Abstract
Abstract  2513
2513
Autophagy, like apoptosis is a programmed cell death pathway that can be pharmacologically targeted. Apoptosis and autophagy pathways interact at the level of Bcl-2 family of proteins. Autophagy helps transformed cells to survive hypoxia, nutrient lack and chemotherapeutic stress. While expressions of pro/anti-apoptotic genes/proteins in acute myelogenous leukemia (AML) blasts have been linked to clinical outcome (Kornblau et al. Clin. Cancer Res 1999; 5:1758, Hess et al. JCO 2007;10:1209, Carter et al. Blood 2011;117:1280), the expression of autophagy related proteins (ARP) in primary AML cells and their impact on prognosis in AML has not been reported.
Quantitative expression of ARP in blast cells from 511 patients with newly diagnosed AML (divided into 3 cytogenetic risk groups) was determined by reverse phase protein array (RPPA). Analysis used unbiased clustering, perturbation bootstrap clustering, and principle component analysis. The values for ARP were divided into thirds (low, medium, and high cohorts) based on the range of expression of all 500 samples. Kaplan-Meier product limit method was used to generate overall survival (OS) and complete remission duration (CRD) curves and log-rank test was used to assess the difference between patients with high and low expressions of ARP. Univariate and multivariate (MVA) Cox proportional hazard modeling included clinical and laboratory parameters relevant to AML outcome (categorized or continuous) and protein levels as categorized variables.
We also correlated expression of ARP with 186 other proteins (encompassing signal transduction, metabolism, apoptosis pathways) that were included in RPPA.
ARP analyzed by RPPA included Beclin1, LKB1, phospho and total AMPK and p62. While Beclin 1 and p62 are proteins integral to autophagosome development and ‘cargo’ delivery to autophagosome, LKB1 and AMPK are kinases upstream of autophagy initiation. Beclin 1 also has dual role in apoptosis and autophagy; caspase mediated cleavage of Beclin 1 suppresses autophagy and enhances apoptosis.
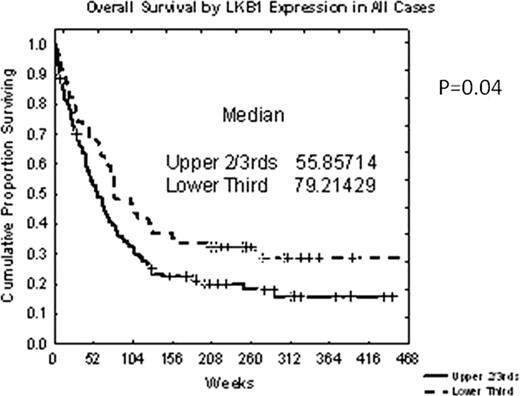
Among all patients with AML, high LKB1 expression (higher 2/3rd versus lower 1/3rd) was associated with significantly shorter OS (p=.037) (Fig. 1). MVA identified higher age, poor-risk cytogenetics, high WBC, low platelet count, low albumin and high LKB1 (p=.01) as variables associated with shorter OS.
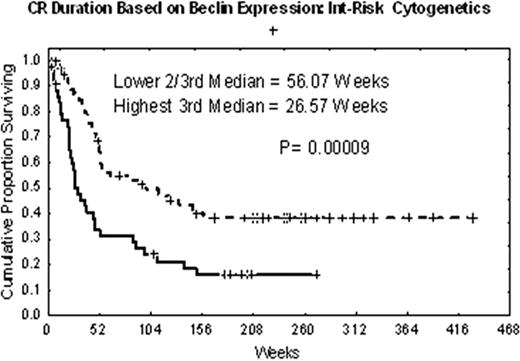
High expression of Beclin1 (high 1/3rd vs lower 2/3rd) was associated with a trend towards worse OS (p=.07) and significantly associated with shorter CRD(p=.0009) (Fig. 2) among patients with intermediate cytogenetic risk AML (AML-IR). This impact on CRD was seen regardless of FLT3-ITD mutation status. By MVA older age, FLT3-ITD mutation, high Beclin1 (p=.019 for Beclin1) were significantly associated with shorter OS, while age, FLT3-ITD and high Beclin 1 expression (p=.0009 for Beclin 1) were significantly associated with shorter CR among patients with AML-IR.
Finally, RPPA analysis restricted to CD34+cells showed highest expression of LKB1 in CD34+CD38-AML “progenitor cell” population (p=0.0000).
Expression of Beclin1 in AML cells correlated positively with Akt, mTOR, PTEN, SHIP2, TSC2, PP2A, JNK2, TAZ etc. and negatively p53, p21, Bcl-XL, HIF1 alpha, FOXO3a etc. LKB1 expression correlated positively with GSK3β, DJ1, TSC2, NFKBp65, PI3Kp110, XIAP, HSP90 etc. and negatively with FOXO3a, Bcl-2, BADp112, AIF etc.
This is the first report of prognostic impact of autophagy related protein expression in AML and their correlation with proteins of other pathways. High expression of LKB1 and Beclin1 were found to be associated with poor clinical outcome. Highest expression of LKB1 is seen in AML “progenitor” cells. Results suggest that targeting of autophagy regulators may have clinical utility in AML therapy. Integration of ARP expression data in to cellular pathways is in progress.
Grant support: ASCO Career Development Award (GB) and CA55164 from NIH(MA)
Borthakur:ASCO Career Development Award: Research Funding. Andreeff:NIH CA55164: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal