Abstract
Hydroxyurea therapy has proven laboratory and clinical efficacies for children with sickle cell anemia (SCA). When administered at maximum tolerated dose (MTD), hydroxyurea increases fetal hemoglobin (HbF) to levels ranging from 10% to 40%. However, interpatient variability of percentage of HbF (%HbF) response is high, MTD itself is variable, and accurate predictors of hydroxyurea responses do not currently exist. HUSTLE (NCT00305175) was designed to provide first-dose pharmacokinetics (PK) data for children with SCA initiating hydroxyurea therapy, to investigate pharmacodynamics (PD) parameters, including HbF response and MTD after standardized dose escalation, and to evaluate pharmacogenetics influences on PK and PD parameters. For 87 children with first-dose PK studies, substantial interpatient variability was observed, plus a novel oral absorption phenotype (rapid or slow) that influenced serum hydroxyurea levels and total hydroxyurea exposure. PD responses in 174 subjects were robust and similar to previous cohorts; %HbF at MTD was best predicted by 5 variables, including baseline %HbF, whereas MTD was best predicted by 5 variables, including serum creatinine. Pharmacogenetics analysis showed single nucleotide polymorphisms influencing baseline %HbF, including 5 within BCL11A, but none influencing MTD %HbF or dose. Accurate prediction of hydroxyurea treatment responses for SCA remains a worthy but elusive goal.
Introduction
Hydroxyurea therapy has proven laboratory and clinical efficacies for ameliorating the signs and symptoms of sickle cell anemia (SCA), primarily by increasing the level of fetal hemoglobin (HbF). Numerous studies over the past 25 years have shown unequivocally that hydroxyurea, especially when escalated to the maximum tolerated dose (MTD, defined by moderate suppression of circulating neutrophils and reticulocytes), can consistently and significantly increase the percentage of HbF (%HbF) level in infants, children, teens, and adults with SCA (reviewed in Ware and Aygun1 and Ware2 ). Beyond the expected changes in %HbF, however, hydroxyurea treatment for SCA also leads to significant and clinically beneficial increases in the hemoglobin concentration, mean corpuscular volume (MCV), and mean corpuscular hemoglobin along with simultaneous significant decreases in white blood cell (WBC) count, absolute neutrophil count (ANC), absolute reticulocyte count (ARC), and platelets because of dose-dependent marrow suppression, plus lower lactate dehydrogenase and total bilirubin levels because of reduced hemolysis.3-7
Most patients with SCA who are adherent to hydroxyurea therapy also will have symptomatic benefits for acute clinical manifestations. The double-blinded placebo-controlled phase 3 Multicenter Study of Hydroxyurea (MSH) documented significantly less vaso-occlusive pain, fewer episodes of acute chest syndrome, reduced numbers of transfusions, and fewer hospitalizations.8 These clinical results from the adult MSH trial were recently confirmed in the multicenter double-blinded placebo-controlled phase 3 Infant Hydroxyurea Study (BABY HUG).9 Long-term benefits of hydroxyurea therapy for SCA recently have been reported, including significantly reduced mortality among American adults,10 Greek adults,11 and Brazilian children12 with SCA.
Despite these robust laboratory and clinical benefits from hydroxyurea treatment for SCA, substantial phenotypic variation is observed; for example, the %HbF achieved in young patients at hydroxyurea MTD ranges from a low of 10%-15% to a high that occasionally exceeds 40% HbF.2-5,13 Additional phenotypic variability is observed in the MTD itself; some patients tolerate hydroxyurea at a dose of only 10-15 mg/kg/d before developing dose-limiting myelosuppression (typically ANC and ARC), whereas others tolerate 30-35 mg/kg/d without excessive myelosuppression.1,2,13 In many cases, a lower MTD limits the efficacy of hydroxyurea but not always; some patients have remarkable HbF responses despite a relatively low MTD.2 Most of this phenotypic variability observed with hydroxyurea treatment in SCA remains unexplained at this time, although clinically relevant differences in pharmacokinetics (PK), pharmacodynamics (PD), and pharmacogenetics (PGx) of hydroxyurea have been postulated.2,14,15
To gain a better understanding of the interpatient variability for hydroxyurea responses and toxicities, we designed a prospective clinical trial for pediatric patients with SCA receiving hydroxyurea treatment, named the Hydroxyurea Study of Long-Term Effects (HUSTLE, NCT00305175). A primary objective of this study was to determine the first-dose PK parameters for children commencing hydroxyurea treatment, using a fixed oral dose of 20 mg/kg. After these children underwent standardized dose escalation to MTD, their PD parameters were evaluated, allowing investigation of predictors of hydroxyurea response. Finally, a candidate gene study was performed to provide initial PGx analyses for the hydroxyurea MTD end points of %HbF and MTD.
Methods
Study population
All subjects with SCA enrolled in the prospective HUSTLE clinical trial were eligible for analysis. This study was approved by the St Jude Institutional Review Board, and all subjects were tested only after written informed consent was given in accordance with the Declaration of Helsinki. Young patients who enrolled before beginning hydroxyurea treatment composed the New Cohort, which included a variety of baseline clinical and laboratory parameters (eg, age, sex, height, weight, body mass index [BMI], body surface area [BSA], blood pressure, complete blood count with WBC differential and reticulocyte count, HbF, alanine aminotransferase [ALT], bilirubin, creatinine, cystatin C, measured glomerular filtration rate) plus the first-dose PK studies. Patients already receiving hydroxyurea treatment at the time of HUSTLE enrollment composed the Old Cohort, which included most baseline testing available through chart review but no opportunity to perform first-dose PK studies. Clinical indications for initiating hydroxyurea therapy were typically recurrent acute vaso-occlusive complications such as pain and acute chest syndrome, although additional laboratory and clinical criteria were also considered.13 All participants were steadily escalated to hydroxyurea MTD with periodic monitoring of laboratory parameters and medication adherence as previously described,5,13 by experienced health care providers who were specifically masked to all knowledge or consideration of the first-dose PK results.
First-dose PK
Subjects in the New Cohort were given a single 20-mg/kg oral dose of hydroxyurea for first-dose PK analysis. Early in the study both capsules and liquid formulations were offered, but subsequently only liquid hydroxyurea was provided to allow consistency and more accurate dosing. Families were instructed to give their child no food for ≥ 6 hours and no liquids for ≥ 2 hours before taking the initial hydroxyurea dose. Approximately 2-3 mL of venous blood was collected by phlebotomy or through a peripheral IV at the following time points: baseline (T = 0), then at 15, 30, 60, 120, 240, and 480 minutes after the hydroxyurea dose. Blood was allowed to clot, and then the serum was separated and stored frozen at −85°C until analysis. The hydroxyurea concentration of each serum specimen was determined with a published colorometric technique16 but with slight modifications from the previously described method, including testing 250-μL aliquots in triplicate, and with the use of a standard curve ranging from 0 to 1000μM.17
On the basis of the hydroxyurea concentrations at each time point, statistical analyses were performed to generate standard PK parameters with the use of the PK Solutions Version 2.0 software program (Summit Research Services). The maximum plasma concentration (Cmax) and the time of maximum plasma concentration were the observed values. The area under the concentration-time curve (AUC) from time zero to the time of the final quantifiable sample (AUC[tf]) was calculated with the log-linear trapezoidal method. In addition, the AUC from time zero to infinity (AUC[inf]) was calculated by extrapolation to infinity by dividing the last measured concentration by the terminal rate constant, λz, which was determined from the slope of the terminal phase of the concentration-time curve with the use of weighted least-squares as the estimation procedure and inverse variance of the output error (linear) as the weighting option. The terminal half-life (t1/2,z) was calculated as 0.693 divided by λz. Estimated PK parameters also included the mean residence time (MRT), apparent volume of distribution, and apparent oral clearance (CL/F). A preliminary analysis on data from the first 27 patients with the use of a linear, 1-compartment model with first-order elimination (no lag-time) provided similar results for CL/F compared with the noncompartmental method (median, 7.35 L/h vs 7.85 L/h). Therefore, noncompartmental approaches were used in subsequent patients. The PK profiles of hydroxyurea after administration of the capsule and liquid formulations were essentially bioequivalent and interchangeable (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), and results from both formulations were combined in subsequent analyses.
Pharmacodynamics
Standard laboratory parameters were collected at baseline and at regular intervals during dose escalation to MTD and periodically thereafter as described.5 The %HbF determination was performed with HPLC and included the acetylated HbF peak. Because some subjects had received erythrocyte transfusions before their hydroxyurea initiation, the %HbF was recalculated as the (HbF)/(HbF + HbS) to remove mathematically any exogenous transfused HbA as previously described.13 The actual MTD was determined by experienced clinical providers, usually after 6-9 months of treatment; typically this was when mild neutropenia (target ANC of 2-3 × 109/L) was achieved, although some subjects had dose-limiting reticulocytopenia (ARC of 50-100 × 109/L). Among the baseline and MTD parameters measured and collected, the baseline %HbF, the MTD %HbF, change in HbF, and the MTD dose itself were the primary end points for PD analyses.
Pharmacogenetics
Candidate genes were identified and selected on the basis of their presumed effects on some aspect of hydroxyurea PK or PD, including ribonucleotide reductase as the primary drug target, plus specific hydroxyurea drug transporters,18 known HbF modifiers,19,20 and other suggested candidate genes.15 For some candidate genes, specific published single nucleotide polymorphisms (SNPs) were available for direct analysis, but for other genes a preliminary discovery phase was required with bidirectional sequencing to identify SNPs within the promoter, exons, or 3′ untranslated regions. In this discovery process, only promoter and exonic SNPs with a minor allele frequency of ≥ 0.10 in African American control DNA specimens were included in the PGx analysis. Because we used a relatively small DNA sample size for this SNP search, we selected an allele frequency > 10% to test rigorously for associations with our phenotypes of interest, while reducing the possibility of finding false-positive associations. All SNP discoveries and genotyping were determined by Big Dye terminator sequencing. A total of 331 SNPs were identified (124 novel) of which 70 SNPs in 18 genes were selected for further genotyping on the basis of allele frequency. All SNPs were typed by Big Dye terminator sequencing or TaqMan probes (Applied Biosystems). Statistical comparisons in the HUSTLE samples were performed only for the best candidate genetic polymorphisms: 12 SNPs in 7 genes in association with PK variables and 23 SNPs in 11 genes in association with PD variables. In addition, α-thalassemia status and β-globin haplotype were determined on all samples.
Statistical methods
Descriptive statistics were used to summarize all clinical and laboratory results, as well as PK parameters, and were typically reported as mean ± SD. Interpatient variability in PK parameters was assessed with the coefficient of variation. The effect of dichotomous variables (eg, sex) on the CL/F was evaluated with a nonparametric Mann-Whitney rank sum test. Associations between continuous variables (eg, weight, height, BSA, BMI, age, ALT, serum creatinine, measured glomerular filtration rate) and CL/F were evaluated with the use of linear regression modeling.
Associations were next tested in univariate analysis between each PK variable and the continuous PD variables baseline %HbF, MTD %HbF, and MTD (mg/kg) with the use of linear regression modeling. These results were considered exploratory and were not adjusted for multiple comparisons. Subsequently, the PK variables were included as covariates to construct models to predict the MTD values; the Lasso method21,22 and the Cp statistics were used to select the best subset of variables for the predictions. Finally, each candidate SNP was tested independently for association with selected PK or PD variables under 3 genetic models (dominant, codominant, and recessive) adjusting for a false discovery rate (FDR) of 0.10.
Results
Subjects
A total of 88 subjects were enrolled in the New Cohort, of whom 61 had reached MTD at the time of data analysis. The remaining 27 subjects did not have MTD variables recorded for the following reasons: inadequate medication adherence (n = 13), insufficient time after drug initiation to reach MTD (n = 7), inability or unwillingness to undergo MTD studies (n = 4), family relocation before reaching MTD (n = 2), and dual therapy with transfusions (n = 1). The average age at hydroxyurea initiation was 9.6 ± 4.8 years, with a male/female ratio of 57:31. For the New Cohort, the average baseline %HbF level was 9.6% ± 6.9% (median, 8.3%; range, 0.0%-30.6%) and increased to 26.5% ± 8.5% at treatment MTD (median, 26.2%; range, 9.4%-55.9%). The average hydroxyurea dose at MTD was 23.9 ± 5.1 mg/kg/d (median, 23.6 mg/kg/d; range, 14.2-35.5 mg/kg/d). All laboratory parameters changed in the predicted direction on hydroxyurea treatment, including significant increases in Hb, MCV, and mean corpuscular hemoglobin as well as significant decreases in WBC, ANC, ARC, platelets, total bilirubin, and lactate dehydrogenase (data not shown).
A total of 86 additional subjects composed the Old Cohort; some were missing baseline HbF values (typically patients who started hydroxyurea many years earlier while on chronic transfusions), but all had current MTD laboratory values that could be used for PD analysis. The %HbF at MTD for the Old Cohort (average, 27.5% ± 7.4%) was similar to the New Cohort subjects, and the MTD dose was also similar (26.6 ± 3.2 mg/kg/d). Altogether there were 174 subjects analyzed, including 157 with all baseline PD laboratory values and 147 with MTD values.
Pharmacokinetics
All 88 New Cohort subjects received first-dose PK with a single oral dose of hydroxyurea at 20 mg/kg. One subject had incomplete PK studies because of a sample collection breakage at the critical 30-minute time point, so these data were excluded from further analysis. Another subject accidentally received an initial dose of 30 mg/kg so her PK parameters were adjusted accordingly to the intended dose of 20 mg/kg.
The first-dose PK values from 87 evaluable patients are summarized in Table 1 and supplemental Figure 1 and clearly show the substantial degree of interpatient variability for hydroxyurea. The CL/F varied ∼ 14-fold, and almost 65% of this variability could be accounted for by weight (supplemental Figure 2). This finding underscores the critical importance of applying a correction for weight differences in the calculation of hydroxyurea dosage in children. The CL/F was also statistically significantly related with age, height, BSA, BMI, bilirubin, ALT, aspartate aminotransferase, blood urea nitrogen, and creatinine, but not with sex. However, in a multiple variable analysis only weight (P < .0001), ALT (P = .0055), and serum creatinine (P = .0098) were retained in the final model as significant covariates for CL/F. In this final model (R2 = 0.709), ALT and serum creatinine contributed little to the observed variability beyond weight.
Univariate summary statistics of first-dose hydroxyurea pharmacokinetics in 87 children with SCA
| Variable . | Mean . | SD . | Median . | Minimum . | Maximum . | CV (%) . |
|---|---|---|---|---|---|---|
| AUC [inf], μg·h/mL | 92.98 | 23.37 | 94.66 | 40.89 | 150.34 | 25.1 |
| CL/F, L/h | 6.92 | 3.17 | 6.46 | 1.57 | 21.59 | 45.8 |
| CL/F, L/h per kg | 0.24 | 0.09 | 0.21 | 0.12 | 0.80 | 37.5 |
| Cmax, μg/mL | 26.13 | 6.83 | 25.40 | 12.85 | 45.48 | 26.1 |
| Half-life, h | 1.70 | 0.53 | 1.67 | 0.65 | 3.05 | 31.2 |
| MRT, h | 3.17 | 0.78 | 2.99 | 2.08 | 6.08 | 24.6 |
| Tmax, h | 0.82 | 0.47 | 0.57 | 0.25 | 2.20 | 57.3 |
| V/F, L | 12.09 | 7.59 | 10.86 | 2.50 | 52.44 | 62.8 |
| Variable . | Mean . | SD . | Median . | Minimum . | Maximum . | CV (%) . |
|---|---|---|---|---|---|---|
| AUC [inf], μg·h/mL | 92.98 | 23.37 | 94.66 | 40.89 | 150.34 | 25.1 |
| CL/F, L/h | 6.92 | 3.17 | 6.46 | 1.57 | 21.59 | 45.8 |
| CL/F, L/h per kg | 0.24 | 0.09 | 0.21 | 0.12 | 0.80 | 37.5 |
| Cmax, μg/mL | 26.13 | 6.83 | 25.40 | 12.85 | 45.48 | 26.1 |
| Half-life, h | 1.70 | 0.53 | 1.67 | 0.65 | 3.05 | 31.2 |
| MRT, h | 3.17 | 0.78 | 2.99 | 2.08 | 6.08 | 24.6 |
| Tmax, h | 0.82 | 0.47 | 0.57 | 0.25 | 2.20 | 57.3 |
| V/F, L | 12.09 | 7.59 | 10.86 | 2.50 | 52.44 | 62.8 |
AUC is the total drug exposure (serum concentration over time) either until the final time point (tf) or extrapolated to infinity (inf), the difference between those two (extra), and D is dose.
CL/F indicates apparent oral clearance; Cmax, the maximum concentration; MRT, mean residence time (average time drug resides in the body); Tmax, time to Cmax; and V/F, apparent volume of distribution.
The majority of patients (51 of 87; 59%) showed a phenotypic “Fast” absorption profile (defined as Cmax at 15 or 30 minutes) whereas 36 of 87 (41%) had a “Slow” absorption profile (Cmax at 60 or 120 minutes), as shown in Figure 1. Compared with the Slow absorption phenotype, persons with the Fast phenotype had a significantly higher Cmax (28.9 ± 6.9 μg/mL vs 22.2 ± 4.3 μg/mL; P < .001) while having a lower MRT (2.9 ± 0.5 hours vs 3.6 ± 0.9 hours; P < .001). This novel finding suggests that the rate of absorption of hydroxyurea is an important determinant of certain measures of systemic exposure.
Concentration-time profiles of hydroxyurea in young patients with SCA Shown are representative examples of “Fast” phenotype with peak concentration at 15-30 minutes (59% of patients) and the “Slow” phenotype with Cmax at either 60 or 120 minutes (41% of patients).
Concentration-time profiles of hydroxyurea in young patients with SCA Shown are representative examples of “Fast” phenotype with peak concentration at 15-30 minutes (59% of patients) and the “Slow” phenotype with Cmax at either 60 or 120 minutes (41% of patients).
Pharmacodynamics
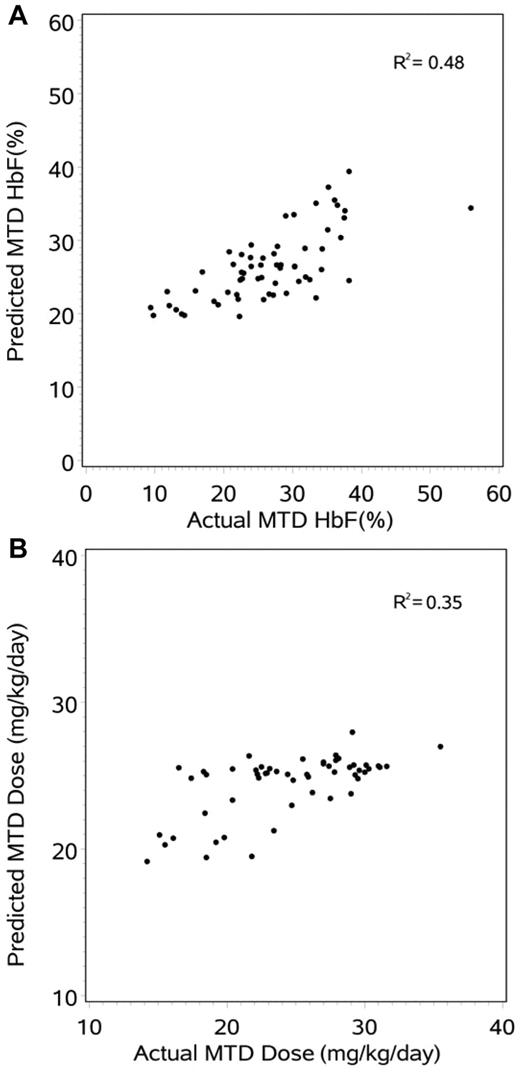
For associations with the laboratory end point of %HbF at MTD, univariate regression modeling identified 6 of 26 clinical or laboratory variables with P values < .10: baseline HbF (P < .0001, R2 = 0.40), baseline total bilirubin (P = .0097, R2 = 0.11), age (P = .0121, R2 = 0.10), height (P = .0491, R2 = 0.06), baseline ARC (P = .0650, R2 = 0.06), and cystatin C (P = .0705, R2 = 0.06). Because the baseline %HbF was such an important and potent predictor of the MTD HbF, these analyses were repeated after controlling for baseline HbF and identified only 2 additional variables that influenced MTD HbF: ARC (P = .0127) and BMI (P = .0534). Next, the individual first-dose PK parameters were tested for associations with the %HbF at MTD and identified 3 of 13 variables with P values < .10: MRT (P = .0124, R2 = 0.10), terminal half-life (P = .0753, R2 = 0.05), and Fast/Slow absorption (P = .0982, R2 = 0.05). After controlling for baseline HbF, however, none of these PK variables was significantly associated with the MTD %HbF. Finally, multiple variable modeling was performed and suggested 5 significant variables for optimal prediction of MTD %HbF (Table 2) that together provided moderate predictive value (R2 = 0.48). Figure 2A shows the predicted %HbF at MTD with the use of this model, compared with the actual MTD HbF.
Multiple variable modeling for hydroxyurea pharmacodynamics variables MTD HbF (%) and dose (mg/kg/d)
| PD variable/order of selection . | Variable . | Direction of the association . |
|---|---|---|
| MTD HbF, % | ||
| 1 | Baseline HbF | Positive |
| 2 | Baseline ARC | Positive |
| 3 | Baseline total bilirubin | Negative |
| 4 | Baseline BMI | Positive |
| 5 | First-dose MRTinf | Negative |
| MTD, mg/kg/d | ||
| 1 | Baseline creatinine | Negative |
| 2 | Baseline BMI | Negative |
| 3 | First-dose half-life | Negative |
| 4 | Baseline ARC | Positive |
| 5 | Fast PK phenotype | Positive |
| PD variable/order of selection . | Variable . | Direction of the association . |
|---|---|---|
| MTD HbF, % | ||
| 1 | Baseline HbF | Positive |
| 2 | Baseline ARC | Positive |
| 3 | Baseline total bilirubin | Negative |
| 4 | Baseline BMI | Positive |
| 5 | First-dose MRTinf | Negative |
| MTD, mg/kg/d | ||
| 1 | Baseline creatinine | Negative |
| 2 | Baseline BMI | Negative |
| 3 | First-dose half-life | Negative |
| 4 | Baseline ARC | Positive |
| 5 | Fast PK phenotype | Positive |
Prediction modeling of the hydroxyurea response. (A) The 5-variable model for predicting %HbF response (R2 = 0.48) which is dominated by baseline HbF. (B) The 5-variable model for predicting MTD dose (R2 = 0.35).
Prediction modeling of the hydroxyurea response. (A) The 5-variable model for predicting %HbF response (R2 = 0.48) which is dominated by baseline HbF. (B) The 5-variable model for predicting MTD dose (R2 = 0.35).
Similar analyses were then performed for the PD variable MTD dose in mg/kg and identified 6 of 26 clinical and laboratory variables with P values < .001: creatinine (P < .0001, R2 = 0.30), weight (P < .0001, R2 = 0.25), BSA (P < .0001, R2 = 0.25), age (P = .0001, R2 = 0.22), height (P = .0001, R2 = 0.22), and BMI (P = .0007, R2 = 0.18). Next, the individual PK parameters were tested for associations with MTD dose and identified 6 of 13 variables with P values < .01: Cmax/D (P < .0001, R2 = 0.28), AUCextra (P = .0001, R2 = 0.22), MRTinf (P = .0002, R2 = 0.22), AUC[inf] (P = .0002, R2 = 0.21), Vz/F (P = .0009, R2 = 0.17), and half-life (P = .0022, R2 = 0.15). Multiple variable modeling suggested 5 significant variables for optimal prediction of MTD dose (Table 2) that together provided modest predictive value (R2 = 0.35). Figure 2B shows the predicted MTD dose with the use of this model, compared with the actual MTD.
Pharmacogenetics
Initially, the associations of α-thalassemia status and β-globin haplotype were investigated with both baseline and MTD %HbF levels. As expected the Senegal haplotype, containing the XmnI γ-globin promoter polymorphism, was associated with higher baseline %HbF (data not shown). However, no β-globin haplotype had any significant association with MTD %HbF, after adjustment for baseline %HbF. Similarly, α-thalassemia status had no significant association with MTD HbF, after adjustment for baseline %HbF, and also no correlation with the MTD dose. A total of 23 SNPs in candidate genes were then compared with these hydroxyurea PD variables. After adjusting for a FDR of 0.10, there were 7 SNPs found to be significantly associated with the baseline %HbF, 2 SNPs significantly associated with the change in %HbF between baseline and MTD, and 1 SNP significantly associated with the MTD dose, but none was associated with the MTD %HbF (Table 3). An additional 12 SNPs were analyzed against 13 first-dose PK variables and baseline HbF. None of these comparisons reached statistical significance after the FDR adjustment, although 6 SNPs showed modest associations with critical PK parameters and had unadjusted P values < .05 (Table 4).
SNP association tests with statistical significance after controlling for the FDR
| PD variable/SNP . | Location . | P . |
|---|---|---|
| Baseline HbF, % | ||
| rs7130110 | Hb Epsilon | 3.28 × 10−5 |
| rs7482144 | XmnI | 3.28 × 10−5 |
| rs1427407 | BCL11A | 3.34 × 10−5 |
| rs766432 | BCL11A | 1.04 × 10−4 |
| rs4671393 | BCL11A | 2.88 × 10−4 |
| rs7557939 | BCL11A | 6.09 × 10−4 |
| rs11886868 | BCL11A | 1.68 × 10−3 |
| HbF change, % | ||
| rs2295644 | ARG2 | 3.33 × 10−3 |
| rs17599586 | ARG1 | 4.04 × 10−3 |
| MTD, mg/kg | ||
| rs1427407 | BCL11A | 4.78 × 10−3 |
| PD variable/SNP . | Location . | P . |
|---|---|---|
| Baseline HbF, % | ||
| rs7130110 | Hb Epsilon | 3.28 × 10−5 |
| rs7482144 | XmnI | 3.28 × 10−5 |
| rs1427407 | BCL11A | 3.34 × 10−5 |
| rs766432 | BCL11A | 1.04 × 10−4 |
| rs4671393 | BCL11A | 2.88 × 10−4 |
| rs7557939 | BCL11A | 6.09 × 10−4 |
| rs11886868 | BCL11A | 1.68 × 10−3 |
| HbF change, % | ||
| rs2295644 | ARG2 | 3.33 × 10−3 |
| rs17599586 | ARG1 | 4.04 × 10−3 |
| MTD, mg/kg | ||
| rs1427407 | BCL11A | 4.78 × 10−3 |
SNPs associated with PK parameters
| PK variable/SNP . | Location . | P . |
|---|---|---|
| AUC, μg·h/mL | ||
| rs16978449 | UTA | .036 |
| Cmax, μg/mL | ||
| rs9960464 | UTA | .008 |
| rs12605147 | UTB | .018 |
| rs2298720 | UTB | .024 |
| CL/F, L/h | ||
| rs12587848 | ARG2 | .025 |
| MRT, h | ||
| rs2295644 | ARG2 | .008 |
| PK variable/SNP . | Location . | P . |
|---|---|---|
| AUC, μg·h/mL | ||
| rs16978449 | UTA | .036 |
| Cmax, μg/mL | ||
| rs9960464 | UTA | .008 |
| rs12605147 | UTB | .018 |
| rs2298720 | UTB | .024 |
| CL/F, L/h | ||
| rs12587848 | ARG2 | .025 |
| MRT, h | ||
| rs2295644 | ARG2 | .008 |
Discussion
In the modern era of medicine emphasizing optimized treatment regimens for individual patients and with “personalized medicine” as a stated goal for the National Institutes of Health and numerous medical conferences, the management of children and adults with SCA is far from ideal. Even in the United States, many patients with SCA do not receive adequate pain management23 and are not prescribed hydroxyurea,24,25 despite its documented laboratory and clinical efficacies,5,6,26-28 mortality reduction10,11 and acceptable safety profile29,30 with long-term use. A variety of treatment barriers still exist for the wider use of hydroxyurea, but not all reside with health care providers and patients.31 With almost 50 years of treatment experience with the use of hydroxyurea in humans, including almost 30 years in SCA, surprisingly little is known about drug transport, metabolism, distribution, and clearance; even less is known about the reasons for interindividual variability in the treatment response.
Hydroxyurea is known to have excellent oral bioavailability32 and relatively rapid clearance with a measured half-life of 2-4 hours in both children and adults,3,33 which primarily depends on renal function14 and recently was shown to use active drug transport mechanisms.18 In the prospective HUSTLE protocol, we investigated the drug PK, PD, and PGx characteristics of hydroxyurea in young patients with SCA, reasoning that a better understanding of interindividual variability might eventually expand and optimize its use in this patient population. The unique study design with both New Cohort and Old Cohort patients allowed careful PK, PD, and PGx data collection on the largest number of patients treated with hydroxyurea to MTD reported to date.
The first-dose PK data provided clear documentation of rapid oral absorption of a fixed dose of 20 mg/kg, with peak serum levels averaging 26 μg/mL (∼ 400μM) that rapidly declined and were typically not detectable after 8 hours. The data also showed substantial interpatient variation as previously reported in adults3,33 ; coefficients of variation > 30% were observed for several PK parameters (Table 1). The average calculated half-life of the drug was < 2 hours, which is slightly lower than reported for adults and probably reflects this young patient population that commonly has glomerular hyperfiltration.34 Somewhat unexpectedly, a variable and binary absorption phenotype for oral hydroxyurea was noted (Figure 1). This observation is novel and potentially relevant clinically, because the Fast/Slow phenotype significantly affected first-dose measures of systemic exposure and was significantly associated with the MTD; children with fast clearance had higher MTD. Future studies should focus on the physiologic and genetic mechanisms underlying this variable absorption phenotype and determine its role in the treatment response to hydroxyurea.
PD parameters were obtained after a standardized schedule of hydroxyurea dose escalation to MTD as previously described.2,13 Importantly, dose escalation to MTD for HUSTLE was performed by experienced health care providers who were masked to any knowledge about first-dose PK data. At MTD, both the average %HbF response and the average hydroxyurea dose were almost identical between the New and Old Cohort subjects and were similar to previously published data for children with SCA at hydroxyurea MTD.1,4,5 Predictors of the hydroxyurea treatment response, particularly the %HbF, have been previously proposed; in the original phase 1/2 trial for adults with SCA, the best HbF responses occurred in subjects with higher baseline HbF and baseline WBC count.5 In the phase 3 MSH study the best responses occurred in adults with higher baseline ANC and ARC, as well as those with greater treatment-associated changes in ANC and MCV, but baseline HbF was not predictive.35 In the phase 1/2 trial for school-aged children with SCA, the highest HbF responses were observed in subjects with higher baseline HbF, WBC count, and ARC, plus those children with the largest treatment-associated changes in ARC, WBC count, and MCV level.36 Published genetic predictors of treatment HbF are even more limited; the lack of CAR haplotype was beneficial in MSH,35 whereas the presence of the XmnI γ-globin promoter polymorphism was not required for a robust effect among Indian patients.37 In the current study, we focused more on baseline clinical laboratory parameters, plus first-dose PK parameters, as potential predictors of either the %HbF response or the MTD dose itself. Although a variety of parameters had significant association in univariate analysis, subsequent multiple variable analysis identified only 5 specific parameters with significant associations with MTD HbF and 5 for MTD dose (Table 2). However, both of these models provided incomplete prediction of the hydroxyurea treatment response at MTD (Figure 2), indicating that a substantial amount of the hydroxyurea effect still remains unexplained.
PGx analysis was performed with candidate genes for baseline and MTD PD parameters, as well as possible associations with the measured and calculated PK parameters. As shown in Table 3, only 7 SNPs were associated with the baseline %HbF, including 5 within the BCL11A gene locus; these data confirm the critical importance of this gene for regulation of baseline HbF levels. However, no SNP tested was associated with the MTD HbF, indicating that these recently elucidated regulators of HbF are probably not critical for the HbF response to hydroxyurea beyond their baseline effects. Two SNPs in ARG1 and ARG2, respectively, were associated with the HbF change, but no pathophysiologic mechanism is clear at this time; however, the rs2295644 marking the ARG2 gene locus was associated with the MRTinf (P = .008) and has been previously implicated in renal disease,38 so it could potentially affect renal clearance of the drug, the AUC, and possibly the MTD dose. In contrast, after adjustment for FDR, no SNPs were predictive of any PK parameter, highlighting the limited current understanding of hydroxyurea absorption, distribution, metabolism, and excretion. However, several comparisons had unadjusted P values < .05 that may warrant future exploration (Table 4). These include associations between measures of exposure to hydroxyurea and SNPs in the genes encoding the urea transporters UTA and UTB, which we reported recently to recognize hydroxyurea as a transported substrate.18 Our candidate gene analysis was necessarily limited by the availability of known or potential genetic modifiers, and newer potential candidates such as catalase39 may also be important. Unbiased genome-wide association studies may ultimately be the most fruitful approach for elucidating the genetic predictors of the hydroxyurea response.
These prospective PK, PD, and PGx analyses provide important data and initial clues to the interindividual variability of hydroxyurea response, especially the %HbF at MTD. However, accurate prediction of hydroxyurea effects and toxicities still remains unavailable, and the standardized dose escalation to mild myelosuppression is currently the best option. Because the use of hydroxyurea probably expands for more and younger children with SCA,40 the development of a predictive model for MTD HbF and MTD dose that potentially includes baseline laboratory and clinical variables, first-dose PK studies, and genetic modifiers remains a worthy but elusive goal. Such a model may help overcome the barriers to hydroxyurea utilization that currently limit its widespread use among children with SCA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the clinical providers, as well as the patients and families, who participated in this study. The St Jude Hartwell Center sequencing core facility and staff are also acknowledged for support of this project.
This work was supported by the National Heart, Lung, and Blood Institute (R01-HL-090941 and U54-HL-070590-07, R.E.W.) and the American Lebanese Syrian Associated Charities (ALSAC).
National Institutes of Health
Authorship
Contribution: R.E.W. and A.S. designed the study, performed research, analyzed data, and wrote the paper; J.M.D., N.A.M., J.M.F., and B.A. performed research and analyzed data; J.H. and A.C.K. performed research; M.P.S. and S.W. analyzed data; and T.H. designed the study, performed research, and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Russell E. Ware, International Hematology Center of Excellence, Baylor College of Medicine, 1102 Bates St, Houston, TX 77030; e-mail: reware@bcm.edu.