In this issue of Blood, Barreira da Silva and colleagues further unravel the mysterious interaction between human NK cells and dendritic cells (DCs) through detailed study of their synaptic interface.1 Here, actin integrity in the DCs works to restrain cytolysis and enable civil cross-talk thus defining a regulatory NK-cell synapse.
The immunologic synapse defines the contact between an immune cell and an object of interest. While most work on immunologic synapses has been performed in T cells, there are many other examples of immune cells that use highly reproducible synapses to access or restrain contact-dependent effector functions. One important example is that of NK cells, known for their ability to mediate contact-dependent cytotoxicity as well as contribute to immune responses through production of cytokines and chemokines. As the major lymphocyte of the innate immune system, NK cells are poised to participate in immunity and are therefore specialized for surveillance and rapid responses. In the course of interacting with potential cells of interest, NK cells form immunologic synapses and use molecular interactions at the synaptic interface to essentially decide whether or not to engage effector functions. These interactions can use both activating and inhibitory NK-cell receptors, thereby generating signals that can either trigger or restrain. Current models of NK-cell engagement hold that the balance between the two determines the outcome. If activating signals predominate, the NK cell will reorganize its actin cytoskeleton at the synapse and polarize its microtubule organizing center (MTOC) along with lytic granules to the synapse to allow for the directed secretion of granule contents onto the target cell to promote cytotoxicity (reviewed in Orange2 ). This is referred to as the NK-cell lytic immunologic synapse. In contrast, if inhibitory signals predominate at the synapse, then actin reorganization is not needed and will be specifically blocked.3 In this case the MTOC and lytic granules will not polarize to the synapse and the targeted cell will not be killed. This type of interaction is referred to as the NK-cell inhibitory synapse (reviewed in Eissmann and Davis4 ).
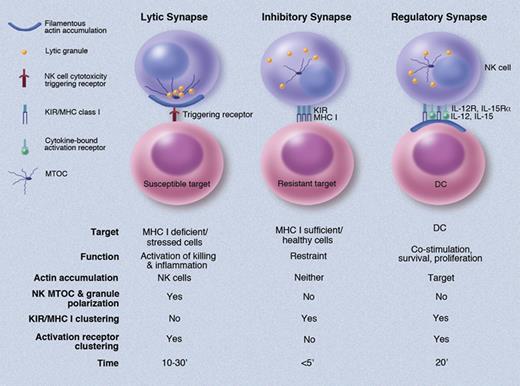
Three distinct types of NK-cell immunologic synapses. Differences between the three types of NK-cell immunologic synapses are depicted schematically with key details noted above each. These include the cell targeted, resulting NK-cell function, synaptic accumulation of actin filaments, polarization of the MTOC and lytic granules, clustering of KIR and MHC ligands, clustering of activation receptors, and the timeframe of formation. With regard to actin accumulation, it is required by the lytic synapse in the NK cell, the regulatory synapse in the DCs, and not at all by the inhibitory synapse. See symbol key in top left corner. Professional illustration by Marie Dauenheimer.
Three distinct types of NK-cell immunologic synapses. Differences between the three types of NK-cell immunologic synapses are depicted schematically with key details noted above each. These include the cell targeted, resulting NK-cell function, synaptic accumulation of actin filaments, polarization of the MTOC and lytic granules, clustering of KIR and MHC ligands, clustering of activation receptors, and the timeframe of formation. With regard to actin accumulation, it is required by the lytic synapse in the NK cell, the regulatory synapse in the DCs, and not at all by the inhibitory synapse. See symbol key in top left corner. Professional illustration by Marie Dauenheimer.
Not all NK-cell synaptic interactions, however, cleanly fit into lytic or inhibitory categories. This is especially true of the interaction between NK cells and DCs. While immature DCs can be destroyed (or edited) by NK cells, the interaction between DCs and NK cells can enable civil cross-talk to promote the functions of both cells.5 In the latter case, the NK cell can be induced by IL-12, IL-15, and IL-18 provided by the DCs and the NK cell can facilitate DC functions via producing regulated amounts of TNF-α, IFN-γ, and HMGB-1.5-8 Importantly, in these types of interactions with mature DCs, NK cells do not kill the DCs owing to the ligation of inhibitory receptors on the NK cell by DC-expressed inhibitory ligands.
In this issue of Blood, Barreria de Silva and colleagues have advanced earlier studies of the interaction between DCs and NK cells to define a third type of NK-cell immunologic synapse; the NK-cell regulatory synapse (see figure). The regulatory synapse is in some ways a mixture of the two earlier-defined types of NK-cell synapses (lytic and inhibitory) plus additional unique characteristics. In particular the regulatory synapse requires active processes within both the NK cell and DCs to enable the regulatory output and is therefore in the truest sense, collaboration. This is best exemplified by the requirement for actin reorganization in the DCs (a previously reported finding6 ). Actin reorganization in the DCs allows for MHC-I clustering at the regulatory synapse, thereby preventing the formation of a lytic synapse in the NK cell.1 This is distinct from inhibitory synapses, where actin function is not a fixed requirement.3 The regulatory synapse, however, is likely to need this extra emphasis from the DCs because it also provides IL-12, IL-18, and IL-15 at the synaptic interface to moderately activate the NK cells.1,6,7 This can serve the purpose of promoting NK-cell survival, proliferation, and priming for further responsiveness.7 Although inhibitory signaling likely occurs through KIR ligation in the NK cell at the regulatory synapse, the lifetime of this synapse is longer than that of the inhibitory synapse and presumably includes the presence of activating receptors.1,9,10 The present study demonstrates that unlike the lytic synapse , the regulatory synaptic cleft is much broader having a greater mean distance between the NK cell and DC membrane.1,10 The exact purpose of this difference is likely to be significant mechanistically. Thus a number of unique characteristics define the regulatory synapse as a specific and specialized interface using distinct mechanisms to enable particular functions.
This work raises important questions as to the scope of this type of NK-cell regulatory synapse. First, while IL-15 trans-presentation has been shown to be required,7 the full sequence and temporal role of receptor ligand functions at this synapse remains to be determined. This should shed additional insight on the mechanisms of access to the regulatory synapse and will be especially informative given the preference of CD56bright “immature” NK cells for forming this type of synapse.1 Second, because NK cells express a variety of costimulatory ligands, which can promote other immune functions including those of adaptive immunity, it will be important to determine how these interactions compare with the presently defined regulatory synapse. To what extent do other nonlytic contact-dependent functional interactions of NK cells share the presently defined parameters and cellular mechanisms of the regulatory synapse? Finally, while it remains to be demonstrated as to how this third type of NK-cell synapse will fit into coordinated immune responses, it is likely it will prove to be as relevant to host defense and immunity as either of the other two. It also raises the question as to what other types of functionally and mechanistically distinct NK-cell synapses remain to be identified.
Conflict of interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal