC-derived f-actin dynamics are important for stabilization of MHC class I molecules at the synapse and for maintenance of its regulatory features. (A) Mature ΔWASP DC/resting NK-cell cocultures were fixed after 1 or 20 minutes of interaction and f-actin was stained with bodipy conjugated phallacidin (green). DAPI was used to stain nuclear DNA (blue). Arrows indicate the synapse. The graph represents the quantification of f-actin staining intensity at the synapse compared with the staining at the opposite side of the same NK cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (B) Mature ΔWASP DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and stained with bodipy conjugated phallacidin to stain f-actin (green) and anti-KIRs antibodies (red). The fluorescence intensities of KIRs and f-actin stainings were plotted along the indicated trajectory, from A to B. The synapse area in cellular conjugates is indicated by boxes. (C) Mature ΔWASP and control DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and treated for TEM analysis. Original magnifications are 4200×, 13 500×, 4200× and 46 000×, from left to right. The graph represents the size of several regions in the synaptic clefts of 5 DC/NK-cell conjugates for each condition. (D) Mature control or ΔWASP DC co-cultures with resting NK cells were fixed after 20 minutes of interaction. F-actin (green), nuclear DNA (blue) and tubulin, perforin, IL-15Rα, or MHC class I (red) were stained. Arrows point to molecule enrichment at the synapse. The number of conjugates with MTOCs or perforin granules adjacent from the synapse were plotted, in the upper graph, as percentages of total number of conjugates analyzed. The lower graph represents the quantification of IL-15Rα and MHC class I staining intensity at the synapse, compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. Images are representative of 1 experiment for TEM and at least 3 independent experiments for the others. Original magnifications are 100× for all the light microscopy images. Values on graph bars are medians and error bars represent interquartile ranges from the analysis of at least 100 conjugates from at least 3 independent experiments. ↑↑ indicates fold enrichment > 2. *P < .05. Statistics were performed with a null value of 25%, representing random distribution. Scale bars are 10 μm unless specified otherwise.
DC-derived f-actin dynamics are important for stabilization of MHC class I molecules at the synapse and for maintenance of its regulatory features. (A) Mature ΔWASP DC/resting NK-cell cocultures were fixed after 1 or 20 minutes of interaction and f-actin was stained with bodipy conjugated phallacidin (green). DAPI was used to stain nuclear DNA (blue). Arrows indicate the synapse. The graph represents the quantification of f-actin staining intensity at the synapse compared with the staining at the opposite side of the same NK cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (B) Mature ΔWASP DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and stained with bodipy conjugated phallacidin to stain f-actin (green) and anti-KIRs antibodies (red). The fluorescence intensities of KIRs and f-actin stainings were plotted along the indicated trajectory, from A to B. The synapse area in cellular conjugates is indicated by boxes. (C) Mature ΔWASP and control DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and treated for TEM analysis. Original magnifications are 4200×, 13 500×, 4200× and 46 000×, from left to right. The graph represents the size of several regions in the synaptic clefts of 5 DC/NK-cell conjugates for each condition. (D) Mature control or ΔWASP DC co-cultures with resting NK cells were fixed after 20 minutes of interaction. F-actin (green), nuclear DNA (blue) and tubulin, perforin, IL-15Rα, or MHC class I (red) were stained. Arrows point to molecule enrichment at the synapse. The number of conjugates with MTOCs or perforin granules adjacent from the synapse were plotted, in the upper graph, as percentages of total number of conjugates analyzed. The lower graph represents the quantification of IL-15Rα and MHC class I staining intensity at the synapse, compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. Images are representative of 1 experiment for TEM and at least 3 independent experiments for the others. Original magnifications are 100× for all the light microscopy images. Values on graph bars are medians and error bars represent interquartile ranges from the analysis of at least 100 conjugates from at least 3 independent experiments. ↑↑ indicates fold enrichment > 2. *P < .05. Statistics were performed with a null value of 25%, representing random distribution. Scale bars are 10 μm unless specified otherwise.
Pharmacologic inhibition of f-actin polymerization also blocks inhibitory signals and MHC class I polarization at the synapse of mature DCs with resting NK cells
In addition to compromising f-actin polymerization by siRNA mediated silencing of WASP, we treated cocultures of mature DCs and resting NK cells with a pharmacologic inhibitor that specifically inhibits de novo polymerization of f-actin, namely cytochalasin B.31,34 Cytochalasin B treatment disrupted f-actin polymerization at the synapse (supplemental Figure 5A) and the number of conjugated DCs was significantly reduced (supplemental Figure 5B). However, when cytochalasin B was added to cocultures of mature DCs and resting NK cells after 20 minutes of interaction, the number of conjugated DCs was similar to the control conditions (supplemental Figure 5B) and we could observe f-actin polymerization at the synapse (supplemental Figure 5A). We further evaluated the influence of pharmacologic inhibition of cytoskeletal rearrangements on the DC-induced production of IFN-γ by NK cells. In these studies, f-actin polymerization was inhibited right after the start of the coculture (time 0 hours, arresting synapses in an immature stage) or after 2 hours of co-culture (when synapses had matured). As previously described,7,16,21 mature DCs in the co-cultures led to an increase of IFN-γ production by NK cells, compared with cultures of NK cells alone (Figure 6A). Interestingly, addition of cytochalasin B to the co-cultures after 0 hours of interaction resulted in a similar percentage of stimulated IFN-γ+ NK cells (Figure 6A), despite decreased conjugate formation between mature DCs and resting NK cells (supplemental Figure 5B). Furthermore, when f-actin polymerization was inhibited at a later time point (2 hours), a significant increase of IFN-γ producing NK cells was detected (Figure 6A) from the same amount of conjugates as observed in the DMSO control conditions (supplemental Figure 5B). Moreover, we observed a higher percentage of DC conjugated NK cells producing IFN-γ at all time points analyzed, when f-actin dynamics were interrupted (Figure 6B), and this increase was much less pronounced in unconjugated NK cells (supplemental Figure 5C). This confirms the role of f-actin dynamics in the activation of NK cells, on interaction with mature DCs. This increase was not observed when NK-cell single cultures were treated with cytochalasin B (Figure 6A) and this inhibitor did not alter the ability of NK cells to respond to PMA and Ionomycin (supplemental Figure 5D). Consistent with f-actin polymerization in NK cells being important for cytotoxicity, arresting f-actin dynamics with cytochalasin B did not induce NK-cell cytotoxicity against mature DCs, as the percentage of live NK cells and DCs, after 6 hours of coculture, was similar in DMSO and cytochalasin B treated conditions (supplemental Figure 5E). These experiments were repeated using proinflammatory cytokine matured DCs and similar trends were observed (R.B.d.S. and C.M., unpublished data, July 2008). Furthermore, we confirmed enhanced NK-cell activation in cytochalasin B treated cocultures with mature DCs by measuring IFN-γ production in ELISA assays (supplemental Figure 6A). Stimulation of IFN-γ production by NK cells in response to DC-stimulation was cell contact dependent, because it was reduced by transwell separation of the 2 cell types (supplemental Figure 6B).
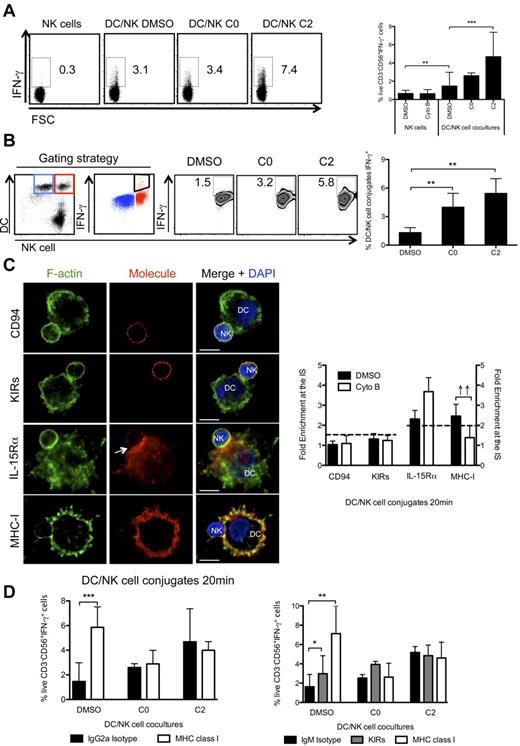
F-actin remodeling is essential for the maintenance of MHC class I/KIRs inhibitory signaling at the mature synapse between mature DCs and resting NK cells. (A) NK cells were cultured alone or with mature DCs for 6h, in the presence of BFA, and IFN-γ production by NK cells was assessed by ICS, followed by flow cytometry. DMSO and cytochalasin B were added at 0 or 2 hours after start of the culture (C0 and C2, respectively). NK cells were gated as live, individual, CD3−CD56+cells. (B) Mature DCs and resting NK cells were prestained with vital dyes and cocultured for 6 hours, in the presence of BFA. IFN-γ production by DC-conjugated NK cells was assessed by ICS in flow cytometry. DMSO or cytochalasin B were added at 0 or 2 hours after start of the coculture (C0 and C2, respectively). IFN-γ+ NK cell-conjugated DCs (in red) were gated as indicated, and could be detected despite the auto-fluorescence of unconjugated DCs (blue). (C) Cytochalasin B was added to cocultures of mature DCs and resting NK cells, and conjugates were fixed after 20 minutes of interaction. F-actin (green), nuclear DNA (blue) and CD94, KIRs, IL-15Rα, or MHC class I (red) were stained. Arrows point to molecule enrichment at the synapse. The graph represents the quantification of molecule staining intensity at the synapse compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (D) Resting NK cells or mature DCs were preincubated with anti-KIRs or anti-MHC class I blocking antibodies (HLA class I specific IgG2a in left and IgM in right panel), respectively, or with the isotype matched antibody controls. DMSO or cytochalasin B were added to cultures as in panel A. Plots are representative of at least 3 independent experiments. Values on graph bars represent medians from at least 3, or 2 for panel B, independent experiments with duplicates. Error bars indicate interquartile ranges. ↑↑ Fold enrichment > 2; *P < .05, **P < .001 and ***P < .0001. P values from Mann-Whitney test, nonparametric, and bi-caudal. Scale bars are 10 μm.
F-actin remodeling is essential for the maintenance of MHC class I/KIRs inhibitory signaling at the mature synapse between mature DCs and resting NK cells. (A) NK cells were cultured alone or with mature DCs for 6h, in the presence of BFA, and IFN-γ production by NK cells was assessed by ICS, followed by flow cytometry. DMSO and cytochalasin B were added at 0 or 2 hours after start of the culture (C0 and C2, respectively). NK cells were gated as live, individual, CD3−CD56+cells. (B) Mature DCs and resting NK cells were prestained with vital dyes and cocultured for 6 hours, in the presence of BFA. IFN-γ production by DC-conjugated NK cells was assessed by ICS in flow cytometry. DMSO or cytochalasin B were added at 0 or 2 hours after start of the coculture (C0 and C2, respectively). IFN-γ+ NK cell-conjugated DCs (in red) were gated as indicated, and could be detected despite the auto-fluorescence of unconjugated DCs (blue). (C) Cytochalasin B was added to cocultures of mature DCs and resting NK cells, and conjugates were fixed after 20 minutes of interaction. F-actin (green), nuclear DNA (blue) and CD94, KIRs, IL-15Rα, or MHC class I (red) were stained. Arrows point to molecule enrichment at the synapse. The graph represents the quantification of molecule staining intensity at the synapse compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (D) Resting NK cells or mature DCs were preincubated with anti-KIRs or anti-MHC class I blocking antibodies (HLA class I specific IgG2a in left and IgM in right panel), respectively, or with the isotype matched antibody controls. DMSO or cytochalasin B were added to cultures as in panel A. Plots are representative of at least 3 independent experiments. Values on graph bars represent medians from at least 3, or 2 for panel B, independent experiments with duplicates. Error bars indicate interquartile ranges. ↑↑ Fold enrichment > 2; *P < .05, **P < .001 and ***P < .0001. P values from Mann-Whitney test, nonparametric, and bi-caudal. Scale bars are 10 μm.
In analogy to our experiments with WASP silencing, we investigated the distribution of MHC class I and IL-15Rα molecules, as well as CD94 and KIRs in conjugates of mature DCs with resting NK cells that had been treated with cytochalasin B. On f-actin dynamics interruption, we observed that the distribution of CD94, KIRs and IL-15Rα was not significantly altered, compared with DMSO treated conditions (Figure 6C). In contrast, cytochalasin B treatment disrupted accumulation of MHC class I molecules at the synapse (Figure 6C). Furthermore, we studied the 3-D distribution of f-actin, IL-15Rα and MHC class I molecules at the synapse (supplemental Figure 6C). In DMSO treated conjugates, f-actin was found segregated from IL-15Rα and MHC class I clusters in the z plane reconstruction of synapses (supplemental Figure 6C). Moreover, a minority of IL-15Rα molecules colocalized with MHC class I molecules, confirming that inhibitory and activating signaling might originate from different synapse domains. The analysis of the DC surface of the cytochalasin B treated conditions showed that, while IL-15Rα retained its distribution (median colocalization with f-actin and MHC class I was only 15.7% and 16.60%, respectively), MHC class I molecules were significantly less segregated from f-actin (40.6% ± 1.8% of colocalized voxels) and less concentrated in distinct surface areas, suggesting that f-actin dynamics are important for the maintenance of inhibitory MHC class I clusters at the synapse.
To provide functional evidence that indeed f-actin stabilized MHC class I dependent inhibitory signaling, we investigated blocking of MHC class I interactions with KIRs under inhibitor treatment. To exclude that the blocking antibodies could lead to CD16 mediated NK-cell activation, IgMs were used in parallel. In these experiments, the percentage of NK cells producing IFN-γ, after blocking MHC class I/KIR interactions, increased (Figure 6D), implicating that MHC class I recognition is an inhibitory event during NK-cell activation by mature DCs and that it down-modulates IFN-γ production by NK cells. Interestingly, when cytochalasin B was added, the blocking antibodies did not further increase the frequency of IFN-γ producing NK cells in the cocultures with DCs (Figure 6D). In contrast, f-actin dynamics interruption did not stimulate IFN-γ production of NK cells cocultured with MHC class I deficient K562 cells (supplemental Figure 7A-B). These functional assays suggest that f-actin stabilizes MHC class I/KIR signaling after synapse maturation between mature DCs and resting NK cells, to limit NK-cell activation in these interactions.
F-actin stabilizes MHC class I molecules at the synapse between mature blood DCs with resting NK cells and represses IFN-γ production by NK cells
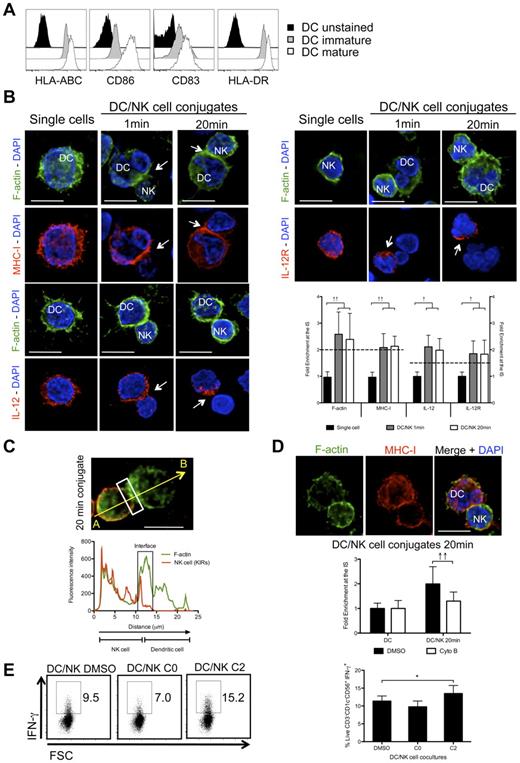
Monocyte-derived DCs have been abundantly used as a model to study human DCs, but might only be representative of one type of DCs, namely inflammatory DCs.35 In contrast, human steady state myeloid DCs (the more abundant CD1c+ (BDCA1+) and less abundant BDCA3+ human blood DCs) were rarely used for NK-cell stimulation.17 On maturation with poly(I:C), CD1c+ DCs up-regulated several surface markers, like the costimulatory molecules CD83 and CD86, as well as the antigen presenting molecules HLA-DR and HLA-ABC (Figure 7A). To analyze their synapse with NK cells, mature blood DCs were conjugated with autologous resting NK cells and the kinetics of f-actin, MHC class I, IL-12, and IL-12R distribution was studied by immunofluorescence (Figure 7B). We were able to see f-actin polymerization at the synapse after 1 minute and 20 minutes of co-culture. This f-actin enrichment seemed to be DC-derived, as it minimally overlapped with the KIR staining of NK cells (79.7% ± 0.9%, P < .05; Figure 7C). In a similar way as f-actin, MHC class I molecules, as well as IL-12 and IL-12R were significantly enriched at the synapse after 1 and 20 minutes of interaction (Figure 7B). These results demonstrate that mature blood DCs can form conjugates with autologous resting NK cells and that the process of synapse maturation is accelerated compared with conjugates with mature monocyte-derived DCs. To characterize the ability of blood DCs to stimulate resting NK cells to produce IFN-γ, cocultures of mature blood DCs and resting NK cells were prepared and IFN-γ was measured after 6 hours of coculture (Figure 7E). Interestingly, mature blood DCs were ∼ 3-fold more efficient in stimulating NK cells to produce IFN-γ, than mature monocyte-derived DCs (Figures 7E and 6A [DMSO treated conditions]). In analogy to Figures 4, 5, and 6, we were furthermore, interested if f-actin dynamics was also playing a role in inhibitory signaling during the crosstalk between mature blood DCs and resting NK cells. Indeed, f-actin dynamics interruption led to an increase in IFN-γ production, correlating with a decrease in MHC class I clustering at the mature synapse (Figure 7D-E).
F-actin dynamics stabilizes MHC class I molecules at the mature synapse of NK cells with human blood DCs. (A) Mature or immature blood DCs were stained with fluorochrome conjugated anti–HLA-ABC, -CD86, -CD83 and –HLA-DR antibodies and compared with unstained blood DCs. (B) Single cell cultures or cocultures of mature blood DCs with resting NK cells were fixed after 1 or 20 minutes of interaction and f-actin (green), nuclear DNA (blue) and MHC class I, IL-12 or IL-12R (red) were stained. Arrows point to molecule enrichment at the synapse. The graph represents the quantification of molecule staining intensity at the synapse compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (C) Mature blood DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and stained with bodipy-conjugated phallacidin to stain f-actin (green) and anti-KIRs antibodies (red). The fluorescence intensities of KIRs and f-actin stainings were plotted along the indicated trajectory, from A to B. The synapse area in cellular conjugates is indicated by boxes. (D) Cytochalasin B was added to cocultures of mature blood DCs with resting NK cells, and conjugates were fixed after 20 minutes of interaction. F-actin (green), nuclear DNA (blue) and MHC class I (red) were stained. Arrows point to molecule enrichment at the synapse. The graph represents the quantification of molecule staining intensity at the synapse compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (E) NK cells were cultured with mature blood DCs for 6 hours and IFN-γ production by NK cells was accessed by ICS in flow cytometry. DMSO or cytochalasin B were added at 0 or 2 hours after start of the cocultures (C0 and C2, respectively). NK cells were gated as live, individual, CD3−CD1c−CD56+cells. Microscopy images are representative of 3 independent experiments. Original magnifications are 100× for all the microscopy images. Values on graph bars (B and D) represent medians from the analysis of at least 100 conjugates from at least 3 independent experiments. Values on graph bars in panel C represent medians from 3 independent experiments with duplicates. Error bars indicate interquartile ranges; ↑↑, fold enrichment > 2 for molecules that exist in both DC and NK cell (IL-15Rα and MHC class I); and ↑, fold enrichment > 1.5 for molecules that exist in only 1 cell (IL-12 and IL-12R); *P < .05. P values from Mann-Whitney test, nonparametric and bi-caudal. Scale bars are 10 μm.
F-actin dynamics stabilizes MHC class I molecules at the mature synapse of NK cells with human blood DCs. (A) Mature or immature blood DCs were stained with fluorochrome conjugated anti–HLA-ABC, -CD86, -CD83 and –HLA-DR antibodies and compared with unstained blood DCs. (B) Single cell cultures or cocultures of mature blood DCs with resting NK cells were fixed after 1 or 20 minutes of interaction and f-actin (green), nuclear DNA (blue) and MHC class I, IL-12 or IL-12R (red) were stained. Arrows point to molecule enrichment at the synapse. The graph represents the quantification of molecule staining intensity at the synapse compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (C) Mature blood DCs were allowed to conjugate with resting NK cells for 20 minutes. Cocultures were fixed and stained with bodipy-conjugated phallacidin to stain f-actin (green) and anti-KIRs antibodies (red). The fluorescence intensities of KIRs and f-actin stainings were plotted along the indicated trajectory, from A to B. The synapse area in cellular conjugates is indicated by boxes. (D) Cytochalasin B was added to cocultures of mature blood DCs with resting NK cells, and conjugates were fixed after 20 minutes of interaction. F-actin (green), nuclear DNA (blue) and MHC class I (red) were stained. Arrows point to molecule enrichment at the synapse. The graph represents the quantification of molecule staining intensity at the synapse compared with the staining at the opposite side of the same conjugated cell. Values were normalized to the values of molecule distribution in unconjugated cells, assigned as 1. (E) NK cells were cultured with mature blood DCs for 6 hours and IFN-γ production by NK cells was accessed by ICS in flow cytometry. DMSO or cytochalasin B were added at 0 or 2 hours after start of the cocultures (C0 and C2, respectively). NK cells were gated as live, individual, CD3−CD1c−CD56+cells. Microscopy images are representative of 3 independent experiments. Original magnifications are 100× for all the microscopy images. Values on graph bars (B and D) represent medians from the analysis of at least 100 conjugates from at least 3 independent experiments. Values on graph bars in panel C represent medians from 3 independent experiments with duplicates. Error bars indicate interquartile ranges; ↑↑, fold enrichment > 2 for molecules that exist in both DC and NK cell (IL-15Rα and MHC class I); and ↑, fold enrichment > 1.5 for molecules that exist in only 1 cell (IL-12 and IL-12R); *P < .05. P values from Mann-Whitney test, nonparametric and bi-caudal. Scale bars are 10 μm.
Discussion
On maturation, human DCs are able to interact and stimulate resting NK cells, without becoming a target for their cytotoxicity. This interaction might occur in several tissues of the body, but seems to be required for NK-cell preactivation in secondary lymphoid organs.10,36 Here, we show that the interaction between mature monocyte-derived DCs and autologous resting NK cells occurs very rapidly and matures with DC-derived f-actin polymerization. While IL-15R accumulation at the conjugate interface was not altered by disruption of f-actin dynamics, this treatment significantly impaired MHC class I polarization, spatial distribution and inhibitory signaling to NK cells. As one possible explanation for these findings, it is tempting to speculate that f-actin might stabilize low affinity interaction at the synapse by fencing in these molecules at the center of the interface. Indeed, the affinity of IL-15 for IL-15Rα or for IL-15Rβγc is higher than the affinity of KIRs for MHC class I molecules (Kd≈0.1-1nM and Kd≈7-100μM, respectively).37-41 Therefore, IL-15Rα might be kept at the synapse by the high-avidity interaction of IL-15 trans-presentation by IL-15Rα to IL-15Rβγc. In contrast, the low affinity interaction of MHC class I molecules with KIRs could require enrichment at the synapse by fence-like partitioning of the f-actin based membrane skeleton (MSK) at the synapse.42 F-actin dynamics in the MSK has been shown to play a role in the diffusion of MHC class II molecules in the plasma membrane, as its disruption lead to an increased diffusion coefficient of these molecules.43 In addition, although inhibitory NK-cell synapses with target cells display no dramatic f-actin polymerization, its pharmacologic inhibition compromises KIR enrichment at these interfaces, suggesting a role for f-actin in maintaining inhibitory signaling even at NK-cell synapses with target cells.44 Therefore, interruption of f-actin dynamics in DC/NK-cell conjugates could lead to an alteration of the MSK fences that could allow MHC class I molecules to diffuse away from the synapse and compromise inhibitory signaling to NK cells at the synapse between mature DCs and resting NK cells.
While several studies on NK-cell and T-cell cytotoxic synapses demonstrated the need of lymphocyte-derived f-actin polymerization and dynamics for NK-cell activation and killing of susceptible target cells,24,26,27,45,46 only a few have analyzed the role of the DC cytoskeleton.31,47 Similar to our work, these previous reports detect DC-derived f-actin polymerization at the synapse in conjugates of mature DC with naive CD4+ T cells and resting NK cells, suggesting that DCs stabilize the synapse during T-cell priming and NK-cell preactivation. Such “imprinting,” that is, synapse organization from the activated APC to the resting effector cell, and later from the primed effector cell to the target cell, has previously been proposed, when it was found that DCs transport MHC class II and costimulatory molecules, prepackaged together in vesicles, to the cell surface,48 presumably for efficient synapse formation with resting CD4+ T cells. Our data give further evidence that DCs stabilize synapses with NK cells during resting NK-cell activation, and thereby orchestrate this interaction.
In summary, our study suggests that DCs stabilize MHC class I molecules via f-actin at their synapse with NK cells to prevent their cytolysis by these innate lymphocytes during NK-cell activation through this interaction. This allows DCs to survive this innate leukocyte interaction for successive priming of adaptive immune responses.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank G. Ferlazzo for the gift of antibodies; S. Mazel for advice on flow cytometry; S. Romao, A. North, U. Ziegler, and T. Bruggman for advice about image acquisition and analyses; and the Center for Microscopy and Image Analysis of the University of Zürich for expert technical support.
R.B.d.S. is a GABBA PhD student and received support from the Portuguese Foundation for Science and Technology, Portugal. This work was in part supported by the National Cancer Institute (R01CA108609), Cancer Research Switzerland (KFS-02 652-08-2010), the Sassella Foundation (10/02), the Vontobel Foundation, the Association for International Cancer Research, and the Swiss National Science Foundation (310030_126995) to C.M.
National Institutes of Health
Authorship
Contribution: R.B.d.S. and C.M. planned, performed, and analyzed the experiments; C.G. provided the HLA-Bw4 expressing K562 cells; and R.B.d.S. and C.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian Münz, Viral Immunobiology, Institute of Experimental Immunology, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland; e-mail: christian.muenz@uzh.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal