Abstract
We previously demonstrated that the gene encoding PTPROt, the truncated form of protein tyrosine phosphatase receptor type O expressed predominantly in hematopoietic cells, is a candidate tumor suppressor and is down-regulated in chronic lymphocytic leukemia (CLL). Here, we show that PTPROt expression is significantly reduced in CD19+ spleen B cells from Eμ-T cell leukemia 1 (TCL1) transgenic mice relative to the wild-type mice. Strikingly, as much as a 60% decrease in PTPROt expression occurs at 7 weeks independently of promoter methylation. To elucidate the potential mechanism for this early suppression of PTPROt in these mice, we explored the role of activating protein-1 (AP-1) in its expression. We first demonstrate that AP-1 activation by 12-O-tetradecanoylphorbol-13-acetate induces PTPROt expression with concurrent recruitment of c-fos and c-jun to its promoter. The PTPROt promoter is also responsive to over- and underexpression of AP-1, confirming the role of AP-1 in PTPROt expression. Next, we demonstrate that TCL1 can repress the PTPROt promoter by altering c-fos expression and c-jun activation state. Finally, using primary CLL cells we have shown an inverse relationship between TCL1 and PTPROt expression. These findings further substantiate the role of TCL1 in PTPROt suppression and its importance in the pathogenesis of CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent form of adult leukemia in the Western world. In view of the relatively large incidence of CLL, the altered expression of specific growth regulatory genes in this leukemia relative to normal B cells is of considerable interest with respect to its potential applications in diagnosis, prognosis, and specific drug targeting. One such gene is that encoding the receptor-type protein tyrosine phosphatase PTPRO (also designated PTP-oc, PTP-U2, PTP-Φ, and Glepp1). Lymphoid cells express a truncated form of PTPRO, termed PTPROt, that is generated by transcription from a distinct promoter.1 PTPROt expression is high in naïve B lymphocytes but is suppressed in primary diffuse large B-cell lymphoma and diffuse large B-cell lymphoma cell lines.2 We have shown previously that the expression of PTPROt is significantly reduced in the majority of a large cohort of primary human CLL samples.3 This study also demonstrated that PTPROt is methylated at a far upstream CpG island (CGI) in the majority of primary CLL samples relative to normal lymphocytes as well as in the WaC3CD5 leukemia cell line and that treatment of this cell line with DNA-hypomethylating agents results in re-expression of the gene. Methylation of this CGI generally correlated inversely with PTPROt expression in a few primary CLL samples tested.3
We also have demonstrated that both full-length4 and the truncated5 forms of this enzyme exhibit the characteristics of a candidate tumor suppressor that include delayed entry of the cells into cell cycle and increased susceptibility to apoptosis. Furthermore, PTPRO is localized to the chromosomal region 12p12.3 that is characterized by loss of heterozygosity in different cancer cell types.6-8 The growth suppressor characteristics of this protein that can lead to altered phosphorylation of its substrates prompted us to explore the mechanisms of regulation of its expression in CLL. Recent studies in our laboratory9 and elsewhere10 have identified the kinases Syk and Lyn, both involved in B-cell receptor signaling, as substrates of PTPROt. These kinases are either up-regulated, constitutively active, or both in CLL and have proven role in the pathogenesis of CLL.11,12 In addition, therapies targeting these specific kinases have demonstrated durable clinical activity in a large proportion of CLL patients.13 These observations raise the possibility that loss of PTPROt expression may contribute to the pathogenesis and progression of CLL and further prompted our interest in understanding the mechanism of PTPROt deregulation in CLL.
Although methylation plays an important role in gene suppression, it is becoming evident that this epigenetic modification may be preceded by transcriptional repression.14 To explore this possibility, it is critical to identify the transcription factors involved in the expression of PTPROt in B cells and the molecular mechanism for its deregulation in CLL. For this purpose, it would be ideal to use a biologic system in which the gene can be studied in its transcriptionally active and inactive states. We have therefore used 2 different models to investigate the mechanism of transcriptional regulation of PTPROt in normal and disease states, namely, the Eμ–T-cell leukemia 1 (TCL1) transgenic (Tg) mouse model of CLL and 12-O-tetradecanoylphorbol-13-acetate (TPA)–inducible expression in U937 cells. The TCL1 gene, which is responsible for prolymphocytic T-cell leukemia development, is also overexpressed in human B-cell neoplasms.15 Tg mice specifically expressing the TCL1 gene in the B-cell compartment16 develop a lymphoproliferative disorder on aging that mimics human CLL17 and have therefore been extensively used to explore different aspects of CLL disease progression14,16,18 and therapy.19 Because of its similarity to human CLL, this model is an ideal system to explore dysregulation of PTPROt expression. The use of the second model system was based on the observation that TPA can dramatically induce PTPROt expression in the monocytic cell line U937.20 This cell culture model provides a unique tool to study its expression and regulation in its uninduced (inactive) and induced (active) states. We have then extended our studies to CLL-like cell lines Mec1 and WaC3CD5 as well as primary CLL samples to confirm that the mechanisms identified using other models are also valid in the B-cell system. Here, we show that activating protein-1 (AP-1) elements play a critical role in PTPROt expression and that their inactivation by TCL1 in CLL is a major mechanism underlying PTPROt suppression in this leukemia.
Methods
Reagents
The antibodies used in this study were as follows: anti–c-jun, anti–c-fos, anti-TCL1 (Santa Cruz Biotechnology), anti–p(S73)-c-jun, anti-p(S383)Elk-1 (Cell Signaling Technology), anti-Ku70 (NeoMarkers), and anti-GAPDH (Millipore). The TCL1, c-fos, and c-jun expression plasmids and PTPt-P-Luc reporter plasmid were described previously.5,21 c-jun and TCL1 small interfering RNA (siRNA) were from Santa Cruz Biotechnology. GST-c-jun (1-79) was a generous gift from Dr Michael Karin (University of California, San Diego). Murine stem cell virus (MSCV) + TCL1 and MSCV were generous gifts from Dr Michael Teitell (University of California, Los Angeles).
Mouse sample preparation
All mouse studies were performed under protocols approved by The Ohio State University Institutional Animal Care and Use Committee. CD19+ spleen B cells were isolated from C3H/B6 (The Jackson Laboratory) and TCL1 Tg mice using MACS cell separation reagents (Miltenyi Biotec). Mice were considered to have CLL if they showed visible signs of hepatosplenomegaly and met other criteria as described previously.16 The B-cell purity (> 95%) was assessed by flow cytometry. RNA was extracted using TRIzol reagent (Invitrogen) following manufacturer's protocol, followed by DNase I treatment. DNA was isolated by standard proteinase K digestion.22,23 Whole cell extracts were prepared in cell lysis buffer (50mM Tris, pH 8.1, 10mM EDTA, and 1% SDS) supplemented with protease and phosphatase inhibitor cocktails.
Human primary CLL samples
Peripheral blood was obtained from CLL patients after obtaining written consent on an Ohio State University Institutional Review Board–approved protocol, according to the Declaration of Helsinki. Patients were newly diagnosed or without treatment for a minimum of 30 days. Patients had a confirmed diagnosis of their disease by National Cancer Institute criteria and significantly elevated peripheral leukocyte counts (> 20 000/μL), but they were otherwise unselected based on disease stage or prognostic subgroup. CD19-positive tumor cells were enriched using RosetteSep (StemCell Technologies) followed by Ficoll density gradient centrifugation. Cells were incubated at 37°C and 5% CO2 in RPMI-1640 with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2mM l-glutamine (Sigma-Aldrich). Fluorescence in situ hybridization (FISH) was performed as described previously.24
Cell lines, treatments, and transfection
U937 and K562 cells were obtained from ATCC and cultured following ATCC recommendations. CLL-like cell line Mec1 (DSMZ) and WaC3CD5 cells25 were cultured in RPMI-1640 supplemented with 10% FBS and antibiotics. For TPA treatment, cells were seeded at 0.2 × 106 mL and treated with 10 ng/mL TPA for 24 hours. U937 cells were transfected by Nucleofection (Lonza) following the manufacturer's recommended protocol. K562 and Mec1 cells were transfected using Lipofectamine 2000 (Invitrogen) following manufacturer's protocol and harvested 48 hours after transfection. K562 cells stably expressing TCL1 were selected with 750 μg/mL G418 for 7 days followed by culture in 375 μg/mL G418-containing media. WaC3CD5 cells expressing PTPROt were generated by retroviral infection3 followed by selection in 1 μg/mL puromycin for 7 days before harvesting them for protein extraction.
Real-time RT-PCR
Expressions of PTPROt, c-fos, and c-jun were analyzed by real-time RT-PCR using SYBR Green chemistry. The primers used were as follows: mPTP-RT-F2, 5′-ATGAACGAAGAGGAAGGAGCAG-3′ and mPTP-RT-R2, 5′-GCAATGGGTTCTTCTGTGAATGG-3′; β-actin-RT-F, 5′-CGTCTTCCCCTCCATCGTG-3′ and β-actin-RT-R, 5′-GGTGTGGTGCCAGATTTTCTCC-3′; hc-jun-RT-F, 5′-ACGACCTTCTATGACGATGC-3′ and hc-jun-RT-R, 5′-TGATGTGCCCGTTGCTGGACTGG-3′; hc-fos-RT-F, 5′-GGGGCAAGGTGGAACAGTTATC-3′ and hc-fos-RT-R, 5′-TTCAGCAGGTTGGCAATCTCGGTC-3′; hPTP-RT-F, 5′-CTCCACCCAAATCACTCTTCGCAG-3′ and hPTP-RT-R, 5′-ACCATTGTTGAGACGGCTATGAACG-3′; and hpre-fos-F, 5′-AATGGTTTCACAGTGGGGTGC-3′ and hpre-fos-R, 5′-ACCGCTTGGAGTGTATCAGTCAGC-3′.
Combined bisulfite restriction analysis
Bisulfite conversion of DNA was performed as described previously.22,23 The PTPRO CpG island was amplified using the nested primers mPTP-BS-F1, 5′-CGGAGGGTTGTTTGTTTG-3′ and mPTP-BS-R1, 5′-AACTTCCAAATCTTTCCAC-3′; and mPTP-BS-F2, 5′-GGTAATCGTTGTAGTTGTGG-3′ and mPTP-BS-R2, 5′-ACGAAATAAATCCCCATC-3′. The PCR product was digested with HinfI. Tsp509I digestion was used to confirm complete conversion of DNA (data not shown).
ChIP assay
ChIP assay was performed essentially as described previously.26 In brief, cells (control or TPA treated) were cross-linked with 1% formaldehyde for 15 minutes followed by neutralization with glycine. Chromatin prepared in cell lysis buffer (50mM Tris, pH 8.1, 10mM EDTA, and 1% SDS) was used for immunoprecipitation with IgG, anti–c-fos, or anti–c-jun. After decross-linking, DNA was recovered by phenol:chloroform:isoamyl alcohol/chloroform:isoamyl alcohol precipitation. The input and pull-down DNA were subjected to PCR with primers specific for the PTPROt promoter: hPTPt-ChIP-F1, 5′-CCTACCTATATTTGAATTGGGGGATGC-3′ and hPTPt-ChIP-R1, 5′-CCTGAAGTTTGGTTTTCTGACTGGAG-3′. The intensity of the PCR product from pull-down DNA was normalized to that obtained from input DNA. Fold enrichment in TPA-treated cells was calculated as increase in association over that observed in untreated control.
JNK assay
GST–c-jun (1-73) was induced in bacteria and purified using glutathione-Sepharose beads as described previously.9 The JNK activity assay was performed following a published protocol.27 In brief, the extracts of K562 cells (vector or TCL1-expressing cells) were prepared in WCE300. An equal amount of extract (400 μg) was diluted 3 times with dilution buffer and incubated with 10 μg GST–c-jun for 3 hours at 4°C with constant agitation. After 5 washes with wash buffer, the beads were equilibrated in kinase buffer. The assay was performed in 30 μL of kinase buffer with 20μM ATP and 1.0 μCi of [γ-32P]ATP for 20 minutes at 30°C. The reaction was stopped by adding 4 × Laemmli loading buffer, and the proteins that were resolved on 10% SDS-PAGE were subjected to autoradiography.
Statistical analysis
All experiments were repeated as indicated in the figure legends. Error bars were generated using mean ± SD of respected data. Comparisons of 2 datasets for statistical significance were performed using the Student t test. For data obtained from primary CLL samples, a linear regression model was used to study the association between PTPRO mRNA expression and its possible upstream factors.
Results
PTPROt is suppressed in the TCL1 Tg mouse model of CLL
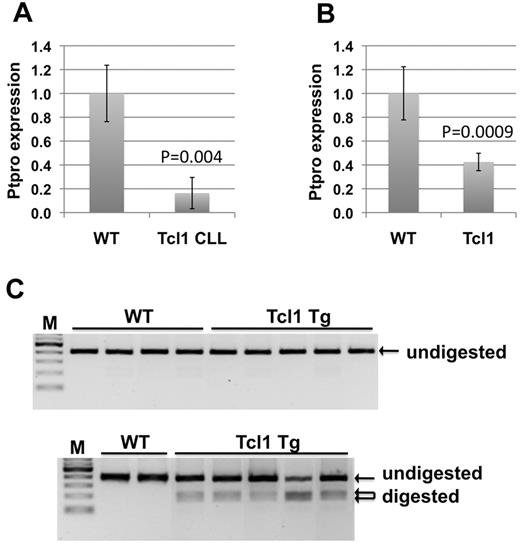
The availability of the well-established Eμ-TCL1 Tg mouse model of CLL provides an ideal system to study regulation of PTPROt expression in CLL. As a first step, we investigated whether PTPRO expression was suppressed in this CLL model. For this purpose, we performed real-time RT-PCR analysis of CLL spleen B lymphocytes from TCL1 Tg mice and normal spleen B cells from syngeneic wild-type (WT) mice. The data indeed revealed ∼ 84% suppression of PTPROt in the CLL B cells relative to normal B cells (Figure 1A). To determine whether PTPRO suppression was an early event in the TCL1 Tg mice, we analyzed its expression in spleen B lymphocytes from 7-week-old TCL1 Tg and syngeneic WT mice. Real-time RT-PCR analysis showed that PTPRO was suppressed by as much as 60% at this early time point (Figure 1B). Because we have observed previously the methylation of PTPRO CGI in primary CLL cells, the next obvious question was whether PTPRO suppression in the mice was accompanied by methylation of its CGI. To test this possibility, we performed combined bisulfite restriction analysis on DNA isolated from the spleen B cells at early stage as well as the CLL stage (same as those used to check its expression). The data showed that the PTPRO CGI was methylated, albeit at a low level, in CLL B lymphocytes but that it remained essentially unmethylated in B lymphocytes from WT syngeneic mice (Figure 1C). Interestingly, no detectable methylation was observed in the B cells from TCL1 Tg mice at 7 weeks.
Transcriptional suppression and methylation of PTPROt in lymphocytes from TCL1 Tg mice. Real-time RT-PCR analysis of RNA from CD19+ selected spleen B cells from WT and TCL1 Tg mice at CLL stage (A) and at 7 weeks (B). Error bars represent SD. P values were calculated using the Student t test. (C) Bisulfite converted DNA from spleen CD19+-selected B cells of WT and TCL1 Tg mice at 7 weeks (top) and at CLL stage (bottom) were subjected to combined bisulfite restriction analysis using HinfI as the restriction enzyme.
Transcriptional suppression and methylation of PTPROt in lymphocytes from TCL1 Tg mice. Real-time RT-PCR analysis of RNA from CD19+ selected spleen B cells from WT and TCL1 Tg mice at CLL stage (A) and at 7 weeks (B). Error bars represent SD. P values were calculated using the Student t test. (C) Bisulfite converted DNA from spleen CD19+-selected B cells of WT and TCL1 Tg mice at 7 weeks (top) and at CLL stage (bottom) were subjected to combined bisulfite restriction analysis using HinfI as the restriction enzyme.
AP-1 elements play a role in PTPROt transcription
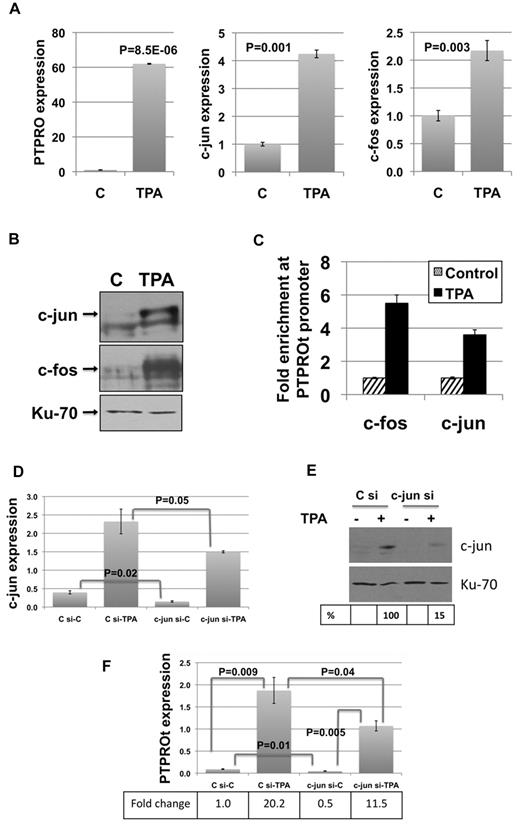
The suppression of PTPRO at an early time point in TCL1 Tg mice in the absence of CGI methylation prompted us to explore its transcriptional regulation. PTPROt is transcribed from a promoter located between exons 12 and 13.1 Promoter analysis using the evolutionarily conserved regions browser28,29 revealed that PTPROt harbors several AP-1 elements that are conserved between mice and humans. Interestingly, TCL1 is known to function as a transcriptional regulator by inhibiting the transactivation potential of AP-1.21 It is noteworthy that PTPROt was initially cloned as a TPA-inducible phosphatase,20 suggesting the involvement of AP-1 in its transcription. To determine whether AP-1 elements c-fos and c-jun play a direct role in PTPROt expression, we used U937 cells where PTPROt expression can be induced by TPA.20 Treatment of U937 cells with 10 ng/mL TPA for 24 hours resulted in dramatic increase in PTPROt expression (Figure 2A). Under this condition, a marked increase in the mRNA and protein levels of c-fos and c-jun also was observed (Figure 2A-B). To confirm potential alteration in association of these AP-1 elements with the PTPROt promoter, ChIP was performed using antibodies specific for c-fos and c-jun. The data revealed 3- to 5-fold enrichment of these factors at the PTPROt promoter following TPA-induced differentiation of U937 cells (Figure 2C), indicating the involvement of AP-1 elements in PTPRO transcription. This notion was further confirmed by siRNA-mediated knockdown of c-jun. After transfection with control or c-jun siRNA, U937 cells were split for control and TPA treatment. Real-time RT-PCR and Western blot analysis demonstrated inhibition of basal as well as TPA-induced up-regulation of c-jun in c-jun siRNA-transfected cells compared with control siRNA-transfected cells (Figure 2D-E). Similarly, real-time RT-PCR showed inhibition of basal and TPA-induced expression of PTPROt in c-jun siRNA-transfected cells (Figure 2F).
AP-1 elements are involved in PTPROt transcription and are essential for its transcription. U937 cells treated with 10 ng/mL TPA for 24 hours were used for expression of PTPROt, c-fos, and c-jun by real-time RT-PCR (A) and expression of c-fos and c-jun by Western blot (B). Error bars represent SD. P values were calculated using the Student t test. (C) Formaldehyde cross-linked chromatin from control and TPA-treated (10 ng/mL) U937 cells was used for immunoprecipitation with antibodies specific for c-fos and c-jun followed by PCR for PTPROt promoter. The data are normalized to input and represented as fold enrichment in TPA-treated cells over control cells. The experiment was repeated twice with similar results. Error bars represent SD. Depletion of c-jun in U937 cells by siRNA was measured by real-time RT-PCR (D) and Western blot (E). (F) Effect of c-jun siRNA on basal and TPA-induced expression of PTPROt was assessed by real-time RT-PCR. The experiment was repeated twice with similar observations. Error bars represent SD. P values were calculated using the Student t test.
AP-1 elements are involved in PTPROt transcription and are essential for its transcription. U937 cells treated with 10 ng/mL TPA for 24 hours were used for expression of PTPROt, c-fos, and c-jun by real-time RT-PCR (A) and expression of c-fos and c-jun by Western blot (B). Error bars represent SD. P values were calculated using the Student t test. (C) Formaldehyde cross-linked chromatin from control and TPA-treated (10 ng/mL) U937 cells was used for immunoprecipitation with antibodies specific for c-fos and c-jun followed by PCR for PTPROt promoter. The data are normalized to input and represented as fold enrichment in TPA-treated cells over control cells. The experiment was repeated twice with similar results. Error bars represent SD. Depletion of c-jun in U937 cells by siRNA was measured by real-time RT-PCR (D) and Western blot (E). (F) Effect of c-jun siRNA on basal and TPA-induced expression of PTPROt was assessed by real-time RT-PCR. The experiment was repeated twice with similar observations. Error bars represent SD. P values were calculated using the Student t test.
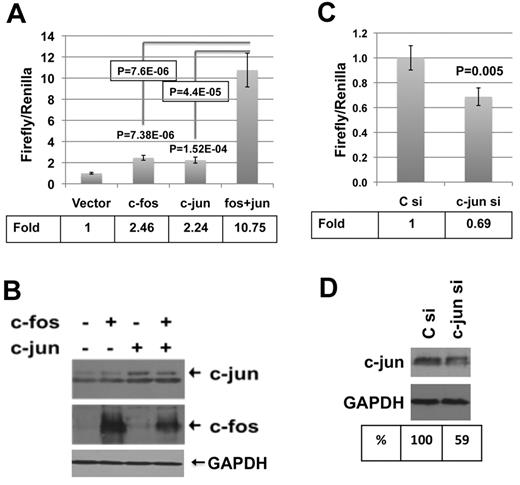
The role of AP-1 elements also was established by transfecting a PTPROt promoter-luciferase reporter construct in the presence or absence of AP-1 elements in K562 cells. Cotransfection of the promoter-reporter plasmid along with either c-fos– or c-jun–expressing plasmid resulted in 2-fold increase in promoter activity (Figure 3A) that further increased 5-fold on coexpression of both c-fos and c-jun (Figure 3A). Western blot analysis confirmed the expression of c-fos, c-jun, or both in these cells (Figure 3B). Conversely, a 30% decrease in promoter activity was observed on transfection of these cells with c-jun siRNA (Figure 3C). Western blot analysis confirmed 40% decrease in c-jun levels in these cells (Figure 3D).
AP-1 regulates PTPROt promoter in K562 cells. K562 cells were transfected with either pGL3-basic or PTPt-P-luc (PTPROt promoter region from −1040 to +261 with respect to transcription start site cloned upstream of firefly luciferase in pGL3-basic5 ) in combination with expression vectors for c-fos, c-jun, or both. Luciferase activity was measured using a dual luciferase assay kit. (A) Normalized promoter activity (firefly/Renilla) is represented as fold change over the activity in the absence of c-fos and c-jun. (B) Overexpression of c-fos and c-jun was confirmed by Western blot analysis. K562 cells were transfected with pGL3-basic or PTPt-P-Luc along with control or c-jun–specific siRNA. Luciferase activity was measured using a dual luciferase assay kit. (C) Normalized promoter activity (firefly/Renilla) is represented as fold change in c-jun siRNA-transfected cells over control siRNA-transfected cells. (D) Knockdown of c-jun was confirmed by Western blot analysis. Each experiment was performed with 5 replicates and repeated twice. Error bars represent SD. P values were calculated using the Student t test.
AP-1 regulates PTPROt promoter in K562 cells. K562 cells were transfected with either pGL3-basic or PTPt-P-luc (PTPROt promoter region from −1040 to +261 with respect to transcription start site cloned upstream of firefly luciferase in pGL3-basic5 ) in combination with expression vectors for c-fos, c-jun, or both. Luciferase activity was measured using a dual luciferase assay kit. (A) Normalized promoter activity (firefly/Renilla) is represented as fold change over the activity in the absence of c-fos and c-jun. (B) Overexpression of c-fos and c-jun was confirmed by Western blot analysis. K562 cells were transfected with pGL3-basic or PTPt-P-Luc along with control or c-jun–specific siRNA. Luciferase activity was measured using a dual luciferase assay kit. (C) Normalized promoter activity (firefly/Renilla) is represented as fold change in c-jun siRNA-transfected cells over control siRNA-transfected cells. (D) Knockdown of c-jun was confirmed by Western blot analysis. Each experiment was performed with 5 replicates and repeated twice. Error bars represent SD. P values were calculated using the Student t test.
TCL1 represses PTPROt promoter by inhibiting phosphorylation of c-jun and expression of c-fos
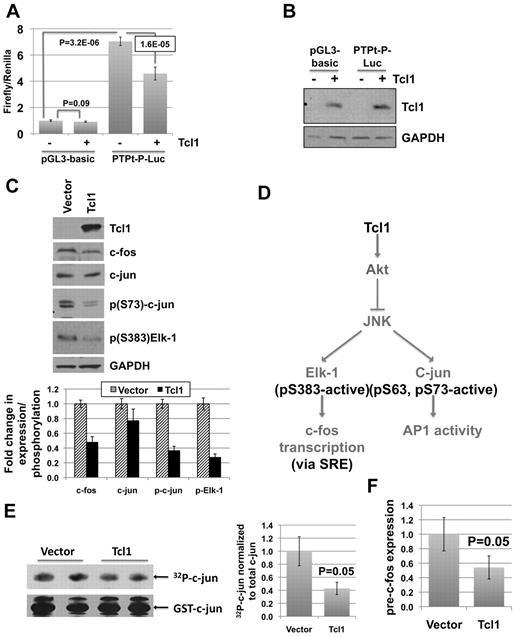
PTPROt suppression in TCL1 Tg mouse model for CLL as well as TCL1-mediated inhibition of the transactivation potential of AP-121 suggested the possibility that TCL1 itself may down-regulate PTPROt expression. To address this issue, TCL1 was cotransfected with either pGL3-basic or PTPt-P-Luc in K562 cells that do not express endogenous TCL1. Although there was no significant effect of TCL1 on the activity of the basic promoterless construct relative to the vector-transfected cells, the PTPROt promoter activity was reduced by 35% (Figure 4A) following ectopic expression of TCL1 in these cells. TCL1 expression in these cells was confirmed by Western blot analysis (Figure 4B).
TCL1 suppresses PTPROt promoter in K562 cells by inhibiting c-fos transcription and c-jun activity. K562 cells were transfected with pGL3-basic or PTPt-P-Luc and TCL1 expression vector as indicated. (A) Normalized luciferase activity (firefly/Renilla) is reported as fold change over pGL3-basic activity. Error bars represent SD. P values were calculated using the Student t test. (B) TCL1 expression in transfected cells was confirmed by Western blot. The experiment was repeated twice with 5 replicates each. (C) Western blot analysis of whole cell extracts prepared from K562 cells stably expressing TCL1 using the antibodies indicated in the figure. Quantification of bands using AlphaImager (Alpha Innotech) represented as column graph. Error bars represent SD. (D) Possible mechanisms of AP-1 inhibition by TCL1. (E) JNK activity assay was performed using extracts from K562 cells transfected with vector or stably expressing TCL1. 32P-Labeled substrate (c-jun) was resolved on 10% SDS-PAGE and subjected to autoradiography and immunoblotting with anti-GST antibody. The experiment was repeated twice with duplicates in each run. Quantification of bands represented as column graph. Error bars represent SD. P values were calculated using the Student t test. (F) Expression of pre-c-fos normalized to β-actin was analyzed by real-time RT-PCR on total RNA from vector control and TCL1-expressing K562 cells. The experiment was repeated twice with triplicate measurements. Error bars represent SD. P values were calculated using the Student t test.
TCL1 suppresses PTPROt promoter in K562 cells by inhibiting c-fos transcription and c-jun activity. K562 cells were transfected with pGL3-basic or PTPt-P-Luc and TCL1 expression vector as indicated. (A) Normalized luciferase activity (firefly/Renilla) is reported as fold change over pGL3-basic activity. Error bars represent SD. P values were calculated using the Student t test. (B) TCL1 expression in transfected cells was confirmed by Western blot. The experiment was repeated twice with 5 replicates each. (C) Western blot analysis of whole cell extracts prepared from K562 cells stably expressing TCL1 using the antibodies indicated in the figure. Quantification of bands using AlphaImager (Alpha Innotech) represented as column graph. Error bars represent SD. (D) Possible mechanisms of AP-1 inhibition by TCL1. (E) JNK activity assay was performed using extracts from K562 cells transfected with vector or stably expressing TCL1. 32P-Labeled substrate (c-jun) was resolved on 10% SDS-PAGE and subjected to autoradiography and immunoblotting with anti-GST antibody. The experiment was repeated twice with duplicates in each run. Quantification of bands represented as column graph. Error bars represent SD. P values were calculated using the Student t test. (F) Expression of pre-c-fos normalized to β-actin was analyzed by real-time RT-PCR on total RNA from vector control and TCL1-expressing K562 cells. The experiment was repeated twice with triplicate measurements. Error bars represent SD. P values were calculated using the Student t test.
To elucidate the mechanism of TCL1-mediated repression of PTPROt, TCL1 was stably expressed in K562 cells to assess its effect on AP-1 elements. The results demonstrated dramatic decrease in the level of c-fos but not c-jun following TCL1 expression (Figure 4C). Interestingly, the regulation of c-fos at the mRNA level in response to mitogenic stimuli has been extensively studied compared with its posttranslational modification.30 The relatively less dramatic decrease in c-jun expression prompted us to focus on changes that may affect its transcriptional activity. JNK-mediated phosphorylation of Ser63 and Ser73 within the N-terminal transactivation domain of c-jun is known to potentiate its activity.30,31 Because TCL1 can activate Akt32 and the activated Akt can reduce JNK activity33,34 it was logical to assume that TCL1 expression would inhibit phosphorylation of c-jun at the N-terminal transactivation domain (see Figure 4D depicting TCL1-mediated inhibition of c-fos transcription and c-jun activity). Western blot analysis using anti–p(S73)-c-jun antibody indeed demonstrated its reduced phosphorylation in TCL1 expressing K562 cells compared with vector transfected cells (Figure 4C). To demonstrate that TCL1 indeed inhibited JNK activity, it was assayed in the extracts from K562 cells stably expressing TCL1 using GST–c-jun (1-79) as the substrate. Phosphorylation of GST–c-jun was significantly lower (P = .05) in TCL1-expressing cells than the control (vector-transfected) cells indicating that the activity of JNK was compromised because of TCL1 expression (Figure 4E).
Interestingly, JNK also can phosphorylate and activate Elk-1,27,35 a transcription factor involved in c-fos expression27,36 (Figure 4D). To determine whether suppression of c-fos by TCL1 also was because of inactivation of JNK, we checked the phosphorylation of Elk-1 by Western blot analysis and we indeed observed reduced Elk-1 phosphorylation in K562 cells expressing TCL1 compared with the control cells (Figure 4C). Furthermore, real-time RT-PCR analysis with primers specific to pre-mRNA confirmed that c-fos suppression occurred at the transcriptional level (Figure 4F). The suppression of c-fos and inhibition of c-jun phosphorylation by TCL1 could therefore explain the transcriptional repressor effect of TCL1 on PTPROt expression.
TCL1 also inactivates AP-1 in B-CLL cell lines
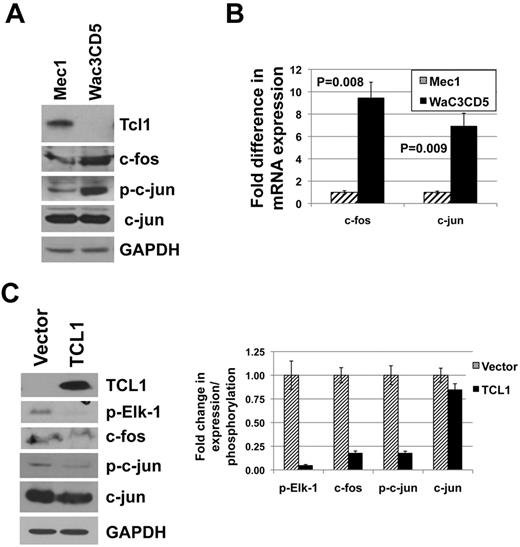
To confirm that TCL1-mediated inactivation of AP-1 and suppression of PTPROt similarly occur in B-cell (B)-CLL, we initially determined the expression of TCL1 in 2 CLL-like cell lines Mec1 and WaC3CD5. The data demonstrated that while Mec1 expressed TCL1, WaC3CD5 cells did not (Figure 5A). Comparison of the status of AP-1 elements between these cells indeed showed that the expression of c-fos (at both protein and RNA level) as well as phosphorylation of c-jun was higher in the TCL1 nonexpressing WaC3CD5 cells (Figure 5A-B). Although we observed a dramatically lower expression of c-jun mRNA in the TCL1 expressing Mec1 cells (Figure 5B), c-jun protein was comparable in both the cell lines (Figure 5A). Next, TCL1 was ectopically expressed in WaC3CD5 cells (Figure 5C). As observed previously in K562 cells expressing TCL1, the expression of c-fos and phosphorylation of c-jun as well as Elk-1 were considerably reduced (by 95% for p-Elk-1 and by 85% for c-fos and p-c-jun) with no dramatic change in c-jun protein (reduced by 15%) on TCL1 expression in WaC3CD5 cells (Figure 5C).
TCL1 expression in CLL-like cell lines inversely correlates with expression of c-fos and phosphorylation of c-jun. (A) Whole cell extracts of CLL-like cell lines Mec1 and WaC3CD5 were subjected to immunoblot analysis using antibodies indicated in the figure. (B) Real-time RT-PCR analysis of c-fos and c-jun in total RNA extracted from Mec1 and WaC3CD5 cells. The experiment was repeated twice with triplicate measurements. Error bars represent SD. P values were calculated using the Student t test. (C) WaC3CD5 cells ectopically expressing TCL1 were generated by retroviral infection. Western blot analysis using antibodies indicated in the figure was performed on whole cells extracts from vector control and TCL1 expressing WaC3CD5 cells. Quantification of bands represented as column graph.
TCL1 expression in CLL-like cell lines inversely correlates with expression of c-fos and phosphorylation of c-jun. (A) Whole cell extracts of CLL-like cell lines Mec1 and WaC3CD5 were subjected to immunoblot analysis using antibodies indicated in the figure. (B) Real-time RT-PCR analysis of c-fos and c-jun in total RNA extracted from Mec1 and WaC3CD5 cells. The experiment was repeated twice with triplicate measurements. Error bars represent SD. P values were calculated using the Student t test. (C) WaC3CD5 cells ectopically expressing TCL1 were generated by retroviral infection. Western blot analysis using antibodies indicated in the figure was performed on whole cells extracts from vector control and TCL1 expressing WaC3CD5 cells. Quantification of bands represented as column graph.
AP-1 and TCL1 regulate PTPROt promoter activity in B-CLL cell lines
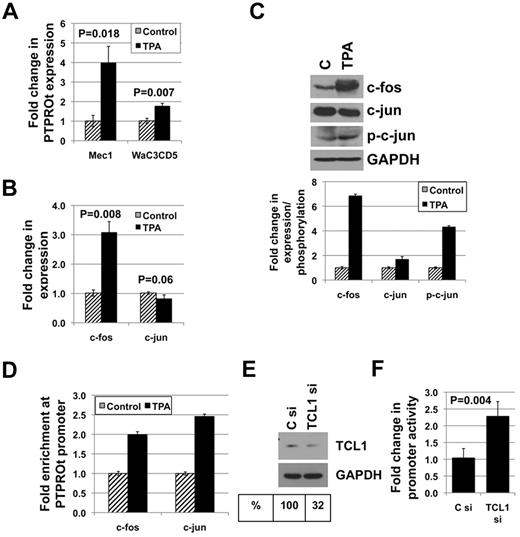
Next, we investigated whether AP-1 elements can regulate PTPROt promoter activity in B cells. For this purpose, both Mec1 and WaC3CD5 cells were treated with TPA to activate AP-1. Measurement of PTPROt expression by real-time RT-PCR demonstrated a significant induction in both cell lines (Figure 6A). Despite the expression of TCL1 in Mec1 cells, TPA-mediated induction of PTPROt was higher in these cells relative to the TCL1 nonexpressing WaC3CD5 cells, probably because of TPA-mediated inactivation of Akt that is downstream of TCL1.37,38 Furthermore, the mechanism of PTPROt suppression in WaC3CD5 cells seems to be independent of AP-1 elements that respond to TPA (see “Discussion”). It should be noted however that TPA-mediated up-regulation of PTPROt in either of these CLL-like cell lines was not as dramatic as that observed in U937 cells (compare Figures 2A and 6A). Although we have demonstrated previously PTPRO CGI methylation in both Mec1 and WaC3CD5 cells,3 we focused on transcriptional regulation by TPA because of lack of evidence for the control of PTPROt expression by methylation. Mec1 cells were used for further studies on AP-1–mediated regulation of PTPROt because of relatively higher PTPROt induction in these cells. Real-time RT-PCR (Figure 6B) and Western blot analysis (Figure 6C) of control and TPA-treated cells showed up-regulation of c-fos (3-fold at RNA level and 7-fold at protein level) but not c-jun following TPA treatment. We therefore determined the phosphorylation state of c-jun and observed an increase in c-jun phosphorylation (by 4-fold) in TPA-treated cells (Figure 6C). We also performed ChIP with antibodies against c-fos and c-jun to determine their association with the PTPROt promoter. As expected, both c-fos and c-jun were enriched at the promoter in TPA-treated cells (Figure 6D), suggesting their involvement in PTPROt induction by TPA. To confirm that AP-1 can regulate PTPROt promoter activity in Mec1 cells, a promoter-reporter construct was cotransfected along with expression constructs for c-fos, c-jun, or both. As observed previously in K562 cells, c-fos or c-jun protein alone was able to activate the promoter ∼ 2.5 fold whereas both proteins in combination had a synergistic effect on the promoter activity (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Real-time RT-PCR (supplemental Figure 1B) and Western blot analysis (supplemental Figure 1C) were performed to confirm the expression of c-fos, c-jun, or both. These data reinforce the involvement of AP-1 in regulation of PTPROt in B cells. Furthermore, regulation of PTPROt promoter by TCL1 was demonstrated by cotransfecting the promoter-reporter construct with either control siRNA or siRNA against TCL1. Knockdown of TCL1 (by ∼ 60%; Figure 6E) was associated with an ∼ 2.2-fold increase in promoter activity (Figure 6F), supporting the role of TCL1 as a transcriptional repressor of PTPROt.
AP-1 elements and TCL1 are involved in the regulation of PTPROt in CLL-like cell line Mec1. (A) Mec1 and WaC3Cd5 cells treated with 10 ng/mL TPA for 24 hours were used for real-time RT-PCR analysis of PTPROt. (B) Expression of c-fos and c-jun in Mec1 cells treated with TPA was measured by real-time RT-PCR. (C) Western blot analysis was performed to determine expression of c-fos, c-jun, p-c-jun, and GAPDH as well as phosphorylation of c-jun. Quantification of bands is represented as column graph. (D) Chromatin immunoprecipitation was performed on untreated and TPA-treated Mec1 cells using antibodies against c-fos and c-jun followed by PCR for PTPROt promoter. The data are normalized to input and represented as fold enrichment in TPA-treated cells over control cells. Mec1 cells were transfected with PTPt-P-Luc promoter reporter construct along with either control siRNA or siRNA against TCL1. (E) Western blot analysis was performed to confirm knockdown of TCL1. (F) Luciferase activity measured by a dual luciferase assay kit was normalized (firefly/Renilla) and is represented as fold change in TCL1 siRNA-transfected cells over that in control siRNA-transfected cells.
AP-1 elements and TCL1 are involved in the regulation of PTPROt in CLL-like cell line Mec1. (A) Mec1 and WaC3Cd5 cells treated with 10 ng/mL TPA for 24 hours were used for real-time RT-PCR analysis of PTPROt. (B) Expression of c-fos and c-jun in Mec1 cells treated with TPA was measured by real-time RT-PCR. (C) Western blot analysis was performed to determine expression of c-fos, c-jun, p-c-jun, and GAPDH as well as phosphorylation of c-jun. Quantification of bands is represented as column graph. (D) Chromatin immunoprecipitation was performed on untreated and TPA-treated Mec1 cells using antibodies against c-fos and c-jun followed by PCR for PTPROt promoter. The data are normalized to input and represented as fold enrichment in TPA-treated cells over control cells. Mec1 cells were transfected with PTPt-P-Luc promoter reporter construct along with either control siRNA or siRNA against TCL1. (E) Western blot analysis was performed to confirm knockdown of TCL1. (F) Luciferase activity measured by a dual luciferase assay kit was normalized (firefly/Renilla) and is represented as fold change in TCL1 siRNA-transfected cells over that in control siRNA-transfected cells.
TCL1 expression in primary CLL cells correlates with suppression of PTPROt
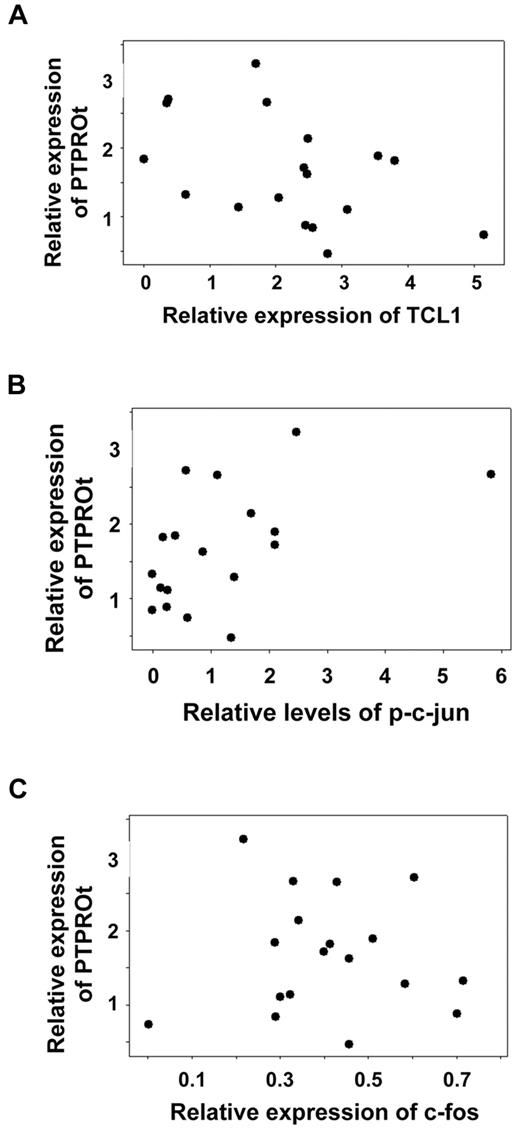
Having demonstrated the role of TCL1 and AP-1 in modulating the expression of PTPROt, it was important to determine whether similar mode of control occurs in primary CLL cells. To test this, a small cohort of primary CLL samples that were highly enriched in B cells (n = 18) was examined for expression of PTPROt by real-time RT-PCR and expression of TCL1, c-fos, and c-jun, as well as phosphorylation of c-jun by Western blot analysis (supplemental Figure 2). Using a linear regression model, a significant negative association for PTPROt expression and the level of TCL1 protein was observed (Figure 7A). A 1-unit increase in TCL1 (normalized to internal control GAPDH) corresponded to a 0.31-unit decrease in PTPROt mRNA (normalized to internal control GAPDH; P = .0228). This model also identified a significant positive association between PTPROt mRNA expression and phosphorylation of c-jun (Figure 7B). A 1-unit increase in p-c-jun (normalized to total c-jun and internal control GAPDH) corresponded to a 0.28-unit increase in PTPROt mRNA (P = .0204). However, the association between c-fos expression and PTPROt was not found to be statistically significant (P = .46; Figure 7C). It therefore seems that modulation of the phosphorylation status of c-jun may be an important determinant in the regulation of PTPROt expression in primary CLL.
Inverse correlation between expression of TCL1 and PTPROt. Whole cell extracts of primary CLL cells were subjected to Western blot analysis with antibodies against TCL1, c-fos, c-jun, p(S73)-c-jun, and GAPDH (normalizer). The autoradiographs were scanned and signal intensities were quantified using AlphaImager. Total RNA from the same samples was used for real-time RT-PCR analysis of PTPROt and GAPDH (normalizer). Each assay was performed in triplicate. Linear regression model was used for statistical correlation between normalized expression values for PTPROt mRNA and TCL1 protein (A), p-c-jun (B), or c-fos (C).
Inverse correlation between expression of TCL1 and PTPROt. Whole cell extracts of primary CLL cells were subjected to Western blot analysis with antibodies against TCL1, c-fos, c-jun, p(S73)-c-jun, and GAPDH (normalizer). The autoradiographs were scanned and signal intensities were quantified using AlphaImager. Total RNA from the same samples was used for real-time RT-PCR analysis of PTPROt and GAPDH (normalizer). Each assay was performed in triplicate. Linear regression model was used for statistical correlation between normalized expression values for PTPROt mRNA and TCL1 protein (A), p-c-jun (B), or c-fos (C).
Because PTPROt is located on chromosome 12 and trisomy 12 is a frequent alteration in CLL,39 we analyzed a set of samples with and without trisomy 12 to determine whether an extra copy of chromosome 12 in samples with detected trisomy 12 had higher expression of PTPROt. A 2-sample t test did not reveal any significant differences (P = .735) in PTPROt expression between these 2 groups of samples (supplemental Figure 3). These data suggest that despite the presence of an extra copy of chromosome 12, PTPROt remains under negative regulation by repressive mechanisms (including TCL1) in CLL.
Discussion
In this study, we used the TCL1 Tg mouse model of CLL to explore the transcriptional regulation of PTPROt in CLL. Although we have observed hypermethylation of the only CpG island located 220 kb upstream of PTPROt promoter, this region is closer to the PTPRO (encoding the full-length form expressed in cells of epithelial origin) promoter than the downstream PTPROt promoter. Furthermore, a quantitative correlation between the extent of methylation and gene suppression has not been immediately evident by analyzing methylation of CpG residues in 50 CLL samples by Massarray (R. Claus, T.M., J.C.B., C. Plass, and S.T.J., unpublished data, November 1, 2011). It therefore seems that methylation probably participates in permanent silencing of PTPROt in some primary CLL samples after initial transcriptional suppression. Although the commonly used DNA-hypomethylating agents, such as 5-AzaC or 5-AzadC (Decitabine), demethylate the C-5 methyl groups at the upstream promoter and re-express the silenced PTPROt in leukemia cells,3,5 it is not known whether or how this distant element can regulate the downstream promoter. Furthermore, there are no CpG islands in the core promoter region of PTPROt, the isoform expressed in hematopoietic cells. Extensive analysis of the 5 CpG residues in the downstream PTPROt promoter region did not reveal any inverse relationship between their methylation and PTPROt expression. Further studies could provide a mechanism for this lack of inverse correlation, a topic that is beyond the scope of the present study. An important finding of the present study is the suppression of PTPROt in the TCL1 Tg mouse model of CLL at a very early stage of development in the absence of detectable methylation at its CGI, suggesting that the major or primary control of PTPROt suppression occurs at the transcriptional level. In this context, it should be noted that recent studies using the TCL1 Tg mouse model have demonstrated early transcriptional suppression of Foxd3 that precedes methylation of its target genes.14
Previous studies have characterized promoter elements immediately upstream of the transcription start site of PTPROt1,40 ; no specific transcription factors with a well-defined role in PTPROt expression have been identified. Recent study has shown that BCL6 functions as a transcriptional repressor of PTPROt in diffuse large B-cell lymphoma.41 Database analysis for the expression of BCL6 did not reveal its up-regulation in CLL (oncomine, http://www.compendiabio.com/products/ore.htm). Furthermore, mutations at the BCL6 locus did not seem to affect its expression in CLL, suggesting that BCL6 probably does not play a significant role in CLL.42 We therefore focused our attention to identify factors that mediate PTPROt expression in normal B cells. Being a TPA-responsive gene, PTPROt was probably regulated by TPA-responsive AP-1 elements. To our knowledge, the present study is the first report to establish the direct involvement of AP-1 elements c-fos and c-jun in the transcription of PTPROt. In addition, through its inhibition of AP-1 function we have established that TCL1 is a transcriptional repressor of PTPROt. This study also has demonstrated that besides its interaction with AP-1 factors21 ; TCL1 can regulate their expression and activation state (Figure 4D). Importantly, this study also unravels an inverse relationship between the expression of TCL1 and PTPROt as well as a positive relationship between phosphorylation of c-jun and PTPROt expression in primary CLL cells. Based on this observation coupled with our previous studies demonstrating the tumor suppressor function of PTPROt5 and its down-regulation in many primary human CLL samples,3 TCL1 suppression is probably a prerequisite to PTPROt expression.
Initial transcriptional suppression followed by epigenetic changes in TCL1 target genes involves the regulation of AP-1 or NF-κB activity. It can therefore be envisioned that hypomethylation of the gene promoter may relieve epigenetic suppression but not the transcriptional repression because of TCL1 expression. We have demonstrated reactivation of PTPROt after treatment of the CLL-like WaC3CD5 cell line with 5-aza-deoxycytidine.3,14 Interestingly, this study has demonstrated that WaC3CD5 cells do not express TCL1. On the contrary, we have not observed decitabine-mediated reactivation of PTPROt in another CLL-like cell line Mec1 that expresses TCL1. It therefore seems that suppression of PTPROt and its reactivation by decitabine in WaC3CD5 cells occurs via epigenetic mechanisms independently of TCL1 expression. It would be of interest to explore other transcriptional or epigenetic factors that contribute to regulation of PTPROt expression in CLL.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Kalpana Ghoshal and Sarmila Majumder (The Ohio State University) for valuable discussions and comments. They also thank Drs Michael Karin (University of California, San Diego) for the GST-c-jun (1-79) construct; Michael Teitell (University of California, Los Angeles) for the MSCV and MSCV+TCL1 constructs; and Yuri Pekarsky for the TCL1, c-fos, and c-jun constructs as well as helpful discussions.
This work was supported, in part, by National Cancer Institute grants CA101956, CA86978 (S.T.J.), and 1K12 CA133250 (A.J.J.).
National Institutes of Health
Authorship
Contribution: T.M. and S.T.J. developed the concept, designed the experiments, and wrote the manuscript; T.M., M.K., J.D., H.K., S.R., A.M.C., Y.Z., and J.H. performed the experiments and analyzed the data; N.Z. provided large number of WT and TCL1 Tg samples as well as useful information on technical issues regarding use of the samples; A.J.J. provided some CLL samples from the TCL1 mice and useful hints regarding use of these samples; D.M.L. provided primary CLL samples highly enriched in B cells; N.A.H. provided trisomy 12 information for CLL samples; X.M. and D.J. performed statistical analysis; and J.C.B. and C.M.C. gave many useful suggestions in the course of this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samson T. Jacob, Tzagournis Medical Research Facility, 420 W 12th Ave, Suite 646D, Columbus, OH 43210; e-mail: samson.jacob@osumc.edu or Tasneem Motiwala, tasneem.motiwala@osumc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal